Ramanathan Sh. (Ed.) Thin Film Metal-Oxides: Fundamentals and Applications in Electronics and Energy
Подождите немного. Документ загружается.

304 T.P. Holme et al.
10.1 Introduction
Selection of catalyst materials with optimal properties is a combinatorial problem.
Trial and error by brute force searches are expensive and do not yield greater sci-
entific understanding about what makes a good catalyst. The efficiency and at least
semiquantitative accuracy of ab initio simulations imply that calculations may help
to search the large space of materials combinations, and furthermore calculations
may give insight that enable intelligent catalyst design. The work of Hammer and
N¨orskov laid the foundations for this field by elucidating the relation between the
transition metal electron d-band energy with the adsorption strength for adsorption
reactions [1].
The oxygen dissociation reaction is especially important in intermediate and low-
temperature fuel cells such as solid oxide fuel cells (SOFCs) that are promising
devices for clean, efficient energy conversion. To reduce cost, SOFCs that run effi-
ciently at lower operating temperatures must be developed.
Sluggishness of the electrochemical oxygen dissociation reaction at the cathode
is a significant barrier to lower temperature operation. Commercial SOFC cathodes
are typically mixed electronic-ionic conductors such as La
x
Sr
1x
Co
y
Fe
1y
O
3
.To
enhance intermediate temperature catalysis, noble metal cathodes have been in-
vestigated [2]. The present contribution presents a method to design catalysts for
heterogeneous dissociative adsorption reactions, and demonstrates the technique to
find a compound Ag–Pt catalyst that is superior under nearly reversible conditions
to the standard Pt catalyst for oxygen dissociative adsorption.
Quantum simulations are an effective tool to study catalysis because they allow
the direct calculation of transition-state structures and energies, and visualization
of orbitals participating in the catalytic reaction to build intuition in the design of
optimal catalysts. Among many previous studies of Pt clusters, work by Xu shows
that clusters assume metallic behavior for clusters larger than seven atoms [3]. Stud-
ies have examined oxygen reduction on Pt clusters [4], Pt slabs [5],andAgslabs
[6]. In this study, quantum chemical simulations were used to determine the relative
ability of Pt
n
Ag
m
clusters and PtAg
x
slabs of differing composition to catalyze the
dissociative adsorption of oxygen on catalysts that occurs at an SOFC cathode. As
a benchmark, similar calculations were performed on clusters and slabs of pure Pt
and Ag of varying size.
Catalytic activity of a metal cluster depends upon the size of the catalyst particle:
as a cluster grows in size from one atom, the metallic bonding forms low-energy
states for catalysis; however, there is an optimum in catalytic activity, as surface
atoms in nanoclusters are more reactive than bulk material. This optimum was found
experimentally for Au clusters [7]. Therefore this study considers Ag and Pt clusters
of varying sizes.
To enable fine-tuning of control over desired properties that may be not found
in elemental compounds, creation of alloys and bilayers has been used to achieve
higher catalytic activity [8]. Precise control over bilayers with surface layers of one
or less than one monolayer has been demonstrated [9]. This study examines a Pt/Ag
bilayer to study the electronic structure of such bilayers. A random alloy is also
studied for comparison with bilayer structures.
10 Design of Heterogeneous Catalysts and Application to Oxygen Reduction Reaction 305
Finally, as Mavrikakis shows, adsorption strength depends upon the strain state
of the metal surface [10]. The strain of PtAg
x
surfaces was examined by X-ray
diffraction (XRD), and the strain effect was taken into account in calculations to
show that the alloying effect, rather than the strain effect, is responsible for the
enhanced activity of PtAg
x
catalysts.
From the base cases of Ag
8
and Pt
8
clusters, this study varies the following
parameters one by one: cluster size and composition, strain, and system spin multi-
plicity. The study considers Ag, Pt, and AgPt random alloy low surface energy (111)
slabs and an Ag/Pt bilayer. Oxygen adsorption, and dissociation, when possible, is
studied and compared with experiment.
10.2 Theory
Optimal catalytic activity is often exhibited by materials that are able to form bonds
of intermediate strength with adsorbates.
A balance should be struck by the metal surface: it must be reactive enough
with the adsorbate to catalyze the reaction but for a sufficiently weak bond with the
reaction products that they are free to desorb. One way to refine this criteria is to
require that the adsorption process be thermodynamically reversible .G
rxn
D 0/
to minimize losses. From this requirement, we may derive an optimal adsorption
energy. For an isothermal, reversible reaction,
G
rxn
D H
rxn
TS
rxn
D 0:
As the entropy of adsorbed species is much lower than the entropy of gaseous
species, S
rxn
is approximated to be the entropy of the adsorbate in the gas phase
S
rxn
S
.g/
. For oxygen, at T D 200
ı
Cand400
ı
C, a reasonable range of operat-
ing temperatures for low-temperature SOFCs, this translates to adsorption energies
of H
rxn
1:1 and 1.6 eV, respectively. It is noted that this simplistic estimate
neglects the entropy of adsorbed species, resulting in a prediction of reversible ad-
sorption at somewhat greater adsorption energies than should be targeted.
Transition metal valence electron states are characterized by a half-filled s-band
and increasingly filled d-band states across the series. The s-band is relatively broad
and constant for different transition metals; trends in the chemistry of transition
metals arise mainly as a result of d-electron interaction. According to the model
proposed by Hammer and Nørskov [1], metals with d-electrons of low energy are
unable to donate much charge to adsorbed molecules, resulting in a weak bond.
Metals with intermediate d-band levels donate more charge to adsorbates, resulting
in a stronger bond. Metals with very energetic d-electrons are strong charge donors
to adsorbates, resulting in a weaker bond as charge is donated into antibonding
orbitals. Therefore, a requirement on the bond strength between an adsorbate and a
metal implies a requirement on metal d-band level.
306 T.P. Holme et al.
Table 10.1 Electron d-band centroid and Fermi level for common catalyst transition metals
Fe Co Ni Cu Zn
"
f
D4:40 "
f
D5:06 "
f
D5:27 "
f
D4:87 "
f
D4:05
"
d
D1:42 "
d
D1:30 "
d
D1:27 "
d
D2:20 "
d
D7:14
Ru Rh Pd Ag Cd
"
f
D5:06 "
f
D5:14 "
f
D5:37 "
f
D4:54 "
f
D3:75
"
d
D2:08 "
d
D2:09 "
d
D1:71 "
d
D3:94 "
d
D8:66
Os Ir Pt Au Hg (liquid)
"
f
D5:34 "
f
D5:58 "
f
D5:77 "
f
D5:33
"
d
D2:48 "
d
D2:61 "
d
D2:24 "
d
D3:27
Fermi level ."
f
/ and d-band centroid ."
d
/ givenineV,"
f
referenced to vacuum, "
d
referenced to the
Fermi level
Using the metal d-band as a guide to reactivity with adsorbates, an algorithm
to design a reversible catalyst may be developed. Table 10.1 shows the valence
d-band center and Fermi level ("
d
and "
f
, respectively) for common catalyst tran-
sition metals. When combined with experiments, calculations, or published values
of adsorption strength of a reactant on different metal surfaces, a volcano plot of cat-
alytic activity may be constructed by plotting the d-band center against adsorption
strength. A catalyst may then be designed to have an "
d
that gives high activity by
alloying elements on either side of the peak in the volcano plot. The alloying effect
of mixing two elements is to form a material with an intermediate "
d
. An alternative
strategy is to use the strain effect explained in Ref. [10], which describes that ma-
terials under tensile strain have a higher "
d
, whereas materials under compressive
strain exhibit lower "
d
. One may envision depositing nonequilibrium or nonlattice-
matched epitaxial structures of an element with a nearly ideal "
d
to optimize "
d
with
the strain effect.
As an example, this method is applied for the test case of oxygen dissociation.
Pure Pt forms a bond with oxygen that is too strong for oxygen to be readily released
after dissociation. To weaken the O–Pt bond, the d-band of Pt should be lowered, so
it may be alloyed with an element with a lower d-band. Ag is chosen in this case.
10.3 Details of Calculations
10.3.1 Cluster Model
The B3LYP method [11, 12] was chosen for its good performance/efficiency ratio.
The selection of basis sets is limited to basis sets that are available for the relatively
heavy metals Ag and Pt. The available basis sets were tested for their accuracy in
predicting the adsorption energy .E
ads
/ of oxygen on Ag
4
:
O
2
C Ag
4
! Ag
4
O
2.ads/
:
10 Design of Heterogeneous Catalysts and Application to Oxygen Reduction Reaction 307
These single-point calculations were performed on geometry optimized at the
B3LYP/LANL2DZ level. By comparing with the highly accurate QCISD(T)/
LANL2DZ results, it was found that the LANL2DZ basis set [13] afforded the
best compromise between accuracy and efficiency. The addition of basis functions
to oxygen beyond 6–21G further increased the predicted absolute adsorption en-
ergy beyond B3LYP/LANL2DZ, lowering accuracy, as density functional methods
tend to overestimate bond energy. Therefore, LANL2DZ was chosen to represent
oxygen as well. Gaussian ’03 was used for all cluster calculations [14].
The adsorption energy of oxygen on metal clusters was calculated from the
formula
E
ads
D E
Ag
n
O
2
E
O
2
E
Ag
n
:
With this definition, stronger adsorption is more negative.
To find adsorption-, dissociation-, and transition-state energies, the following
procedure was utilized:
1. Individually relax the geometry of O
2
and the metal cluster
2. Introduce oxygen to the vicinity of the metal cluster and relax the geometry
3. Search for the transition state for O–O cleaving
4. Split molecular oxygen to 2O
.ads/
and relax the geometry
10.3.2 Slab Model
Electronic states were expanded in plane waves with energy cutoffs of 400eV for
all simulations. For small supercells used for lattice relaxation, a Monkhorst-Pack
[15] 8 8 8k-point sampling scheme was used; for larger supercells used for
surface relaxation, a Monkhorst-Pack 8 8 1 sampling was used, and for the
largest supercells considered for oxygen adsorption, a Monkhorst-Pack 4 4 1
sampling was employed.
Ultrasoft pseudopotentials in the GGA scheme were used with the Perdew-Wang
91 exchange correlation functional [16]. Methfessel-Paxton order 1 smearing was
used with a smearing width of 0.2 eV. The ab initio code VASP was used for all slab
calculations [17].
Lattice constants were found for primitive cells for each material by relaxing the
supercell volume. Slabs of four atomic planes were constructed where the bottom
two layers were fixed to their bulk distances and the top two layers were allowed
to relax. Slabs were separated by at least 15
˚
A of vacuum to minimize interaction
between slabs. For adsorption calculations, molecular oxygen was brought near an
adsorption site in a vertical (superoxo-) or horizontal (peroxo-) configuration and
allowed to relax, while the top two metal layers were simultaneously relaxed. Dif-
ferent adsorption sites were tested, and results presented are for the most favorable
site found.
Adsorption energy is found by
E
ads
D E
MO
2
E
M
E
O
2
;
308 T.P. Holme et al.
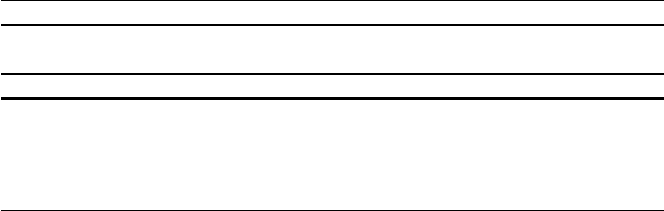
Fig. 10.1 Potential energy of an electron above the Ag(111) surface as a function of z, the surface
normal, found by averaging the potential in the xy plane. Positions of the ions are marked with
arrows, as is the vacuum potential
where E
M
is the energy of the clean surface, E
O
2
is the energy of O
2
in the gas
phase, and E
MO
2
is the energy of the adsorbed system.
The work function of each system was found from the following equation:
D "
f
E
vac
;
where "
f
is the calculated Fermi level of the system and the potential energy of an
electron in vacuum, E
vac
was found from the potential energy determined by the
self-consistent electron density. The potential energy was averaged in the xy plane
and plotted as a function of z, the surface normal direction, as shown in Fig. 10.1,
illustrated for silver.
The center of the d-band, "
d
, was found from the usual definition of the centroid:
"
d
D
R
˛
˛
"n
d
."/d"
R
"
f
1
n
d
."/d"
;
where n
d
."/ is the density of d-band states.
10.4 Details of Experiments
Sputter targets of Pt, Ag, Pt
0:4
Ag
0:6
,andPt
0:25
Ag
0:75
were fabricated by Kurt
Lesker Co. (99.99%) and used for DC sputtering of porous electrodes on thin-
film solid oxide supports according to the method described by Huang [18]. I–V
performance data of the fuel cells was measured using a Solartron 1260/1287 in
galvanodynamic mode at a scan rate of 0:2 A=s.
10 Design of Heterogeneous Catalysts and Application to Oxygen Reduction Reaction 309
Analysis of electronic structure of the valence band of catalysts was done with
X-ray photoemission spectroscopy (XPS) with an SSI S-Probe Monochromatized
XPS Spectrometer, which uses Al.K
˛
/ radiation (1486 eV) as a probe. XPS was
done on samples after a thin surface layer was sputtered off in UHV to ensure there
was no oxide or other surface contamination; survey scans confirmed that only the
pure metals remained.
Analysis of strain was performed with XRD (Phillips PANalytical X’Pert PRO)
using the Cu K
’
line (1.54184
˚
A) at 45 kV and 40 mA. XRD was performed on the
bulk sputtering targets; 2 scans were performed after the zero of 2 was aligned
to the detector.
10.5 Results
The following sections present the results of calculations on clusters and slabs of Pt,
Ag, and PtAg
x
compounds.
10.5.1 Cluster Model
The effect of varying cluster size, composition, and spin are explored for the oxygen
adsorption reaction on Ag
n
.n D 4; 6; 8; 14/; Pt
m
.m D 2; 4; 8/,andAg
n
Pt
m
[.n; m/ D .4; 2/, (6, 2), (4, 4)].
The clusters were constructed by extracting the desired number of atoms from
their bulk configuration and relaxing the ion positions. Stable geometries found for
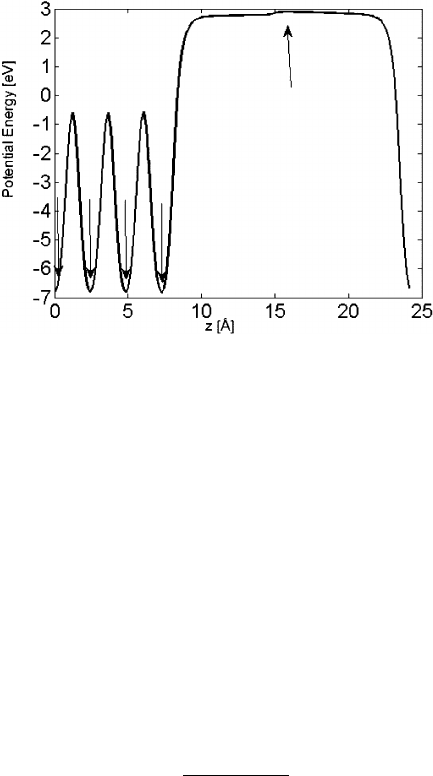
the Ag clusters are shown in Fig. 10.2.ForAg
4
and Ag
6
, the atoms assume a config-
uration similar to what they would have in a close-packed plane. In Ag
8
, the atoms
Fig. 10.2 Stable states for
(from left to right and top
to bottom) Ag
4
; Ag
6
; Ag
8
,
and Ag
14
310 T.P. Holme et al.
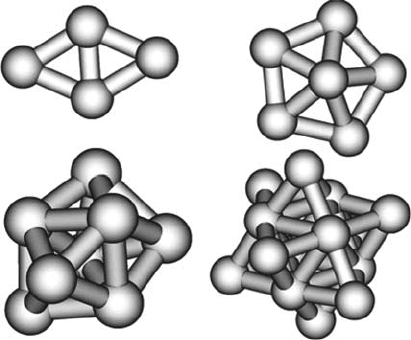
Fig. 10.3 Stable states for
Pt
2
; Pt
4
,andPt
8
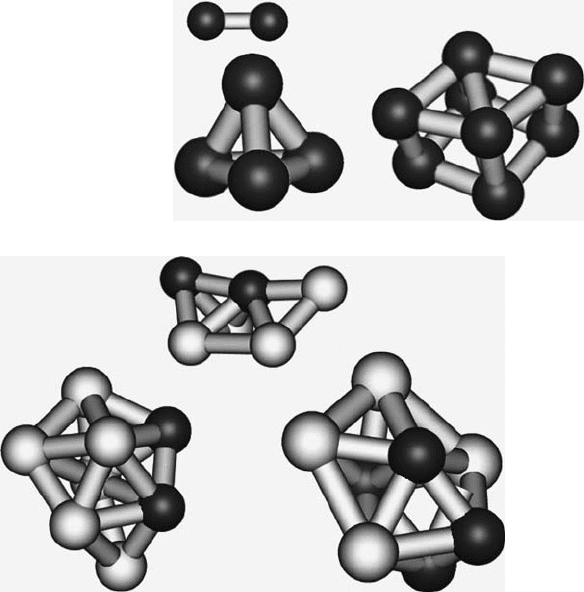
Fig. 10.4 Stable states for Ag
4
Pt
2
; Ag
6
Pt
2
,andAg
4
Pt
4
. Ag atoms are shown in white and
Pt in gray
begin to assume the tetrahedral coordination they would have in the fcc structure,
and the Ag
14
structure is very close to a full unit cell of fcc bulk Ag. Ag clusters are
more stable in low-spin states of multiplicity 1.
The geometries of the Pt clusters are shown in Fig. 10.3. Again, the similarity of
the optimum geometry of the cluster to the bulk configuration is evident. Pt clus-
ters were found to be more stable in high-spin states, in agreement with Kua and
Goddard [19].
The geometries of the AgPt alloys are shown in Fig. 10.4. The clusters were
made by taking a silver cluster of the appropriate size and randomly substituting Pt
atoms for Ag atoms. The geometry was then allowed to relax. As noted by Chris-
tensen et al. [20], and in agreement with the phase diagram by Okamoto [21], Pt
impurities in an Ag matrix are thermodynamically driven to form a bulk alloy. As
can be seen in Fig. 10.4, Pt atoms are incorporated into the Ag cluster. One possible
driving force for this reaction is that the Pt gains a negative charge in Ag (shown
by Mulliken charge analysis [22]) and experience a Columbic repulsion between
negatively charged Pt atoms.
10 Design of Heterogeneous Catalysts and Application to Oxygen Reduction Reaction 311
The geometries and energies of stable structures are highly dependent upon the
spin state of the system. Singlet silver clusters are most stable, whereas platinum
clusters favor high spin states. The alloyed clusters considered also favor singlet
states. Oxygen is most stable in the triplet state when isolated, but when it nears
the catalyst cluster, lower energy configurations are found for singlet states in some
cases. In all the figures, the energies and geometries given correspond to the spin
state with lowest energy.
10.5.1.1 Cluster Size
Electronic Structure
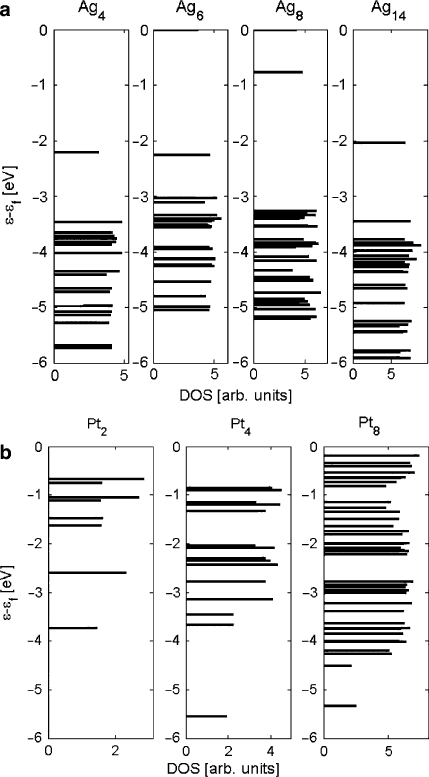
The energy of occupied electron eigenstates in silver and platinum clusters is shown
in Fig. 10.5. It is clear that by Ag
8
, the cluster has not converged to metallic proper-
ties, as the DOS of Ag
14
has a considerably lower HOMO-1 energy than does Ag
8
.
Without simulating a larger cluster, it cannot be determined whether the Ag
14
clus-
ter can be considered to represent bulk silver. The eigenvalues of HOMO-1 states
are s-states. The relatively higher eigenvalue of the s-states of Ag
8
may account for
the very weak bonding with O as will be discussed later.
The DOS of Pt
4
and Pt
8
are significantly different, showing that Pt
4
is not fully
metallic. According to Xu, once Pt clusters grow above seven atoms, their elec-
tronic structure becomes largely similar to bulk Pt [3]. Therefore this Pt
8
cluster is
considered to represent metallic Pt.
Oxygen Adsorption
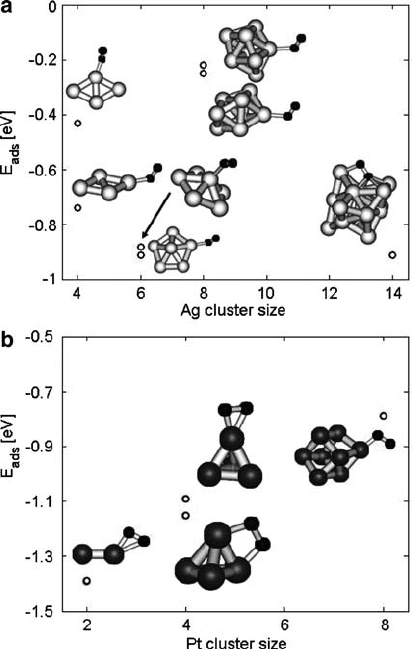
Geometries and energies of oxygen adsorption on Ag clusters and on Pt clusters
are shown in Fig. 10.6. The coordination of the site consistently influences the
properties of the adsorption reaction. The site with lower coordination is the more
energetically favorable site for adsorption. The atoms of lower coordination are
more able to donate electrons to oxygen because they share electrons with other
metal atoms in the cluster to a lesser degree than sites with higher coordination.
Adsorbed oxygen on Ag
8
has a shorter O–O bond and a longer O–Ag bond
than the other adsorbed states, correlating with higher adsorption energy. Weak ad-
sorptiononAg
8
may be explained by referring to Fig. 10.5, which shows that the
HOMO-1 eigenstates have higher energy in the Ag
8
cluster than the other silver
clusters. An orbital analysis shows that the HOMO of Ag
4
O
2
; Ag
6
O
2
,andAg
14
O
2
is primarily composed of Ag-s mixed with O-p states, whereas Ag
8
O
2
HOMO-3
to HOMO states have very little contribution from O states. The HOMO-4 level
of Ag
8
O
2
has mostly O-p to O-p bonding and lies 2.9 eV below the HOMO level,
explaining the weak adsorption on Ag
8
.
312 T.P. Holme et al.
Fig. 10.5 Energy of
eigenstates of electrons in
(a)Agclustersfor
Ag
4
; Ag
6
; Ag
8
,andAg
14
and (b)Ptclustersfor
Pt
2
; Pt
4
,andPt
8
Energy of adsorption on Ag clusters falls within the range of experimental values
0.4–1.1eV reported by Campbell [23] and Wang [24], respectively. An exception is
the unusually weak bonding on Ag
8
as explained earlier.
Adsorption on Pt clusters becomes less favorable for larger clusters, as the Pt
atoms become less reactive when they share more bonds with neighboring Pt atoms.
Adsorption is generally in a side-on configuration, as found experimentally by
Gland et al. [25]. The steady-state adsorption of O
2
on Pt at high coverage is ex-
perimentally 1:5 ˙ :4 eV from Brown and King [26]. The high-coverage regime
most closely corresponds to adsorption on Pt
2
, for which the adsorption is found to
be 1.4 eV, in good agreement with Brown and King.
10 Design of Heterogeneous Catalysts and Application to Oxygen Reduction Reaction 313
Fig. 10.6 Stable energies
and structures of O
2.ads/
M
n
for oxygen adsorbed at
different sites on (a)Ag
clusters and (b) Pt clusters.
Agatomsinwhite,Ptingray,
and O in black
O–O and O–M bond lengths, as well as total Mulliken charge on the two oxy-
gen atoms, are given in the Table 10.2. In all cases, adsorbed oxygen gains a partial
negative charge. The degree of charge transfer correlates relatively well with adsorp-
tion strength, in contrast with the model proposed by Hammer and N¨orskov. In the
Hammer-N¨orskov model, weaker adsorption on silver entails more charge transfer
to adsorbates. The reader is referred to Ref. [1] for more details.
The correlation between adsorption energy and charge, and adsorption energy
and O–M and O–O length is notable: a greater O
2
net charge, longer O–O bond,
and shorter O–M bond correlate well with stronger adsorption. The extra charge
density donated to the oxygen p orbitals lengthens and weakens the O–O bond in
the adsorbed state. Therefore, in systems with strong adsorption, there is greater
charge donation, weakening of the O–O bond, and stronger O–M bonding.
