Pugnaire F.I. Valladares F. Functional Plant Ecology
Подождите немного. Документ загружается.

productivity. However, it appears certain that the severity of any effect depends on the
normal habitat of the plant, in particular how xeric it is.
PHOTOSYNTHESIS AT SUBZERO TEMPERATURES
Lichens have long been known to carry out photosynthesis at subzero temperatures. Lange
(1965) surveyed a wide range of species including some from Antarctica and found photo-
synthesis to 248CbyC. alcornis, a temperate lichen. Other species, Umbilicaria decussata
(Vill.) Zahlbr., and P. caesia (Hoffm.) Fuernr. (Parmelia coreyi in article) from Cape Hallett,
had limits of 118C and 148C, respectively, values that are similar to species from hot
deserts (Lange and Kappen 1972). Schroeter et al. (1994) have since measured photosynthesis
of U. aprina to 178C in the field. In their analysis of the response of NP to subzero
temperatures they proposed that tolerance of subzero temperatures had the same physio-
logical basis as tolerance to low water potentials. This impression was gained from the very
similar response shown by photosynthesis to low water potentials whether generated by
subzero temperatures or by equilibration with low atmospheric humidity (Kappen 1993b).
In both cases water is removed from the cells either to intercellular ice, lichens have external
ice-nucleating agents with freezing initiating at around 58C (Ashworth and Kieft 1992,
Schroeter and Scheidegger 1995), or to the atmosphere. Schroeter et al. (1994) suggested
that this explained the absence of cyanobacterial lichens in continental Antarctica because
they cannot photosynthesize in equilibrium with humid air but need liquid water (Lange et al.
1988, Schroeter 1994). In addition, Schroeter and Scheidegger (1995) demonstrated convin-
cingly that lichen thalli could rehydrate at subzero temperatures only in the presence of ice.
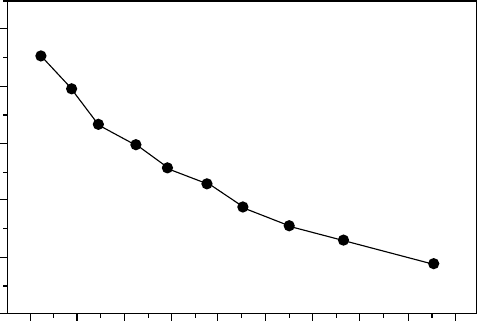
Thallus water content depended on the temperature being only 7% of maximal WC at 218C,
18% at 4.58C, and nearly 100% at 88C (Figure 13.13). Concurrent use of a low temperature
scanning electron microscope showed the progressive refilling of the algal and fungal cells as
the temperature was increased with the water coming, via the atmosphere, from extracellular
ice (Schroeter and Scheidegger 1995). At the same time photosynthesis commenced and
increased in line with thallus WC.
60
50
40
30
20
Water content (% d.wt.)
10
–4 –6 –8 –10 –12
Thallus temperature (⬚C)
–14 –16 –18 –20 –22
FIGURE 13.13 The relationship between thallus water content (% d.wt.) and thallus temperature for
Umbilicaria aprina (Schroeter and Scheidegger 1995). Dry lichen thalli where equilibrated in the dark
for 24 h at the selected temperature and in the presence of ice.
Francisco Pugnaire/Functional Plant Ecology 7488_C013 Final Proof page 410 16.4.2007 2:34pm Compositor Name: BMani
410 Functional Plant Ecology
A common mechanism would also explain the correlation between cold resistance and dry
habitats, such as deserts, found for lichens. The concept has even more far-reaching interest
when it is realized that their distribution pattern of Antarctic plants better reflects their rank
order for subzero photosynthesis than their desiccation or cold tolerance (Table 13.3). For
instance, whereas cyanobacterial lichens have very good resistance to desiccation and to low
temperatures, they have nearly no ability to photosynthesize at subzero temperatures, and are
excluded from continental Antarctica.
PHOTOSYNTHESIS UNDER SNOW
Kappen (1989) demonstrated that rehydration and reactivation of U. sphacelata occurred in
the field under snow and at subzero temperatures in the maritime Antarctic. This was an
important observation because, taken with the later studies of Schroeter and Scheidegger
(1995) it appeared that green algal lichens can reactivate photosynthesis under snow without
liquid water present that is, without the need of a thaw cycle. The influence of snow cover has
been reviewed by Kappen (1993b, 2000). Snow can provide an efficient insulation against wind
and extreme temperatures. Considerable light, equivalent to around 10%–30% of incident
values, can penetrate to the base of a 15 cm snow pack (Kappen and Breuer 1991), and this is
more than sufficient to saturate NP at the low temperatures. The performances of northern
maritime Antarctic mosses under snow (Collins and Callaghan 1980) and Cetraria nivalis in
Sweden (Kappen et al. 1996) were modeled and considerable photosynthetic production was
found. It seems almost certain that substantial photosynthesis can occur long before the
temperatures reach above freezing and while the lichens are still covered with snow.
The situation, however, appears to be different in continental Antarctica. In an innovative
experiment Pannewitz et al. (2003b) constructed fiber optics to measure lichen and moss
activity in late summer at Granite Harbour (778 S) and left them to be covered by winter
snow. In the following early summer it was then possible to follow the chlorophyll a
fluorescence activity of the samples under the snow without disturbance. In a complete
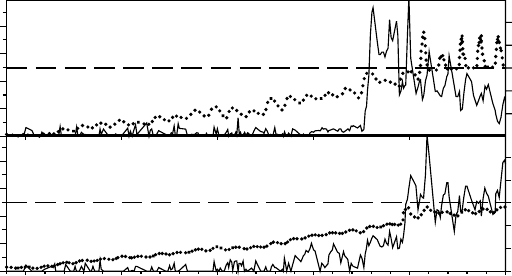
contrast to the maritime Antarctic there was no evidence of reactivation until liquid water
formed when temperatures reached freezing point (Figure 13.14). Temperatures under the
snow had, over the long winter, equilibrated with those above the snow and the insulating
TABLE 13.3
A Summary of Physiological Abilities of Cyanobacterial Lichens, Green Algal Lichens,
Liverworts, and Mosses with Respect to Photosynthetic Performance at Subzero
Temperatures
Plant Type
Cyanobacterial
Lichens Liverworts Mosses
Green Algal
Lichens
Distribution Maritime only Mainly maritime Throughout Antarctica
Subzero photosynthesis to 2
C Little to 8
Cto24
C
Positive NP In humid air None ? Little ability Above 80% rh
Desiccation=
Cold tolerance: WET
High Low Low=medium High
DRY Very high Low High Very high
Note: Positive NP in humid air refers to the ability of the group to attain positive net photosynthesis from a dry
condition only in the presence of humid (90%–95% relative humidity) air. Desiccation=cold tolerances are given in
two rows, the upper when wet and the lower when dry.
Francisco Pugnaire/Functional Plant Ecology 7488_C013 Final Proof page 411 16.4.2007 2:34pm Compositor Name: BMani
Plant Life in Antarctica 411
properties of the snow then prevented rapid warming in the early summer of the buried
plants. Far from providing a suitable environment for production the snow delayed any
activity until it was completely melted. Increases in snow fall could, therefore, have a very
negative effect on lichen and moss biodiversity by shortening the productive season. The
effect of large snow banks is clearly visible at Cape Hallett, where there is high lichen
abundance at the outer edges of the snow banks but a zone with no lichens appears when
the snow bank edge retreats later in the season.
REVERSE DIEL CYCLE OF PHOTOSYNTHESIS AND HIGH LIGHT STRESS
Being poikilohydric, lichens and mosses depend on water to rehydrate and become active.
Typically, even in Antarctica, this means that liquid water is required (Hovenden and Seppelt
1995); the exception would be the rehydration by lichens at subzero temperatures. For
mosses, in particular, liquid water is certainly required and the larger biomasses are found
where consistent water flows occur. In temperate zones water is normally provided by rain,
which typically occurs at cloudy times. Mosses and lichens rapidly dry out as soon as brighter
light conditions and full sunshine reoccur. Desiccation has often been given as one of
the means used by poikilohydric plants to avoid high light and high temperature stress
(Kappen 1988).
The situation in the Antarctic tends to be the opposite, melt water only occurs when
insolation and temperatures are high so that the lichens and mosses are active when the light
is brightest. This can be seen in Figure 13.10 where the photosynthetic activity of B. frigida
was monitored using chlorophyll a fluorescence (Schroeter et al. 1997). Initially when sunlight
reaches the thalli in the early morning they rapidly dry and become inactive. Then, about
noon, the snowmelt rehydrates the thalli and maximal activity occurs. The thalli then remain
photosynthetically active, even when frozen overnight, until drying again occurs at sunrise the
next morning. Mosses also display this reverse pattern since water flow from snow or glacier
melt is always at its maximum during the day and when irradiance is greatest.
Date (2000)
10/11 15/11 20/11 25/11 30/11 5/12
0
20
40
60
80
Temperature (°C)
–15
–10
–5
0
5
10
0
20
40
60
80
–10
–5
0
5
10
Candelariella flava
Physcia dubia
FIGURE 13.14 Insulating effect of a snow bank at Cape Geology, Granite Harbour (778 S): chlorophyll
a fluorescence (solid line) and thallus temperature (dotted line) were measured for the two lichens
Candelariella flava (upper panel) and Physcia dubia (lower panel) using probes put in place the previous
year and allowed to become naturally covered with snow. Note how the thallus temperature slowly rises
(ambient temperatures were around zero degrees) showing a slight daily fluctuation due to incident
radiation. Photosynthetic activity does not start until the temperature is close to zero and free water
appears.
Francisco Pugnaire/Functional Plant Ecology 7488_C013 Final Proof page 412 16.4.2007 2:34pm Compositor Name: BMani
412 Functional Plant Ecology
High light stress is, therefore, of unexpected importance in Antarctica. The severity of the
conditions can be seen in Table 13.4 that shows the mean conditions when the lichens studied
were active. There is a major difference between the continental site at Granite Harbour,
mean PPFD about 55% of full sunlight, and the temperate and maritime sites, mean PPFD
about 5% of full sunlight.
A combination of cold temperatures and high light has been found to be particularly
likely to cause photoinhibition in some higher plants and a similar response is sometimes
suggested for Antarctic mosses and lichens. In mosses, photoinhibition has, indeed, been
reported several times in studies in both the maritime and continental Antarctica. A midday
depression in NP was consistently found under high light by Collins (1977) and was built into
models of photosynthetic production by Collins and Callaghan (1980) and Davis (1983).
Adamson et al. (1988) demonstrated severe photoinhibition in Grimmia (Schistidium) antarctici.
Depressed photosynthesis occurred after 100 min at 500 mmol m
2
s
1
and CO
2
exchange was
negative after 150 min at 1000---1800 mmol m
2
s
1
and there was a corresponding fall in F
v
,
the variable fluorescence.
Later studies have tended not to confirm these earlier results and especially not so in
continental Antarctica. Lovelock et al. (1995a,b) demonstrated the occurrence of photoin-
hibition, measured with chlorophyll a fluorescence, during freeze=thaw cycles. Plants that
were originally under snow showed severe photoinhibition if the snow was removed and they
became exposed to full irradiance. Lovelock et al. (1995a,b) showed full recovery to occur
under warmer temperatures and under low light, and they suggested that photoinhibition was
more of a protective process than one of damage. Post et al. (1990) showed similar photo-
inhibition and recovery for C. purpureus. Post (1990) also showed that the ginger pigment
found in exposed plants of C. purpureus appeared to serve a protective function against high
irradiance. In studies on sun- and shade-adapted B. subrotundifolium, Green et al. (2000)
showed that only the shade-adapted form, which was bright green, was susceptible to high
light. It appeared that the silvery shoot points and nonphotochemical quenching within the
photosystems could protect the sun form against several hours of full sunlight.
Although Kappen et al. (1991) reported photoinhibition, indicated by depressed NP, for
U. sphacelata under high light, no signs of photoinhibition were found when U. aprina was
monitored for 2 days under full natural sunlight even though the thalli had been dug from
under 70 cm of snow before receiving any sunlight in that summer (Kappen et al. 1998a).
Similarly, Schlensog et al. (1997b) found no signs of photoinhibition for L. puberulum after
TABLE 13.4
Mean Values for Incident Radiation and Thallus Temperature for Three Species of Lichens
in Spain, the Maritime Antarctic and the Continental Antarctic
Spain, Guadarrama
Summit, Madrid,
(2000 m) 418 N
Lasallia hispanica
Antarctica
Livingston I.
(10 m) 628 S
Usnea
aurantiaco-atra
Antarctica Granite
Harbour (10 m) 778 S
Umbilicaria aprina
Radiation mean
PAR (mol m
2
day
1
)
All values 17.5 9.8 38
Active only 2.7 3.8 77
Mean temperature (8C) All values 9.7 2.2 9.74
Active only 4.5 1.1 2.9
Note: The mean values have been calculated in each case either for the entire measuring period (1, 14, and 3 years,
respectively) and for times when the lichens were active (Active only).
Francisco Pugnaire/Functional Plant Ecology 7488_C013 Final Proof page 413 16.4.2007 2:34pm Compositor Name: BMani
Plant Life in Antarctica 413
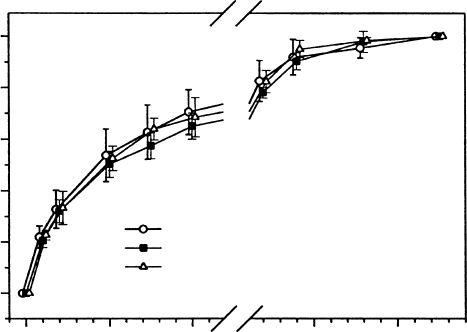
extensive treatment with high light (Figure 13.15). It seems that pigments in lichens act to
reduce the light level within the thallus and thus protect the photobionts (Schroeter et al.
1992, Buedel and Lange 1994, Rikkinen 1995, Schlensog et al. 1997b).
UV RADIATION
There has been considerable interest in the possible effects of UV (especially UV-B) radiation
on Antarctic plants since the presence of the ozone hole in spring over Antarctica since the
1980s (Robinson et al. 2003, Convey and Smith 2006). Many experiments have been carried
out usually involving the removal of UV-B by filters (Robinson et al. 2003). The typical
response is subtle and by changed growth suggesting reallocation of resources (Ruhland and
Day 2000, Lud et al. 2002, Robinson et al. 2005). Despite earlier worries that Antarctic plants
would suffer extensively because they had evolved under very low UV-B levels (not actually
correct because levels are high around midday) and because they were simple in structure
(Gehrke 1998, Gwynn-Jones et al. 1999) recent studies indicate otherwise. Newsham et al.
(2002) have shown that several bryophytes on the Antarctic Peninsula can rapidly (within a
day) adjust their UV-B protection to match UV-B incidence. Green et al. (2005) have shown
that the sun form of B. subrotundifolium is well protected against incident UV-A and that,
when this protection is lowered by shading, it is reinstated within a few days following
reexposure with little obvious effect on plant performance (Figure 13.16). Studies on DNA
damage by the formation of cyclobutyl pyrimidine dimer formation showed that the moss
Sanionia uncinata (Hedw.) at Leonie Island in the maritime Antarctic (678 35
0
S) showed that
any negative effects were transitory and that the species appeared to be well adapted to
ambient levels of UV-B radiation (Lud et al. 2002, 2003). When these results from mosses are
coupled with the extreme protection to incident radiation possessed by lichens, no effect of
full sunlight for 2 days was found on U. aprina samples that were immediately exposed after
uncovering from snow following winter (Kappen et al. 1998a), it must now be concluded that
UV radiation is unlikely to be a stress for these plants except for manipulated situations where
UV levels have been rapidly changed.
100
80
60
40
20
GP (% maximal rate)
0
0 50 100
Before strong light treatment
Directly after strong light treatment
After 24 h of recovery
PPFD (µmol m
–2
s
–1
)
500 1000
FIGURE 13.15 The almost complete lack of photoinhibition in the cyanobacterial lichen Leptogium
puberulum despite treatment with strong light (3 h at 1600 mmol m
2
s
1
PPFD). Immediately after the
treatment there was a slight but insignificant decline in NP at intermediate irradiances but no change in
apparent quantum efficiency (initial slope of the response to irradiance). (Modified from Schlensog, M.,
Schroeter, B., Sancho, L.G., Pintado, A., and Kappen, L., Bibliotheca Lichenologica, 67, 235, 1997b.)
Francisco Pugnaire/Functional Plant Ecology 7488_C013 Final Proof page 414 16.4.2007 2:34pm Compositor Name: BMani
414 Functional Plant Ecology
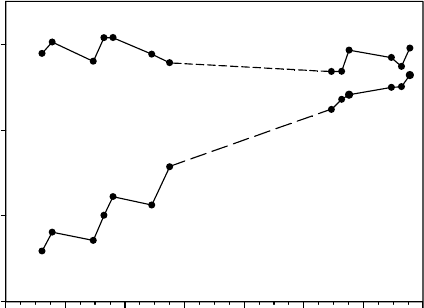
ENDOLITHIC LICHEN COMMUNITY
Under extremely dry conditions in Antarctica, and in other deserts, lichens can adopt an
endolithic growth form where they live within the pores of rocks composed of materials like
sandstone and limestone (Friedmann and Galun 1974). The most studied endoliths are the
‘‘cryptoendoliths’’ that penetrate to 2 cm in the Beacon Sandstone of the Dry Valleys,
Southern Victoria Land, 778 S, (Friedmann 1982, Kappen 1988). Other species such as
Lecidea phillipsiana also grow widely in East Antarctica in granites where they produce a
prominent brown color on the surface and cause extensive rock flaking (Hale 1987). The
lichens form a layered structure within the rock with the upper layer, containing fungal
hyphae and the lichen green algal symbiont Trebouxia, that had a dark color, possibly to
reduce light intensities. The endoliths grow only on the faces of rocks where higher insolation
is received, north-facing or horizontal. On a sunny day the temperature of the rock can
reach þ88C, whereas the air temperature is still lower than 58C below freezing (Kappen et al.
1981). Humidity within the rock is around 80%–90%, which is considerably higher than air,
which is normally around 30%–40% rh. Because the temperature is so strongly dependent on
insolation it can fluctuate markedly during a day and freeze=thaw transitions are common.
A typical daily pattern of the internal rock environment is shown in Figure 13.17 and a
freeze=thaw or wet=dry transition is expected to occur at least once on all days when
metabolic activity occurs, around 120–150 per year. Water is provided to the rock by blowing
snow which then melts into the rock when it is warmed by sunlight. The endoliths are wetted
either by equilibration with the high humidity within the rock or by direct moisture uptake
after snow melt. The latter method is thought to be the most important in the Dry Valley
region (Friedmann 1978).
Carbon metabolism (incorporation of H
14
CO
3
) was saturated at 150 mmol m
2
s
1
at
08C, and had an optimum at 158C (Vestal 1988). Measurements of CO
2
exchange showed
it to be maximal at 38 C68C and to be still positive at 108 C. In a classic long-term study
of the nanoclimate using automated recording systems reporting over satellites, 3 years
UV-A Protection (%)
50
60
70
80
23 24 25 26 27 28 29
January
Sun form
Shade form
FIGURE 13.16 Changes in UV-A protection (% depression of chlorophyll a fluorescence) of the moss
Bryum subrotundifolium measured in situ with a UV-A PAM chlorophyll a fluorometer (Gademann.
Instruments, Wu
¨
rzburg, Germany). The UV-A protection of the sun form of the moss remained
constant, whereas the protection of a shade form (created by shading the moss for 10 days) rapidly
increased back to normal over 6 days. (Modified from Green, T.G.A., Kulle, D., Pannewitz, S., Sancho,
L.G., and Schroeter, B., Pol. Biol., 28, 822, 2005.)
Francisco Pugnaire/Functional Plant Ecology 7488_C013 Final Proof page 415 16.4.2007 2:34pm Compositor Name: BMani
Plant Life in Antarctica 415
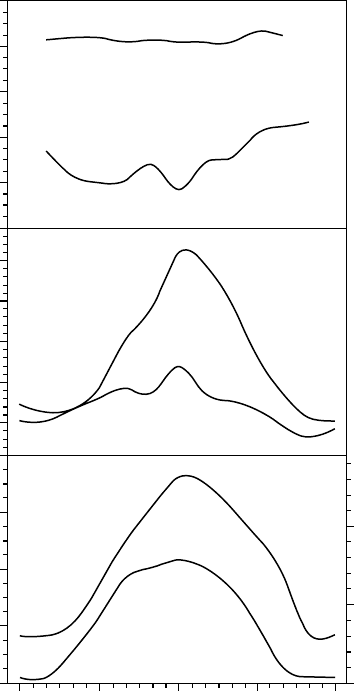
of continuous data were obtained by Friedmann et al. (1987) allowing modeling of aspects
like the thermal environment (Nienow et al. 1988). This was then connected to the CO
2
exchange studies to produce estimates by modeling of the community productivity through-
out the year (Friedmann et al. 1993). The cryptoendoliths showed positive photosynthesis
for around 13 h day
1
with annual totals reaching around 1000 h depending on aspect
and slope (Kappen and Friedmann 1983). Net productivity was greatest around 2
to þ28C with significant gains down to 8to108C. Horizontal surfaces proved more
productive than north-facing sloping surfaces, although colder, because of better water
relations. One unusual feature of the community is the relative unimportance of respiratory
carbon loss during the dark winter, the system is simply too cold. Despite the long productive
periods and the low respiration the estimated carbon gain of 106 mg C m
2
year
1
only
translates into an actual gain of around 3mgCm
2
year
1
(estimated from carbon dating
0
06
Local time (h) (Dec. 9, 1979)
In rock
Ambient
In rock
Ambient
In rock
Ambient
12 18 24
0
10
PPFD in rock
20
400
PPFD ambient
Temperature (⬚C)
rH (%)
800
1200
1600
–15
–10
–5
0
5
0
20
40
60
80
100
FIGURE 13.17 An example of the diel pattern of humidity (upper panel, % RH), temperature (middle
panel, 8C), and irradiance (lower panel), PPFD in mmol Photons m
2
s
1
) both within the rock and in
the surroundings of a north-facing endolithic lichen community on December 9, 1979 at Linnaeus
Terrace, Aasgard Range, McMurdo Dry Valleys. (Modified from Kappen, L., Friedmann, E.I., and
Garty, J., Flora 171, 216, 1981.) In the lower panel note the very different scales for the incident PPFD
(left-hand axis) and PPFD within the rock (right-hand axis).
Francisco Pugnaire/Functional Plant Ecology 7488_C013 Final Proof page 416 16.4.2007 2:34pm Compositor Name: BMani
416 Functional Plant Ecology

techniques and metabolic turnover rates) probably because of the high stresses including
possible loss of metabolites in the freeze=thaw or wet=dry cycles (Greenfield 1988). Because
the endoliths can grow on almost all north-facing rock faces (porous rock types), where
epilithic species are excluded because of the low temperatures and, in particular the abrasive
wind, they are the most common terrestrial vegetation community in the Dry Valleys
(Friedmann 1982). Endolithic communities are one of the better demonstrations of the
importance of shelter, aspect, and water supply in continental Antarctica. As a contrast, it
appears that endolithic communities may also contribute extensively to weathering of their
host rock by dissolving the silica matrix by the production of locally high pH during
photosynthesis (Buedel et al. 2004).
More recently, studying the lithobiontic microorganisms has concentrated in some
ultrastructural aspects. It has been demonstrated that these lithobiontic communities can
be defined as complex biofilms (Figure 13.18) exhibiting a high diversity and different
ecological requirements (De los Rı
´
os et al. 2005b). Accurate identification of lithobiontic
microorganisms was possible by means of molecular techniques (De los Rı
´
os et al. 2005a)
and unexpectedly high genetic diversities have been found (De la Torre et al. 2003). It was also
demonstrated that some minerals in Antarctic rocks are biogenically transformed to
generate inorganic biomarkers—traces left by living microorganisms due to their biological
activity (Wierzchos and Ascaso, 2001, Wierzchos et al., 2003, 2006). Further, in situ
microscopy studies have for the first time demonstrated the presence of microbial fossils
within Antarctic sandstone rocks from the Dry Valleys (Ascaso and Wierzchos, 2003,
Wierzchos et al. 2005, Ascaso et al. 2005), which has important implications for the detection
of endolithic microfossils everywhere in the world or even in extraterrestrial probes (Friedmann
et al. 2001, Ascaso and Wierzchos 2002, Wierzchos and Ascaso 2002). These studies have
been concentrated in the McMurdo Sound to date. However, the lithobiontic habitats of
many important Antarctic areas, such as the Antarctic Peninsula and Transantarctic
Mountains and some ice-free oasis on land remain almost completely unexplored.
~200 µm
FIGURE 13.18 SEM–BSE image of a transverse section of granite from Granite Harbour showing an
algae-rich biofilm colonizing the rock fissures. Scale ¼200 mm.
Francisco Pugnaire/Functional Plant Ecology 7488_C013 Final Proof page 417 16.4.2007 2:34pm Compositor Name: BMani
Plant Life in Antarctica 417
ACTIVE VERSUS INACTIVE
Typically, when looking at performance by plants across Antarctica, it is necessary to use
normal meteorological data that do not reflect local microclimates. In addition, even when
microclimate data are available, all lichens and mosses in Antarctica are poikilohydric and are
inactive for variable, but often large, periods and, at those times, are almost totally pro-
tected from the extremes of climate. Data sets are now available that have recorded activity
of lichens using chlorophyll a fluorescence techniques as well as thallus parameters such as
temperature and incident PPFD. It is now possible to make preliminary analyses of the
conditions when these organisms are active and to see how substantially they differ from
the annual means that include inactive periods as well. Mean annual temperature ranges
from þ9.78C, at the summit of Guadarrama Mountains near Madrid, to 9.78C Granite
Harbour, at a range of 19.4 K. In contrast, the mean temperatures during the active periods
show only a 3.4 K range with the coldest being the maritime Antarctic site, Livingston Island,
and the difference between the Guadarrama summit and Granite Harbour being only 1.6 K.
For total daily radiation during the active period the Guadarrama and maritime sites are
practically identical, whereas the Granite Harbour site stands out with an exceptionally high
value equivalent to a mean instantaneous rate of about 55% full sunlight. This confirms the
high light stress that comes from the reverse diel pattern (Section ‘‘The Reverse Diel Cycle of
Photosynthesis and High Light Stress’’) but shows that it is a feature of continental Antarc-
tica and not of the maritime Antarctic.
The net result is that the habitats of the active plants are by no means as extreme as the
ambient conditions would suggest and, in fact, may be remarkably constant over large
latitudinal ranges, the relative constancy of habitat conditions, first demonstrated by Poelt
(1987) with lichens growing from the Mediterranean to Greenland. If this concept proves
correct for Antarctica then it means that restriction to suitable habitats controls distributions
and adaptation may not play as major role a originally expected. This is certainly an area
requiring future investigation.
METABOLIC AGILITY
The majority of the vegetation in Antarctica is composed of lichens and bryophytes, which
have considerably simpler morphology than higher plants. However, it is a mistake to equate
simpler morphology with simpler metabolism as has been done in the past, for example:
for UV-B protection (Gehrke 1998, Gwynn-Jones et al. 1999). There is growing evidence
that lichens and mosses can be agile in their metabolism. The rapid change in protection
against UV-B found by Newsham et al. (2002) and Green et al. (2005) are two examples. The
moss B. subrotundifolium, and no doubt other mosses like C. purpureus, are capable of
changing from sun to shade forms and back, again within days to weeks. Lichens are
known to be able to alter their dark respiration rate at such a rate that they are almost
fully acclimated (Lange and Green 2005). This agility poses obvious problems if samples
are taken from the field and kept in some form of storage before use. The extreme shade
response to PPFD of photosynthesis by the mosses studied by Rastorfer (1970) is a probable
result of prestorage of the material in the laboratory before use.
INTEGRATING PERFORMANCE
A
NNUAL PRODUCTIVITY
Considerable efforts have been made to obtain values for the productivity, the seasonal net
carbon gain, for Antarctic plants. Unfortunately most estimates have been made in the
Francisco Pugnaire/Functional Plant Ecology 7488_C013 Final Proof page 418 16.4.2007 2:34pm Compositor Name: BMani
418 Functional Plant Ecology
northern maritime area (Signy Island). However, since no species have had their photosyn-
thesis monitored for complete years the estimates have been produced by the application of
models constructed by linking CO
2
exchange and microclimate data sets. The two largest data
sets are those for the cryptoendolithic community in the Dry Valleys (see Section ‘‘The
Endolithic Lichen Community’’) and for Usnea aurantiaco-atra on Livingston Island in the
South Shetlands where microclimate, CO
2
exchange and activity (from chlorophyll fluores-
cence) data sets have been linked (Schroeter et al. 1991, 1997a). In the latter case productivity
gains are predicted throughout the year with spring and autumn with the higher rates and
winter and summer limited by cold and drought, respectively. Considerable variation in
annual total production was also found from year to year (Schroeter et al. 1997a). When
modeled CO
2
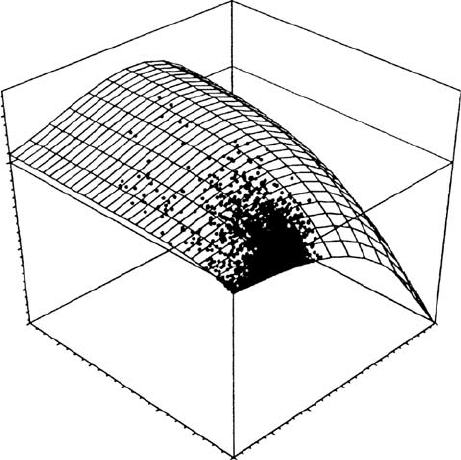
gain is plotted against actual PPFD and temperature data it is clear that the
lichen is rarely active under conditions suitable for optimal photosynthesis but is usually
limited by drying out at high PPFD and high temperatures (Figure 13.19). Most other
productivity estimates are from small data sets and with many and varied assumptions.
One common assertion is that increased winter temperatures will reduce productivity because
of greater respiration rates. However, despite substantial winter warming in the northern
Antarctic Peninsula there appears to have been no effect on lichen growth rates, in fact some
seem to have slightly increased (Sancho and Pintado. 2004). It is known from a temperate
study that some lichens can fully acclimate their respiration to seasonal temperature and it
appears that this may also be happening in Antarctica (Lange and Green 2005).
1000
CO
2
-exchange (mg ChI
–1
h
–1
)
–3
–2
–1
0
1
800
600
PPFD (µ
mol m
–2
s
–1
)
400
200
0
0
5
10
TT (
⬚C)
15
20
25
–5
FIGURE 13.19 The relationship between thallus temperature, PPFD and CO
2
exchange for Usnea
aurantiaco-atra at Livingston Island, maritime Antarctica. Thallus temperature (8C), PPFD
(mmol Photons m
2
s
1
), and photosynthetic activity (from chlorophyll a fluorescence measurements)
were recorded at 10 min intervals for 1 year. Combinations of temperature and PPFD when the lichen
was active were then plotted on a NP response surface to PPFD and temperature generated in the
laboratory. The lichen was rarely active under optimal conditions for PPFD and temperature. (Modified
from Schroeter, B., Grundlagen der Stoffproduktion von Kryptogamen unter besonderer Beru
¨
cksichtigung
der Flechten—eine Synopse—Habilitationsschrift der Mathematisch—Naturwissenschaftliche Fakulta
¨
t
der Christian—Albrechts—Universita
¨
t zu Kiel, 1997.)
Francisco Pugnaire/Functional Plant Ecology 7488_C013 Final Proof page 419 16.4.2007 2:34pm Compositor Name: BMani
Plant Life in Antarctica 419
