Poole Ch.P., Jr. Handbook of Superconductivity
Подождите немного. Документ загружается.


276
Chapter 8: Crystal Structures of High-T c Superconducting Cuprates
observed between the number of atom layers of different kinds in the stacking
unit:
o
2.
3.
.
n > 1
(002
layers must always be present)
m + 1 >
n (002
layers cannot be stacked directly upon each other)
m -p(n
- 1) (a constant number of C layers is required between conse-
cutive DO 2 layers)
/- 1 or 2 (if k-76 0 then l- 2) (imposed by the specificity of the BO
layers)
Ifp- 1 (single C layers between consecutive DO 2 layers), the general formula
can be simplified to
A kBlCn- l DnOk+l+2n .
As stated previously, the number of cation-containing layers in the transla-
tion period must be even, in order to compensate for the relative shift of the cation
sites in consecutive layers. This means that when N = k + 1 + m + n is even, the
conventional cell of the undistorted structure will contain one stacking unit, that
is, one formula unit (Z - 1), whereas when N is odd, it will contain two stacking
units (Z - 2). In the first case the tetragonal cell of a structure without AO' layers
will be primitive (P), and in the second case body-centered (I) because of the
shift of 89 89 observed within the plane for layers separated by half a translation
period along [0 0 1 ]. The symmetry of basic structures will be further discussed
in Section E,b.
d. Limiting Structures
In contrast to the basic structures, limiting structures, as defined here, do not
contain any BO, and consequently no A O layers. Such structures, where DO2
layers alternate with groupings ofp C layers intercalated by 02 layers, have the
general formula
GDO2p .
The structure where DO 2 layers alternate with single C layers (p = 1) is known
for the so-called infinite-layer compound (e.g., (Sr, La)CuO2). Depending on the
parity of p, the translation period of the limiting structures contains either one (p
odd) or two (p even) stacking units.
Well-known structures are in some cases obtained when one or two of the
layers just described are combined. The stacking sequence -C-O2-, for example,
corresponds to the fluorite-type (CaF2) structure, whereas a simple stacking of
AO (or BO) layers leads to the rock-salt-type (NaC1) structure. These "limiting"
structures do not contain
conducting
DO 2 layers and hence do not superconduct;
they will therefore not be considered in the following.

C. Atom Layers and Stacking Rules 277
e. lntergrowth of Basic Structures
As shown earlier, the stacking unit of a basic structure contains one
CmDnO2m+2
and one
AkBlOk+ l
slab. Other structures are known, where the "stacking unit"
contains several slabs with different indices, slabs of DO 2 and C layers alternating
with slabs of BO and AO layers. Such structures are generally considered as an
intergrowth of stacking units of different basic structures. The general formula for
intergrowth structures can be written as
A zk Bzl CzmDzn OEk + :El + 2Zm+ 2q "
where q is the number of intergrown stacking units. Since all but one of the
intergrowth structures refined so far are built up from the stacking units of two
different basic structures, only structures with q = 2 will be considered in the
present work. Note that structures where the "stacking unit" contains more than
two slabs because of a different ordering of the chemical elements are here
considered as superstructures of the basic structures and will be discussed
together with these.
fi
Four-Digit Codes
Basic structures are commonly referred to by a four-digit code that is derived
from the general formula,
AkBlCmDnOk+l+2m+2 ,
considering the numbers of
cation-containing layers of each kind in the stacking unit:
klmn .
The structures of LaCuO2.95 (MoB 1COD
103)
and Tll.64Ba2Ca3Cu4012
(A2B2C304012),
for example, are denoted as 0101 and 2234, respectively. The
code generally reflects the cation ratios in the compound; however, partial
vacancies are ignored and in some cases the same chemical element may be
present in different layers (e.g., Cu in both DO 2 and AO' layers). Mixed
occupation of one or several cation sites is also common. The oxygen content
is not considered in the code and cannot be derived with certainty from it, in
particular since no distinction is made between different types of
additional
layer.
In order to be able to distinguish different chemical families, the four-digit
code is sometimes preceded by the chemical symbol of the cations in the
additional
layers. For the thallium-based compound mentioned earlier, the code
can, for instance, be written as T1-2234. Note that, in the literature, some
compounds are denoted by codes that correspond to coefficients in the conven-
tional chemical formula, but do not take into account the distribution of the
cations among the different kinds of layer. For example, Ba2YCu307 is often
abbreviated as 123 or Y-123 (YBa2Cu307), but is more properly classified as
1212 or Cu-1212 (CuBa2YCu207).
278
Chapter 8: Crystal Structures of High-T c Superconducting Cuprates
The four-digit code can also be extended to the limiting structures with the
general formula CpDOzp. For these phases the number of DO 2 layers in the
stacking unit equal 1, so that the code takes the general expression 00pl.
Following this scheme,
0Nld, Ce)zCuO3.92,
for example, is denoted as 0021. The
code for an intergrowth structure contains the four-digit codes of the basic
structures it derived from, for example, 1201/2201 for CBizSr4Cu2011
(CSr2CuO5/BizSr2CuO6).
D
High-T~ Superconductor Family Tree
A family tree, in which all basic structures of high-T c superconducting cuprates
find their place, can be built by applying three structural operations to the ideal
structure of perovskite. These consist in inserting, one by one, the layers
described earlier, respecting the stacking rules.
a. Structural Operations
As explained previously, the structure of perovskite contains one DO 2 (TiO2) and
one BO (CaO) layer, which corresponds to the generalized formula B1D103 and
the four-digit code 0101. All basic structures of superconducting cuprates can be
generated from the ideal structure of perovskite by applying one or a combination
of three different structural operations, which consist in adding one of the
following:
o
2.
3.
An additional AO (or a bridging BO) layer
A conducting DO 2 and a separating C layer
A separating C and an O2 layer
Each operation may be performed several times. Note that an AO layer can only
be added into a stacking unit that already contains two bridging BO layers. In a
similar way, the structural operation (3) can only be applied to structures that
already contain two or more conducting DO 2 layers in the stacking unit.
b. Application of the Three Structural Operations
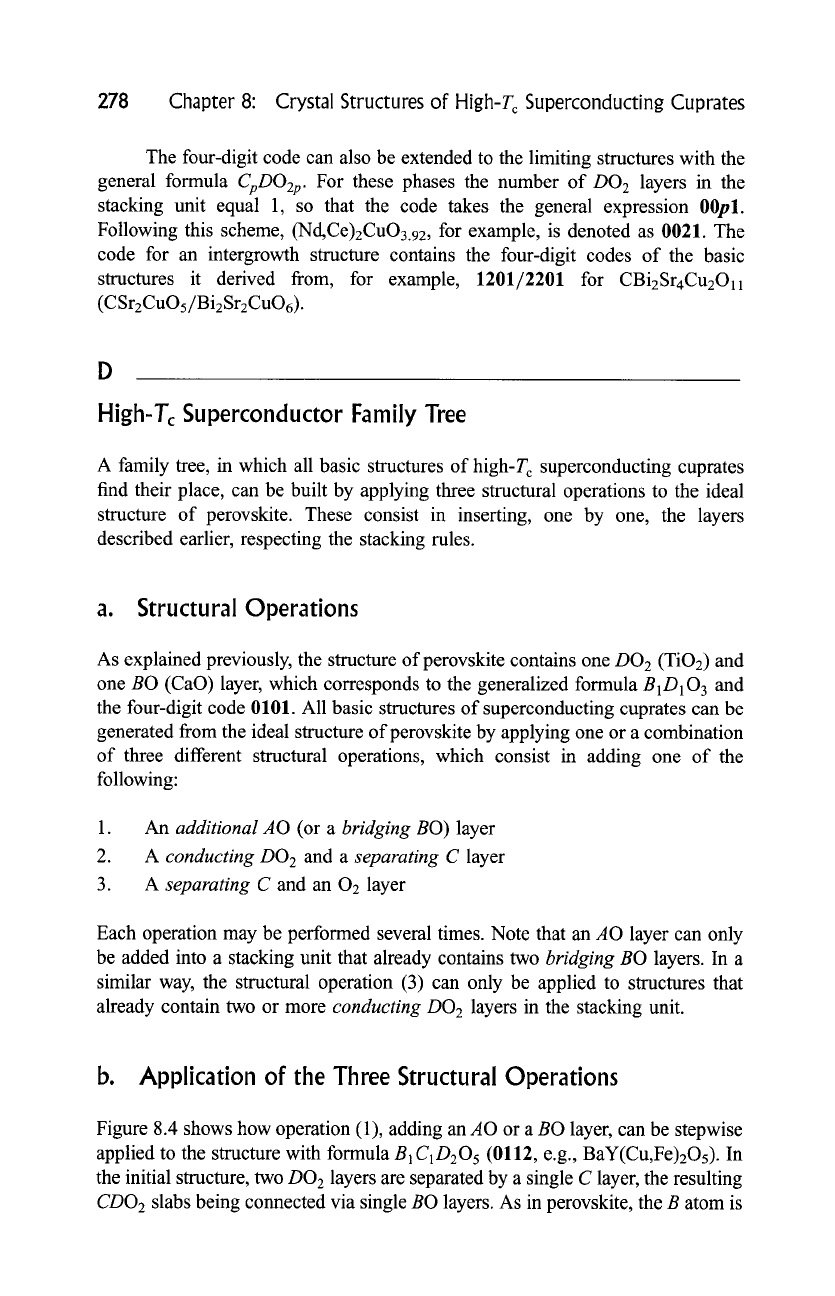
Figure 8.4 shows how operation (1), adding an AO or a BO layer, can be stepwise
applied to the structure with formula B 1C1O205 (0112, e.g., BaY(Cu,Fe)2Os). In
the initial structure, two DO 2 layers are separated by a single C layer, the resulting
CDO 2 slabs being connected via single BO layers. As in perovskite, the B atom is
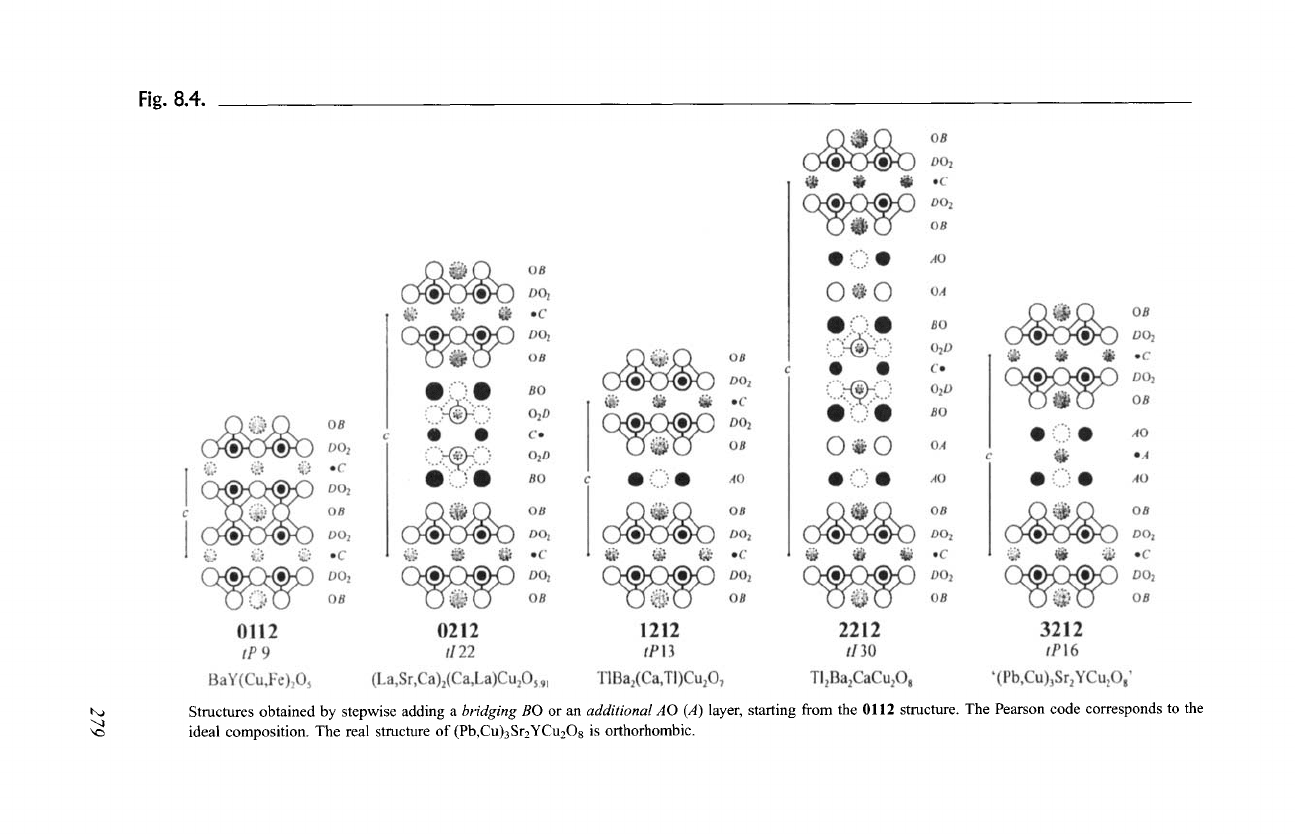
Fig. 8.4.
-,q
Structures obtained by stepwise adding a
bridging BO
or an
additional AO (A)
layer, starting from the 0112 structure. The Pearson code corresponds to the
ideal composition. The real structure of (Pb,Cu)3Sr2YCu208 is orthorhombic.

280
Chapter 8: Crystal Structures of High-Tc Superconducting Cuprates
12-fold coordinated. The metal atoms from the DO 2 layers are 5-fold coordinated,
the surrounding oxygen atoms forming square pyramids. The C atom is located at
the center of a tetragonal prism formed by eight oxygen atoms. When a second
bridging BO
layer is inserted between the BO and DO2 layers, a structure with the
formula
B2C1D206
(0212, (La, Sr, Ca)2(Ca,La)Cu2Os.91) is obtained. The tetra-
gonal cell of the resulting structure contains two stacking units and the two
neighboring BO layers form a rock-salt-type atom arrangement. The coordination
numbers of the C and D atoms remain the same as in 0112, whereas the B atom is
here 9-fold coordinated. An
additional AO
layer can now be inserted between
the two consecutive BO layers, leading to the formula
A1B2C1D207
(1212,
T1Ba2(Ca,T1)Cu2OT). The coordination numbers of the C and D cations remain
the same as in 0212, whereas the coordination of the B and A cations will depend
on the actual composition of the
additional
layer. When the operation consisting
in adding an AO layer is repeated, other members of the structure series with
formula
AkB2C1D2Ok+ 6
are generated (k = 0 and 1 for the 0212 and 1212,
respectively). In Fig. 8.4 schematic drawings of the structures of the members
with k = 2 and 3, 2212 (T12Ba2CaCu208) and 3212 ((Pb,Cu)3Sr2YCu2Os),
respectively, are also shown. For 2212, the cations in both
AO
layers belong to
the same crystallographic site and have octahedral environment. In the example
chosen to illustrate the formula 3212, the inner
additional
layer is built up from
cations only. In this structure the cations in the outer AO layers are 5-fold
coordinates, whereas those in the inner A layer have two neighboring oxygen
atoms in linear coordination.
The operation (2), consisting in adding a DO2 and a C layer, is illustrated in
Fig. 8.5 on Hg-based superconductors. The stacking unit of
A1B2DO 4
(1201,
HgBa2CuO4.18), chosen as starting point, contains one DO2 layer and one A layer
surrounded by a BO layer on each side. Simultaneous insertion of a
conducting
DO2 and a
separating C
layer produces a structure with the formula A 1B2 C1D206
(1212, HgBa2CaCu206.26). The environments of the A and B cations in 1212
are the same as in 1201 (2- and 8-fold coordination, respectively), whereas
the coordination number of the metal atoms in the DO2 layers is reduced
from six to five ("splitting" of the octahedron). The C atom is located inside a
tetragonal prism. The structures of the members of the structure series
A1B2Cn_IDnO2n+2
with n = 3 and 4, 1223 (HgBa2Ca2Cu308.44) and 1234
((Hg,Pb)BaECa3Cu4010.14), respectively, are also shown (n = 1 and 2 for 1201
and 1212, respectively). In the case where there are three or more
conducting
DO 2 layers in the stacking unit, the metal atoms of the external DO 2 layers are
5-fold coordinated, whereas those of the internal DO2 layers have four neighbor-
ing oxygen atoms in square planar coordination.
The structure with formula
B2C1D206
(0212), as shown in Fig. 8.6,
contains CD204 slabs where two DO 2 layers are separated by a single C layer
and B202 slabs formed by two BO layers. The structural operation (3) is
illustrated in the same figure with schematic drawings of the three first members
(m = 1, 2 and 3) of the structure series with the formula
B2CmD202m+4.
If a
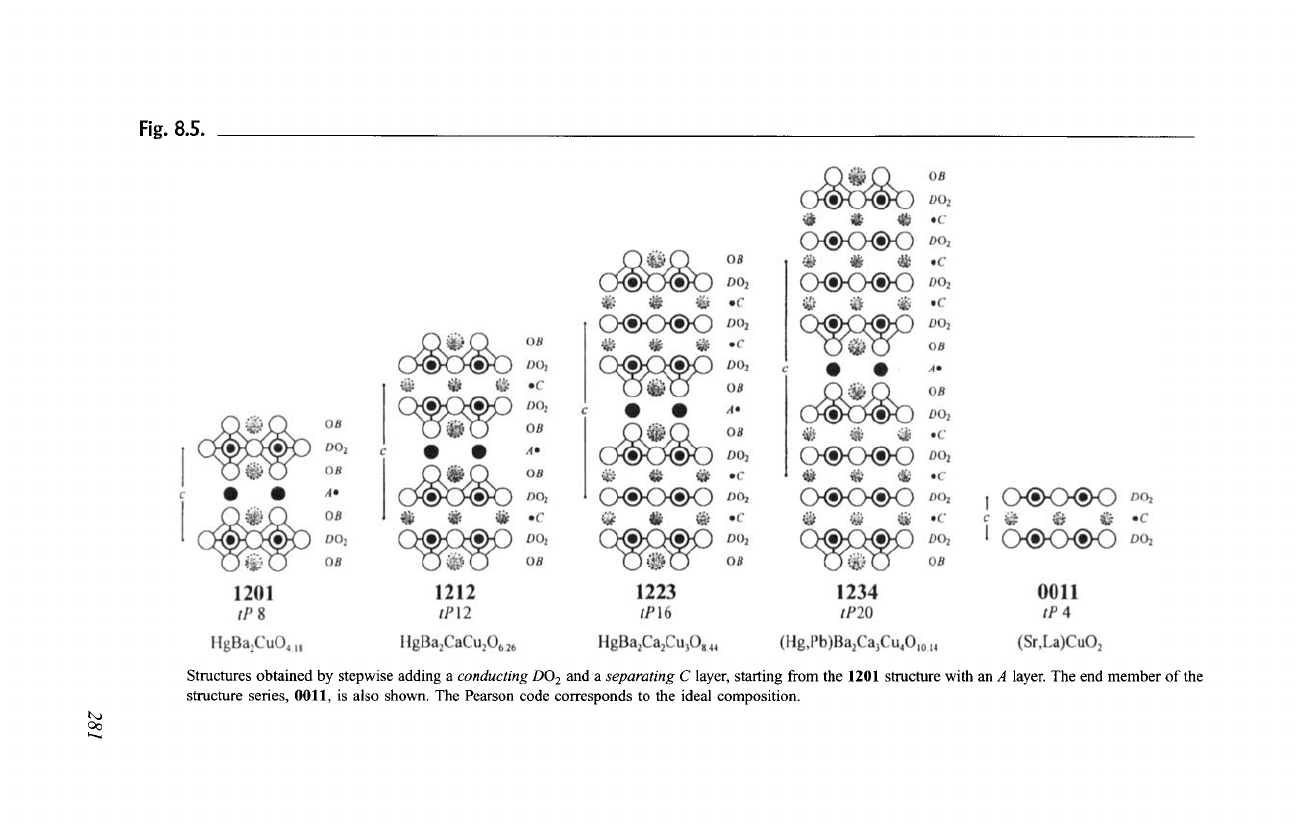
Fig. 8.5.
t,,a
Structures obtained by stepwise adding a
conducting
DO 2 and a
separating C
layer, starting from the 1201 structure with an A layer. The end member of the
structure series, 0011, is also shown. The Pearson code corresponds to the ideal composition.
282 Chapter 8:
Fig. 8.6.
Crystal Structures of High-Tr Superconducting Cuprates
Structures obtained by stepwise adding a
separating C
and an 02 layer, starting from the 0212
structure. The Pearson code corresponds to the ideal composition.
second separating C layer is inserted between the C layer and one of the DO2
layers in 0212, adding simultaneously an 02 layer, a structure with the formula
B2C2D208
(0222, (Nd, Sr)2(Nd, Ce)2Cu207.84)
is obtained. The coordination
of the metal atoms is unchanged through this structural operation, that is, the
B, C, and D cations remain 9-, 8-, and 5-fold coordinated, respectively. If the
operation is repeated, a structure with the formula
B2C3D201o
(0232,
Sr2(Ce,Y)3(Cu,Fe)2010)
is built (two stacking units in the translation period as
in 0212), again leaving the cation coordination unchanged. Note that indepen-
dently of the number of
separating C layers, the C atoms remain 8-fold
coordinated, since the oxygen atoms in the 02 layer are arranged as in the
DO2 layers.
C and 02 layers can also be added to structures that contain
n DO 2
and
p(n - 1) C layers (n >_ 2). For a basic structure, the number of C layers between

D. High-T~ Superconductor Family Tree
283
consecutive 002 layers (p) must be constant and, hence, (n - 1) C and (n - 1)
O2 layers must be added simultaneously. When, for example, this operation is
performed on A1BzC2D309 (1223), a structure with the formula A1BzC4D3013
(1243, hypothetical) is obtained. Inserting pairs of C and O2 layers into the
structure of the so-called infinite-layer compound C1DIO 2 (0011, (Sr, La)CuO2,
Fig. 8.5), gives a structure with the formula
C2OlO 4 (0021, (Nd, Ce)zCuO3.92).
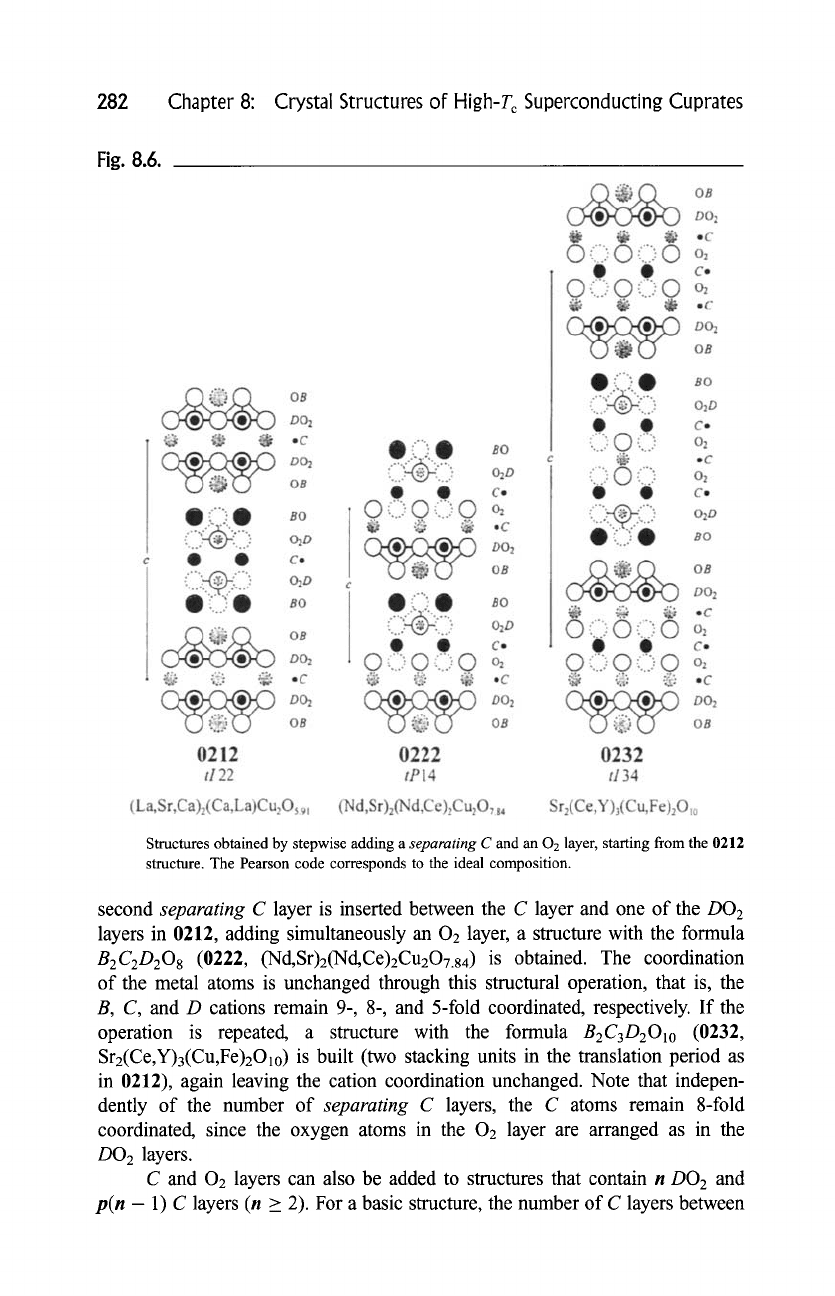
c. Generation of the Family Tree
Starting from the ideal structure of perovskite, or from the tetragonally distorted
structure of the cuprate LaCuO2.95 (0101), and performing, one by one, the
structural operations defined in Section D,a, one can generate the high-T c
superconductor family tree shown in Fig. 8.7. The four-digit codes representing
structures with one and two stacking units in the translation period are placed
within open and shaded rectangles, respectively. Horizontal lines correspond to
the structural operation which consists in adding a bridging BO or an additional
AO layer. When AO layers are progressively added, the members of structure
series with the formulas AkB2CcstDcstOk+cs t are generated. The end points of the
vectors (infinite number of AO layers) correspond to a rock-salt-type (NaC1) atom
arrangement. The simultaneous insertion of a conducting DO2 and a separating C
layer is represented by vertical lines. Repeating the operation generates members
of structure series with the formulas
AcstBcstCn_lDnO2n+cst.
The end member of
each series is the structure of the infinite-layer compound (0011, (Sr, La)CuO2).
Diagonal lines represent the structural operation consisting in adding a separating
C layer and an 02 layer. The structure series grouping the structures produced
along one of these lines can be described by the formulas
AcstBcstCmDcstO2m+cst.
Infinite adding of layers leads in this case to the structure of fluorite (CaF2). This
last operation is also applied, at the bottom of the figure, to the limiting structure
0011. Only a certain number of basic structures are indicated in the diagram;
however, structures with higher numbers in the four-digit code, such as 1256 and
5222,
are also known.
It follows from the diagram that, for example, the structure of
1232
((Bi,Cu)Sr2(Ce,Nd)3Cu2Oll) can be obtained from 0101 (LaCuO2.95) in 10
different ways, one of which corresponds to the following path:
0101 --~ 0201 --~ 1201 --~ 1212 --~ 1222 --~ 1232.
The simultaneous adding of one DO 2 layer and p C (p > 1) layers, which
would correspond to vertical lines ending with the structures 0021 (p = 2,
(Nd, Ce)2CuO3.92),
0031 (p = 3, hypothetical), etc., is not illustrated in the
diagram.
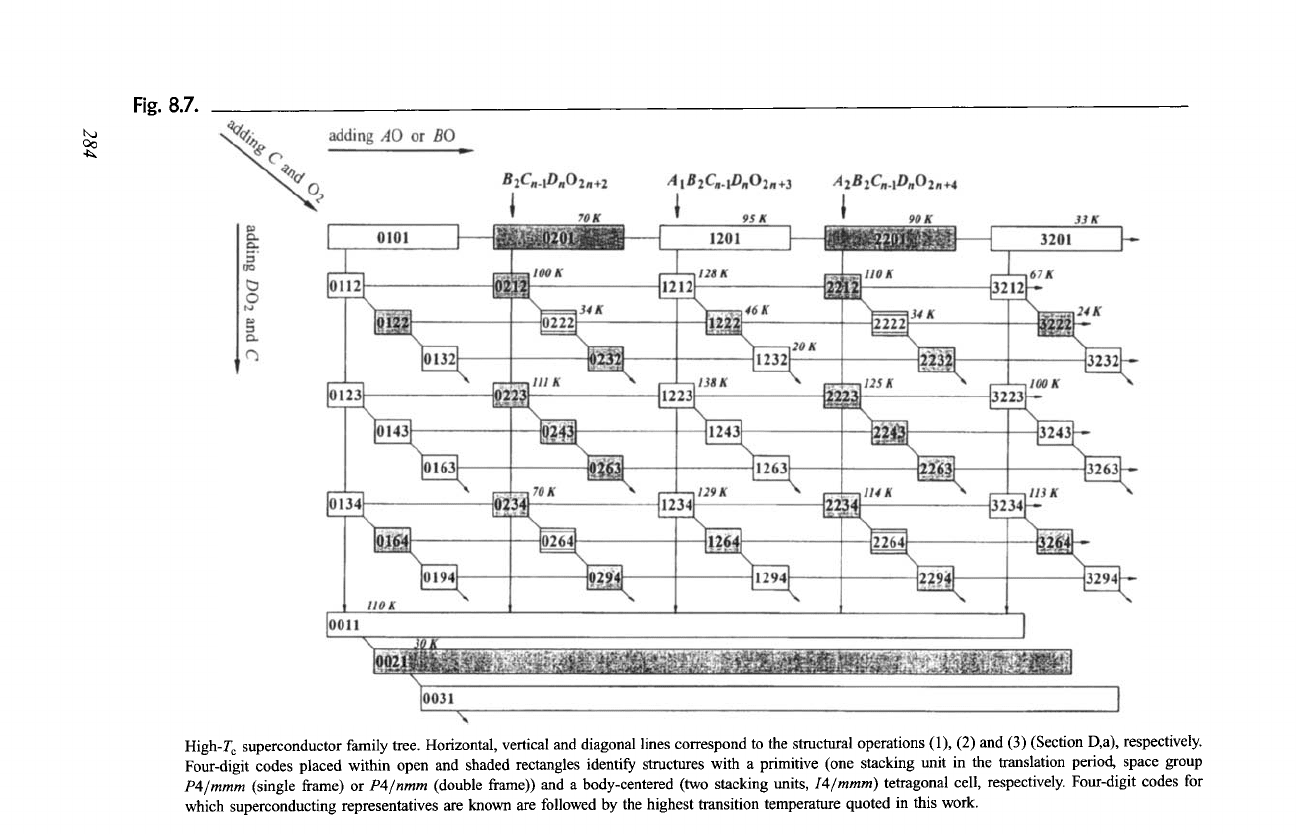
Fig. 8.7.
t,,a
4~
High-T c superconductor family tree. Horizontal, vertical and diagonal lines correspond to the structural operations (1), (2) and (3) (Section D,a), respectively.
Four-digit codes placed within open and shaded rectangles identify structures with a primitive (one stacking unit in the translation period, space group
P4/mmm
(single frame) or
P4/nmm
(double frame)) and a body-centered (two stacking units,
I4/mmm)
tetragonal cell, respectively. Four-digit codes for
which superconducting representatives are known are followed by the highest transition temperature quoted in this work.

E. Symmetry 285
Symmetry
Undistorted basic structures are described in a limited number of space groups,
most of them tetragonal. In the following section, the symmetry of the individual
atom layers is discussed. It is then shown how the space group for ideal basic,
limiting, and intergrowth structures may be derived from the four-digit code.
Distortions to, for example, orthorhombic are common in the real structures. We
will clarify the relationships between the space groups of the undistorted parent
types and space groups of lower symmetry, which have been used in the literature
to refine structures of high-T c superconducting cuprates.
a. Plane Groups for Individual Atom Layers
All atom layers defined in Section C,a and shown in Fig. 8.2 except AO', can be
described in the square plane group
p4mm,
with cell parameters a = b ~ 3.85 A.
There are 4-fold rotation points at the origin and the center of the cell, and mirror
lines along the cell axes ([0 1], [1 0]) and the cell diagonals ([1 1], [1 1]). The
cation sites are located at 4-fold rotation points (site symmetry
4mm),
either at
0 0 or at i 89 The oxygen atoms occupy the following positions:
.
2.
3.
Either I 1 or 0 0 for AO and BO layers
1 0 and 0 1 for AO2, DO 2 and
0 2
layers
1 89 (or 0 0), 1 0 and 0 l, with partial occupancy, for AO" layers
Some structures contain distorted layers, in particular distorted
additional
layers, which can generally be described in the rectangular plane groups
p2mm
or
c2mm,
as shown in Fig. 8.8. In the latter case the cell vectors correspond to the
diagonals of the small cell (a' ~ b' ~ 5.4 A) and the number of atoms in the cell
doubles. The AO' layer, shown in Fig. 8.2, is also described in the rectangular
plane group
p2mm.
The cell parameter ratio
b/a
> 1, if, by convention, the
oxygen atoms are located along [0 1].
b. Space Groups for Basic Structures
When the lattice of perovskite is distorted so that a = b < c, all 3-fold axes are
suppressed and of the three mutually perpendicular 4-fold rotation axes, only the
axis along [0 0 1] remains. Such a B1D10 3 structure (0101, LaCuO2.95),
containing two layers in the translation unit like the cubic perovskite structure,
is described in the tetragonal space group
P4/mmm,
which is a maximal
nonisomorphic subgroup of
Pm3m.
The cation sites are located at the intersec-
