Powers D.L. Boundary Value Problems and Partial Differential Equations
Подождите немного. Документ загружается.

128 Chapter 1 Fourier Series and Integrals
22. Find the Fourier sine and cosine integral representations of the function
given by
f (x ) =
a −x
a
, 0 < x < a,
0, a < x.
23. Find the Fourier sine integral representation of the function
f (x ) =
sin(x), 0 < x <π,
0,π<x.
24. Find the Fourier integral representation of the function
f (x) =
1/, α < x <α+,
0, elsewhere.
25. Use integration by parts to establish the equality
∞
0
e
−λ
cos(λx) dλ =
1
1 +x
2
.
26. TheequationinExercise25isvalidforallx. Explain why its validity
implies that
2
π
∞
0
cos(λx)
1 +x
2
dx = e
−λ
,λ>0.
27. Integrate both sides of the equality in Exercise 25 from 0 to t to derive
the equality
∞
0
e
−λ
sin(λt)
λ
dλ = tan
−1
(t).
28. Does the equality in Exercise 27 imply that
2
π
∞
0
tan
−1
(t) sin(λt) dt =
e
−λ
λ
?
29. FromExercise27derivetheequality
∞
0
1 −e
−λ
λ
sin(λx) dλ =
π
2
−tan
−1
(x), x > 0.
30. Without using integration, obtain the Fourier series (period 2π )ofeach
of the following functions:
Miscellaneous Exercises 129
a. 2 + 4sin(50x) −12 cos(41x);
c. sin(4x +2);
e. cos
3
(x);
b. sin
2
(5x);
d. sin(3x) cos(5x);
f. cos(2x +
1
3
π).
31. Let the function f (x) be given in the interval 0 < x < 1 by the formula
f (x) = 1 −x.
Find (a) a sine series, (b) a cosine series, (c) a sine integral, and (d) a
cosine integral that equals the given function for 0 < x < 1. In each case,
sketch the function to which the series or integral converges in the inter-
val −2 < x < 2.
32. Verify the Fourier integral
∞
0
cos(λq) exp
−λ
2
t
dλ =
π
4t
exp
−
q
2
4t
, t > 0,
by transforming the left-hand side according to these steps: (a) Con-
vert to an integral from −∞ to ∞ by using the evenness of the inte-
grand; (b) replace cos(λq) by exp(iλq) (justify this step); (c) complete
the square in the exponent; (d) change the variable of integration; (e) use
the equality
∞
−∞
exp
−u
2
du =
√
π.
33. Approximate the first seven cosine coefficients (ˆa
0
, ˆa
1
,...,ˆa
6
) of the
function
f (x) =
1
1 +x
2
, 0 < x < 1.
34. Use Fourier sine series representations of u(x) and of the function f (x) =
x ,0< x < a, to solve the boundary value problem
d
2
u
dx
2
−γ
2
u =−x, 0 < x < a,
u(0) =0, u(a) = 0.
35–43. For each of these exercises,
a. find the Fourier cosine series of the function;
b. determine the value to which the series converges at the given values
of x;
130 Chapter 1 Fourier Series and Integrals
c. sketch the even periodic extension of the given function for at least
two periods.
44–52. For each of these exercises,
a. find the Fourier sine series of the function;
b. determine the value to which the series converges at the given values
of x;
c. sketch the odd periodic extension of the given function for at least
two periods.
35. & 44. f (x) =
0, 0 < x <
a
3
,
x −
a
3
,
a
3
< x <
2a
3
, x =0,
a
3
, a, −
a
2
,
a
3
,
2a
3
< x < a.
36. & 45. f (x) =
1
2
, 0 < x <
a
2
, x =
a
2
, 2a, 0, −a,
1,
a
2
< x < a.
37. & 46. f (x) =
2x
a
, 0 < x <
a
2
, x = 0,
a
2
, a,
3a
2
,
(3a −2x)
2a
,
a
2
< x < a.
38. & 47. f (x) =
x, 0 < x <
a
2
, x = 0, a, −
a
2
,
a
2
,
a
2
< x < a.
39. & 48. f (x) =
(a −x)
a
,0< x < a, x = 0, a, −
a
2
.
40. & 49. f (x) =
0, 0 < x <
a
4
,
1,
a
4
< x <
3a
4
, x = 0,
a
4
,
a
2
, a, −
3a
4
,
0,
3a
4
< x < a.
41. & 50. f (x) = x(a −x), 0 < x < a, x = 0, −a, −
a
2
.
42. & 51. f (x) = e
kx
, 0 < x < a, x = 0,
a
2
, a, −a.

Miscellaneous Exercises 131
43. & 52. f (x) =
0, 0 < x <
a
2
, x =−a,
a
2
, a,
1,
a
2
< x < a.
53–58. For each of these exercises,
a. find the Fourier cosine integral representation of the function;
b. sketch the even extension of the function.
59–64. For each of these exercises,
a. find the Fourier sine integral representation of the function;
b. sketch the odd extension of the function.
53. & 59. f (x) = e
−x
, 0 < x.
54. & 60. f (x) =
e
−x
, 0 < x < a,
0, a < x.
55. & 61. f (x) =
1, 0 < x < b,
0, b < x.
56. & 62. f (x) =
cos(x), 0 < x <π,
0,π<x.
57. & 63. f (x) =
1 −x, 0 < x < 1,
0, 1 < x.
58. & 64. f (x) =
1, 0 < x < 1,
2 −x, 1 < x < 2,
0, 2 < x.
65. (Cesaro summability.) Let f (x) be a periodic function with period 2π
whose Fourier coefficients are a
0
, a
1
, b
1
,.... Then, the partial sum
S
N
(x) = a
0
+
N
n=1
a
n
cos(nx) + b
n
sin(nx)
is an approximation to f (x) if f is sectionally smooth and N is large
enough. The average of these approximations is
σ
N
(x) =
1
N
S
1
(x) +···+S
N
(x)
.
It is known that σ
N
(x) converges uniformly to f (x) if f is continuous.
Show that
σ
N
(x) = a
0
+
N
n=1
N + 1 − n
N
a
n
cos(nx) + b
n
sin(nx)
.
132 Chapter 1 Fourier Series and Integrals
66. In analogy to Lemma 2 of Section 7, prove that
N−1
n=0
sin
n +
1
2
y
=
sin
2
(
1
2
Ny)
sin(
1
2
y)
.
67. Following the lines of Section 7, show that
σ
N
(x) −f (x) =
1
2Nπ
π
−π
f (x +y) −f (x)
sin(
1
2
Ny)
sin(
1
2
y)
2
dy.
This equality is the key to the proof of uniform convergence mentioned
in Exercise 65.
68. In a study of river freezing, E.P. Foltyn and H.T. Shen [St. Lawrence River
freeze-up forecast, Journal of Waterway, Po rt, Coastal and Ocean Engi-
neering, 112 (1986): 467–481] use data spanning 33 years to find this
Fourier series representation of the air temperature in Massena, NY:
T(t) = a
0
+
∞
n=1
a
n
cos(2n π t) +b
n
sin(2n πt).
Here T is temperature in
◦
C, t is time in years, and the origin is Oct. 1.
The first coefficients were found to be
a
0
=6.638, a
1
=5.870, b
1
=−13.094, a
2
=0.166, b
2
=0.583,
and the remaining coefficients were all less than 0.3 in absolute value.
The authors decided to exclude all the terms from a
2
and b
2
up, so their
approximation could be written
T(t)
∼
=
a
0
+A sin(2πt +θ).
a. Find the average temperature in Massena.
b. Find A, the amplitude of the annual variation, and the phase angle θ.
c. Find the approximate date when the minimum temperature occurs.
d. Find the dates when the approximate temperature passes through 0.
e. Discuss the effect on the answer to part d if the next two terms of the
series were included.
69. In each part that follows, a function is equated to its Fourier series as
justified by the Theorem of Section 3. By evaluating both sides of the
equality at an appropriate value of x, derive the second equality.
Miscellaneous Exercises 133
a. |x|=
1
2
−
4
π
2
∞
k=0
1
(2k +1)
2
cos
(2k +1)πx
, −1 < x < 1,
π
2
8
=1 +
1
9
+
1
25
+···;
b.
4
π
∞
k=0
1
2k +1
sin
(2k +1)πx
=
1, 0 < x < 1,
−1, −1 < x < 0,
π
4
=1 −
1
3
+
1
5
−
1
7
+···;
c. |sin(x)|=
2
π
−
4
π
∞
n=1
1
4n
2
−1
cos(2nx),
1
2
=
1
3
+
1
15
+
1
35
+···.
This page intentionally left blank

The Heat Equation
CHAPTER
2
2.1 Derivation and Boundary Conditions
As the first example of the derivation of a partial differential equation, we
consider the problem of describing the temperature in a rod or bar of heat-
conducting material. In order to simplify the problem as much as possible, we
shall assume that the rod has a uniform cross section (like an extrusion) and
that the temperature does not vary from point to point on a section. Thus, if
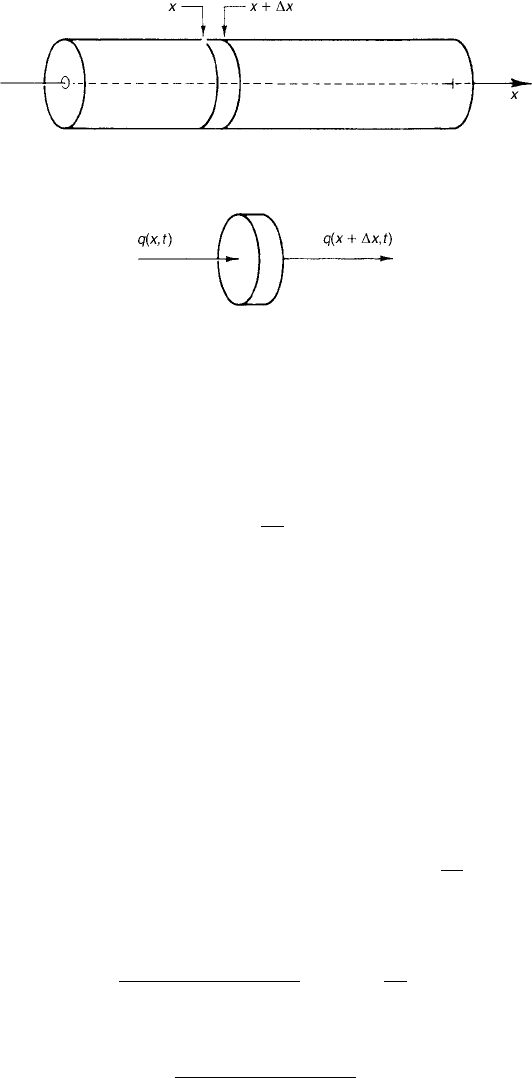
we use a coordinate system as suggested in Fig. 1, we may say that the temper-
ature depends only on position x and time t.
The basic idea in developing the partial differential equation is to apply the
laws of physics to a small piece of the rod. Specifically, we apply the law of
conservation of energy to a slice of the rod that lies between x and x + x
(Fig. 2).
The law of conservation of energy states that the amount of heat that enters
a region plus what is generated inside is equal to the amount of heat that leaves
plus the amount stored. The law is equally valid in terms of rates per unit time
instead of amounts.
Now let q(x, t) be the heat flux at point x and time t. The dimensions of q
are
1
[q] = H/tL
2
,andq is taken to be positive when heat flows to the right.
Therateatwhichheatisenteringtheslicethroughthesurfaceatx is Aq(x, t),
where A is the area of a cross section. The rate at which heat is leaving the slice
through the surface at x +x is Aq(x +x, t).
1
Square brackets are used to symbolize “dimension of.” H = heat energy, t = time, T =
temperature, L = length, m = mass, and so forth.
135
136 Chapter 2 The Heat Equation
Figure 1 Rodofheat-conductingmaterial.
Figure 2 Slice cut from rod.
The rate of heat storage in the slice of material is proportional to the rate of
change of temperature. Thus, if ρ is the density and c is the heat capacity per
unit mass ([c] = H/mT), we may approximate the rate of heat storage in the
slice by
ρcA x
∂u
∂t
(x, t),
where u(x, t) is the temperature.
There are other ways in which heat may enter (or leave) the section of rod
we are looking at. One possibility is that heat is transferred by radiation or
convection from (or to) a surrounding medium. Another is that heat is con-
verted from another form of energy — for instance, by resistance to an elec-
trical current or by chemical or nuclear reaction. All of these possibilities we
lump together in a “generation rate.” If the rate of generation per unit volume
is g,[g] = H/tL
3
, then the rate at which heat is generated in the slice is A xg.
(Note that g may depend on x, t,andevenu.)
We have now quantified the law of conservation of energy for the slice of
rod in the form
Aq(x, t) + A xg= Aq(x + x, t) +A x ρc
∂u
∂t
. (1)
After some algebraic manipulation, we have
q(x, t) − q(x +x, t)
x
+g =ρc
∂u
∂t
.
The ratio
q(x +x , t) −q(x, t)
x
Chapter 2 The Heat Equation 137
should be recognized as a difference quotient. If we allow x to decrease, this
quotient becomes, in the limit,
lim
x→0
q(x +x , t) −q(x, t)
x
=
∂q
∂x
.
The limit process thus leaves the law of conservation of energy in the form
−
∂q
∂x
+g =ρc
∂u
∂t
. (2)
We are not finished, since there are two dependent variables, q and u,inthis
equation. We need another equation relating q and u.ThisrelationisFourier’s
law of heat conduction, which in one dimension may be written
q =−κ
∂u
∂x
.
In words, heat flows downhill (q is positive when ∂u/∂x is negative) at a rate
proportional to the gradient of the temperature. The proportionality factor κ,
called the thermal conductivity,maydependonx if the rod is not uniform and
also may depend on temperature. However, we will usually assume it to be a
constant.
Substituting Fourier’s law in the heat balance equation yields
∂
∂x
κ
∂u
∂x
+g =ρc
∂u
∂t
. (3)
Note that κ, ρ,andc may all be functions. If, however, they are independent
of x, t,andu,wemaywrite
∂
2
u
∂x
2
+
g
κ
=
ρc
κ
∂u
∂t
. (4)
The equation is applicable where the rod is located and after the experiment
starts: for 0 < x < a and for t > 0. The quantity κ/ρc is often written as k
and is called the thermal diffusivity. Table 1 shows approximate values of these
constants for several materials.
For some time we will be working with the heat equation without genera-
tion,
∂
2
u
∂x
2
=
1
k
∂u
∂t
, 0 < x < a, 0 < t, (5)
which,toreview,issupposedtodescribethetemperatureu in a rod of length
a with uniform properties and cross section, in which no heat is generated and
whose cylindrical surface is insulated.
Some qualitative features can be obtained from the partial differential equa-
tion itself. Suppose that u(x, t) satisfies the heat equation, and imagine a graph
