Poole Ch.P., Jr. Handbook of Superconductivity
Подождите немного. Документ загружается.

286
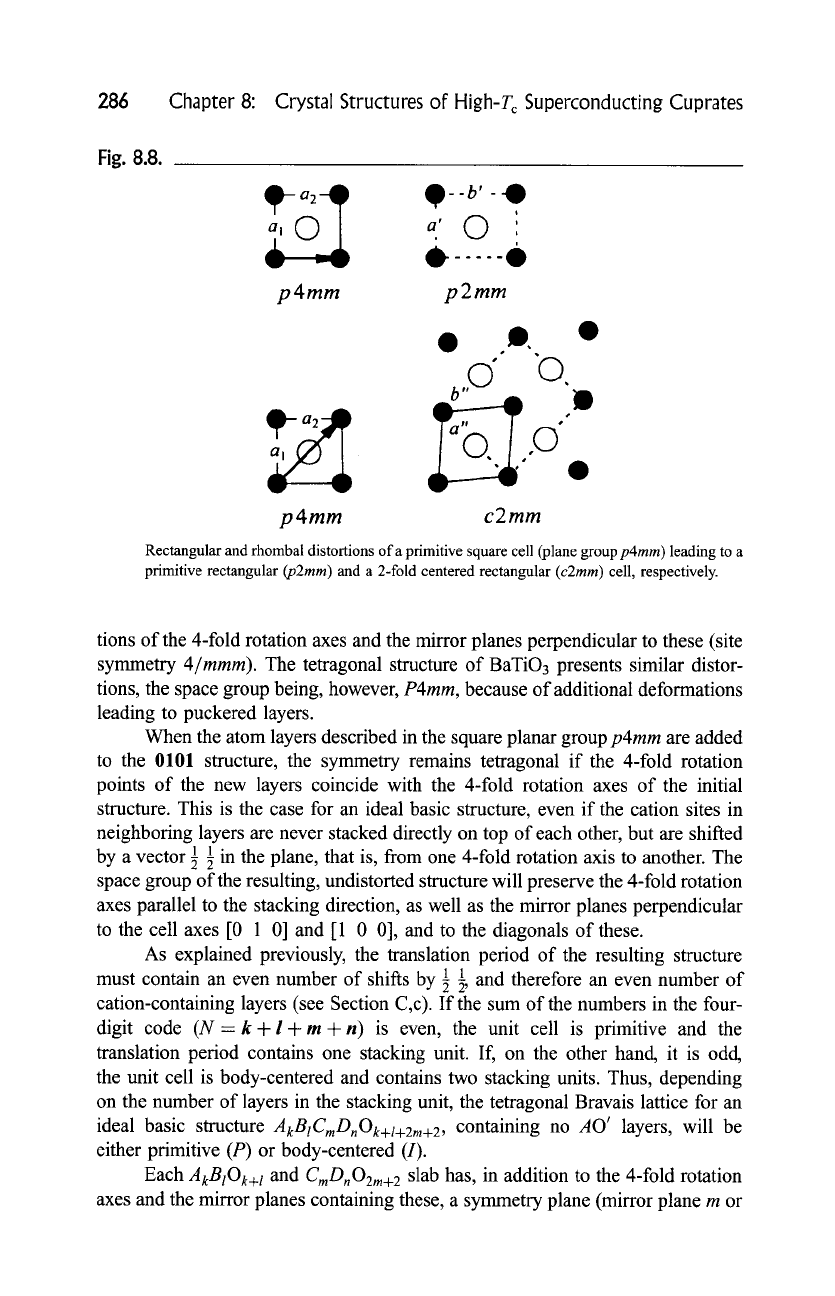
Fig. 8.8.
Chapter 8:
Crystal Structures of High-Tc Superconducting Cuprates
O-'b' O
o,9
O ..... e
p4mm p2mm
9 9
"0,
m
9
0
p4mm c2mm
Rectangular and rhombal distortions of a primitive square cell (plane group
p4mm)
leading to a
primitive rectangular
(p2mm)
and a 2-fold centered rectangular
(c2mm)
cell, respectively.
tions of the 4-fold rotation axes and the mirror planes perpendicular to these (site
symmetry
4/mmm).
The tetragonal structure of BaTiO3 presents similar distor-
tions, the space group being, however,
P4mm,
because of additional deformations
leading to puckered layers.
When the atom layers described in the square planar group
p4mm
are added
to the 0101 structure, the symmetry remains tetragonal if the 4-fold rotation
points of the new layers coincide with the 4-fold rotation axes of the initial
structure. This is the case for an ideal basic structure, even if the cation sites in
neighboring layers are never stacked directly on top of each other, but are shifted
by a vector 89 i in the plane, that is, from one 4-fold rotation axis to another. The
space group of the resulting, undistorted structure will preserve the 4-fold rotation
axes parallel to the stacking direction, as well as the mirror planes perpendicular
to the cell axes [0 1 0] and [1 0 0], and to the diagonals of these.
As explained previously, the translation period of the resulting structure
must contain an even number of shifts by I 1, and therefore an even number of
cation-containing layers (see Section C,c). If the sum of the numbers in the four-
digit code (N = k + 1 + m + n) is even, the unit cell is primitive and the
translation period contains one stacking unit. If, on the other hand, it is odd,
the unit cell is body-centered and contains two stacking units. Thus, depending
on the number of layers in the stacking unit, the tetragonal Bravais lattice for an
ideal basic structure
AkBlCmDnOk+l+2m+2 ,
containing no AO' layers, will be
either primitive (P) or body-centered (I).
Each
AkBtOk+ t
and
CmOnO2m+2
slab has, in addition to the 4-fold rotation
axes and the mirror planes containing these, a symmetry plane (mirror plane m or

E. Symmetry 287
diagonal glide plane n), situated halfway between its external layers. When the
number of layers in the slab, (k + I) or (m + n), is even, the symmetry plane is
located between two cation-containing layers, that is, between two A O or two BO
(k = 0) layers for a AkBlOk+ l slab, and between two C layers, coinciding with an
inner O2 layer, for
a CmOnO2m+2
slab. Because of the shift by ) 1 of the cation
sites in consecutive layers, the plane is in this case a diagonal glide plane n. In
contrast, when the number of layers in the slab is odd, the symmetry plane
coincides with an atom layer. This may be an inner AO or BO (k = 0) layer for
the
AkBtOk+ t
slab and a C (n even) or an inner DO 2 (n odd) layer for the
CmDnOzm+2
slab. The plane is here a simple mirror plane m. Combining the two
kinds of slab, three cases may occur:
.
Both (k + I) and (m + n) are even: The combination of the two glide planes
n results in space group
P4/nmm
with one stacking unit in the translation
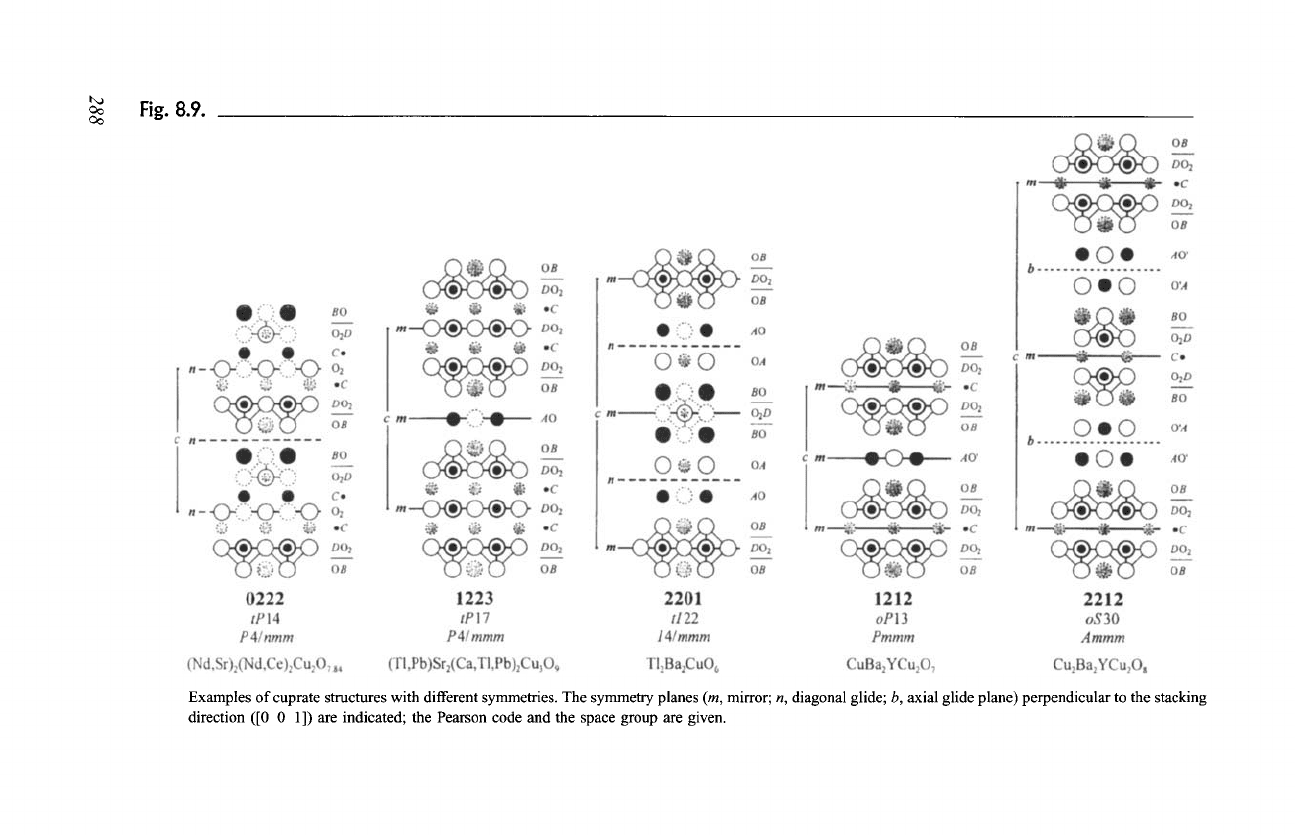
period. The 0222 structure, shown in Fig. 8.9 is an example of this
category.
Both (k + 1) and (m + n) are odd: The two mirror planes m give space
group
P4/mmm
with one stacking unit in the translation period. This case
is illustrated in Fig. 8.9 by the 1223 structure.
(k + I) and (m + n) are of different parity: One mirror plane m and one
diagonal glide plane n lead to space group
I4/mmm
with two stacking units
in the translation period. The 2201 structure is representative for this
category.
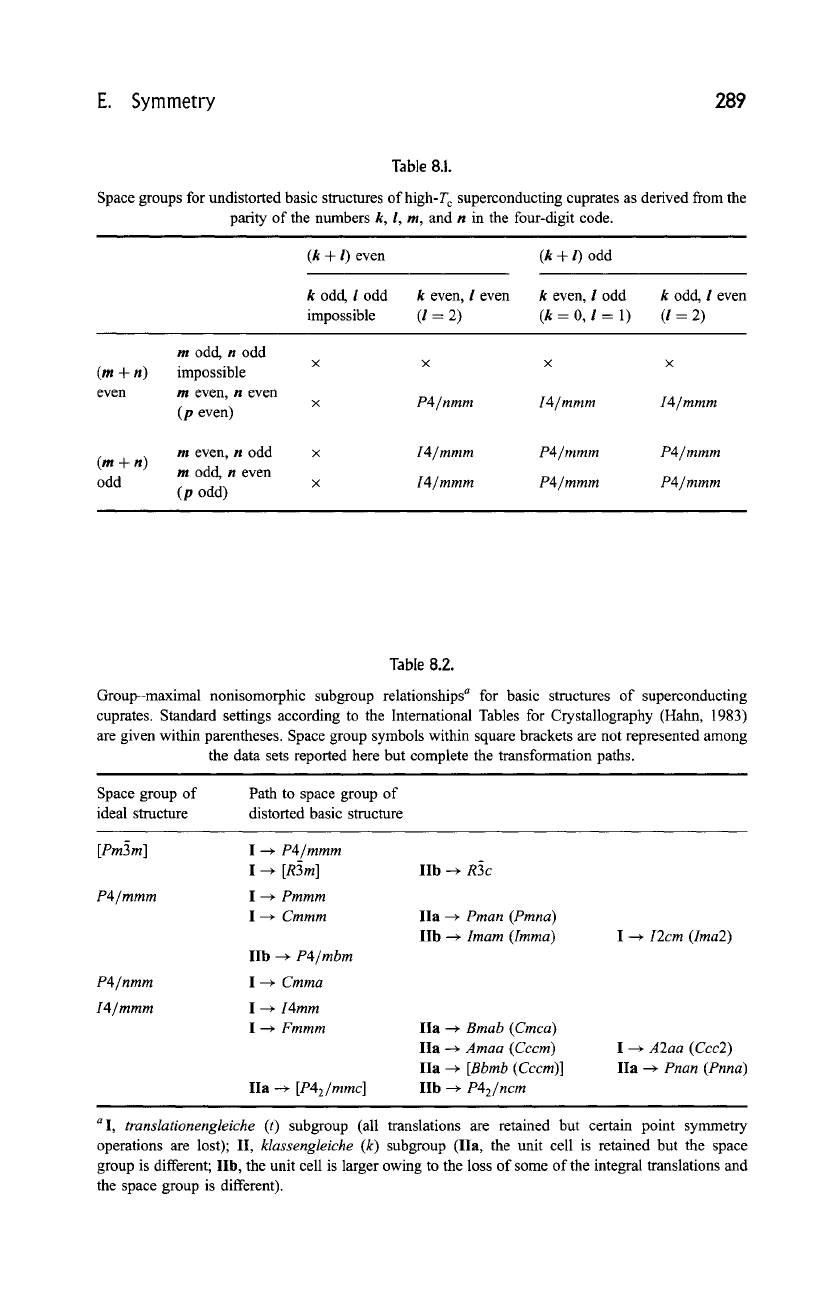
These cases are summarized in Table 8.1, which has been subdivided to consider
the different parity of the individual indices. It results from the stacking rules
described earlier that k and I cannot be odd simultaneously (if I = 1, k = 0). The
same is true for m and n, since m =
p(n-1).
The ideal basic structures can thus be described in one of the following
tetragonal space groups:
P4/mmm
(or cubic
Pm~3m
in the particular case of
idealized perovskite),
P4/nmm,
or
I4/mmm.
Indeed, the crystal structures of the
majority of the high-T c superconducting cuprates known up to now have been
refined in one of these groups. However, space groups of lower symmetry have
been used to describe and refine some crystal structures with significant
distortions. The group-maximal nonisomorphic subgroup relationships that
lead from one of the space groups listed previously to space groups used in
data sets given in Section H,b are indicated in Table 8.2. The lowering of
symmetry observed for the real structures may be caused by any of the following:
Displacements of the atoms from the ideal positions
An ordered arrangement of different cations within layers of the same kind
An ordered arrangement of vacancies
The insertion of extra atoms
t~
Oo
oo
Fig. 8.9.
Examples of cuprate structures with different symmetries. The symmetry planes (m, mirror; n, diagonal glide; b, axial glide plane) perpendicular to the stacking
direction ([0 0 1]) are indicated; the Pearson code and the space group are given.
E.
Symmetry
289
Table 8.1.
Space groups for undistorted basic structures ofhigh-T c superconducting cuprates as derived from the
parity of the numbers k, l, m, and n in the four-digit code.
(k + !) even (k + 1) odd
k odd, 1 odd k even, I even k even, 1 odd k odd, 1 even
impossible (l = 2) (k --- 0, 1 -- 1) (1 = 2)
(m + n)
even
m odd, n odd
impossible
m even, n even
(p even)
X X X X
• P4/nmm 14/mmm 14/mmm
(m + n) m even, n odd • I4/mmm P4/mmm P4/mmm
odd m odd, n even • I4/mmm P4/mmm P4/mmm
(p odd)
Table 8.2.
Group-maximal nonisomorphic subgroup relationships a for basic structures of superconducting
cuprates. Standard settings according to the International Tables for Crystallography (Hahn, 1983)
are given within parentheses. Space group symbols within square brackets are not represented among
the data sets reported here but complete the transformation paths.
Space group of
ideal structure
Path to space group of
distorted basic structure
[Pm3m] I --+ P4/mmm
I ~ [R3m]
P4/mmm I --+ Pmmm
I ~ Cmmm
P4/nmm
I4/mmm
IIb --+ P4/mbm
I --+ Cmma
I --+ I4mm
I --~ Fmmm
IIa -+ [P42/mmc ]
IIb ~ R3c
IIa --+ Pman (Pmna)
IIb ~ Imam (Imma)
IIa --~ Bmab (Cmca)
IIa --~ Amaa (Cccm)
IIa --+ [Bbmb (Cccm)]
lib ~ P42/ncm
I --~ I2cm (Ima2)
I --+ A2aa (Ccc2)
IIa --+ Pnan (Pnna)
a I, translationengleiche (t) subgroup (all translations are retained but certain point symmetry
operations are lost); II, klassengleiche (k) subgroup (IIa, the unit cell is retained but the space
group is different; lib, the unit cell is larger owing to the loss of some of the integral translations and
the space group is different).

290
Chapter 8: Crystal Structures of High-Tr Superconducting Cuprates
These lead, in a majority of cases, to structures that are described in orthorhombic
space groups with cell vectors a + b, -a + b and c (d ~ b' ~ 5.4 A), where a, b,
and c are the cell vectors of the ideal structures. Note that the orthorhombic
structure of BaTiO3 (space group
Cm2m)
presents distortions resulting in a
similar cell. Owing to positional or occupational commensurate modulations,
superstructures with large unit cells are sometimes observed. Incommensurately
modulated structures are also known.
As stated previously, the symmetry considerations up to now do not apply
to structures containing AO' layers. The oxygen atoms in these layers center the
square edges only in one direction, breaking the 4-fold symmetry. Among the
basic structures of superconducting cuprates, there are two well-known examples,
Ba2YCu307 (1212, CuBa2YCu2OT) and Ba2YCu408 (2212, Cu2Ba2YCu208),
where the undistorted structures are orthorhombic. Both structures contain
additional
CuO layers, the copper atoms of which center mutually parallel
squares of oxygen atoms perpendicular to the layer. The replacement of a
single AO layer by a AO' layer, as in Ba2YCu3OT, does not alter the general
stacking scheme, but the 4-fold rotation axis is reduced to a 2-fold one. The
resulting orthorhombic structure, shown in Fig. 8.9, is described in space group
Pmmm,
which is a direct subgroup of
P4/mmm ((k + I)
odd, (m + n) odd). The
cell parameter b is slightly larger than a, because of the presence of oxygen atoms
along [0 1 0] in the AO' layer. The structure can be derived from the structure of
Ba2YCu306.26 with the same four-digit code (1212), space group
P4/mmm,
where one-third of the copper atoms form A layers, considering an ordered
arrangement of extra oxygen atoms in the
additional
layer.
In the case of Ba2YCu408 (2212), the two consecutive AO' layers are
shifted by 0 I with respect to each other if, by convention, the oxygen atoms in
the AO' layers are located along [0 1 0]. Such a shift corresponds to an axial
glide plane b, situated between the two layers, that is, halfway between the BO
layers delimiting the A2B204 slab. Combined with the mirror plane m coinciding
with the central C layer of the CD204 slab, this leads to space group
Ammm
(standard setting
Cmmm).
There are two stacking units in the translation period
along c and the cell parameter ratio
b/a
> 1 (Fig. 8.9).
c. Space Groups for Limiting Structures
Like the majority of the basic structures, the limiting structures with the general
formula
CpDO2p
are described in tetragonal space groups. When p is odd, the
translation period contains two mirror planes m. The first one coincides with the
DO 2 layer and the other one with the inner C layer. This combination yields
space group
P4/mmm
with one stacking unit in the translation period (0011,
(Sr, La)CuO2). When p is even, the mirror planes through the DO 2 layers alternate
with diagonal glide planes n, which coincide with the 02 layer situated between
E. Symmetry 291
the internal C layers. The resulting space group is I4/mmm with two stacking
units in the translation period (0021,
(Nd, Ce)zCuO3.92 ).
d. Space Groups for intergrowth Structures
As for the basic structures, the 4-fold rotation axes and the mirror planes
containing these are preserved in the intergrowth structures, as long as there
are no AO' layers. When the total number of cation-containing layers in all
stacking units forming an intergrowth structure, that is, the sum of the numbers in
all four-digit codes, is even, the tetragonal Bravais lattice is primitive; otherwise,
it is body-centered. Three cases may be distinguished:
,
,
The AkIBI Ok1+l 1 and Ak2Bt2Ok2+l 2 slabs are identical, that is,
k 1 -- k 2
and
I 1 = ! 2
The
CmlDnlOzml+ 2
and
CmzOnzOzm2+ 2
slabs are identical, that is, m I = m 2
and
n 1 = n 2
The corresponding slabs in the two stacking units are different
In cases (1) and (2), only the symmetry planes in the corresponding slabs
that are different will remain. As for the basic structures, the space group is either
P4/mmm, P4/nmm, or I4/mmm, depending in case (1) on the parity of (m I + nl)
and (m 2 -+-n2) and in case (2) on the parity of (k 1 + 11) and (k 2 +/2). This is
summarized in Table 8.3 and illustrated for case (2) by the idealized structure of
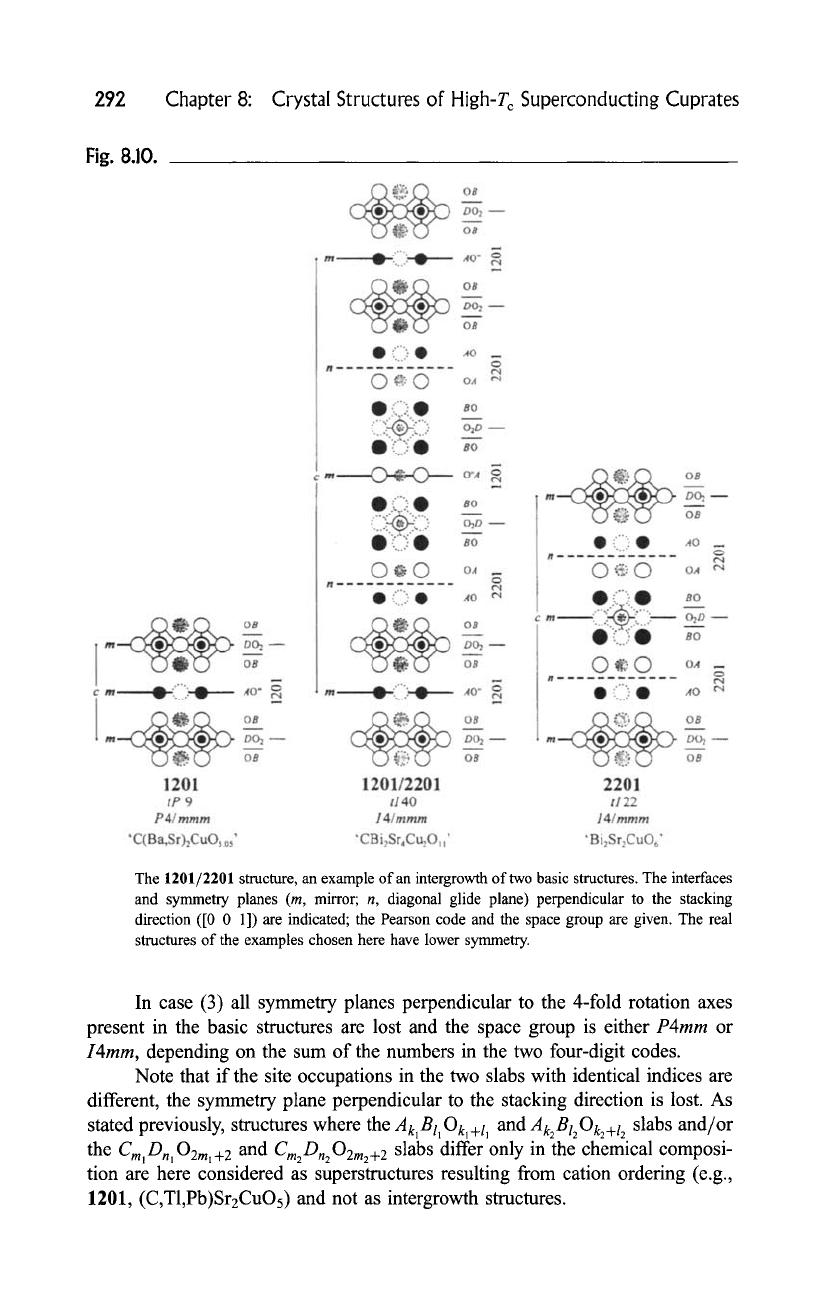
CBizSr4Cu201] (1201/2201) in Fig. 8.10.
Table 8.3.
Space groups for undistorted intergrowth structures of high-T c
superconducting cuprates with identical
AkB110kl+tl
and
Ak2Bt2Ok2+t 2
(or
CmDn O2m~+ 2
and
Cm2Dn202m2+2)
slabs as
derived from the parity of (m 1 4- n2) and (m 2 + n2) [or
(k I 4- il) and (k 2 4-/2)].
k 1 = k2, !1 = ! 2
[m l=m 2,nl=nz]
(m I + nl) even (m 1 4- nl) odd
[(k I 4- ll) even] [(kl + I1) odd]
(m 2 + n2) even
P4/nmm I4/mmm
[(k 2 4-12) even]
(m z + nz) odd
[(k 2 + 12) odd]
I4/mmm P4/mmm
292
Chapter 8: Crystal Structures of High-T~ Superconducting Cuprates
Fig. 8.10.
The 1201/2201 structure, an example of an intergrowth of two basic structures. The interfaces
and symmetry planes (m, mirror; n, diagonal glide plane) perpendicular to the stacking
direction ([0 0 1]) are indicated; the Pearson code and the space group are given. The real
structures of the examples chosen here have lower symmetry.
In case (3) all symmetry planes perpendicular to the 4-fold rotation axes
present in the basic structures are lost and the space group is either P4mm or
I4mm, depending on the sum of the numbers in the two four-digit codes.
Note that if the site occupations in the two slabs with identical indices are
different, the symmetry plane perpendicular to the stacking direction is lost. As
stated previously, structures where the
AkBllOkl+l ~
and
Ak2Bl2
Ok2+/2
slabs and/or
the
CmlOnlO2m1+ 2
and
CmzDn202m2+ 2
slabs differ only in the chemical composi-
tion are here considered as superstructures resulting from cation ordering (e.g.,
1201, (C,T1,Pb)Sr2CuOs) and not as intergrowth structures.
E
Ideal and Real Cation Coordinations
293
Table 8.4.
Group-maximal nonisomorphic subgroup relationships for inter-
growth structures of superconducting cuprates (for notations, see
Table 8.2).
Space group of
ideal structure
Path to space group of
distorted intergrowth structure
[P4/mmm] I ---+ [Cmmm]
IIa --+
Pbmm (Pmma)
I4/mmm I ~ [Fmmm]
IIa ~
Abmm (Cmma)
The majority of the intergrowth structures known up to now are described
in subgroups of the space groups derived for the ideal structures. Group-maximal
nonisomorphic subgroup relationships are presented in Table 8.4.
Intergrowth of Ba2YCu307 (1212) and BazYCu408 (2212), crystallizing in
the orthorhombic space groups
Pmmm
and
Ammm,
respectively, is known for the
compound Ba4YzCu7014.94 (1212/2212, CuBazYCuzO6.94/Cu2BazYCu208).
The total number of layers in the four slabs is odd, that is, there are two stacking
units from each parent structure in the translation period. Since the basic
structures contain identical
CD204 (YCu204) slabs,
the axial glide planes b,
situated between the two consecutive AO' (CuO) layers in the
A2B204
slab from
2212, and the mirror planes m, coinciding with the AO' layers in the AB203 slab
from 1212, remain from the basic structures and alternate along [0 0 1]. The
space group of the resulting structure is
Ammm
and the cell parameter ratio
b/a
> 1 (oxygen atoms in the AO' layers located along [0 1 0]).
Ideal and Real Cation Coordinations
The ideal coordination of the cation derives from the stacking of the layers, the
cation in each layer being surrounded by oxygen atoms from the same and/or the
directly neighboring layers (see Fig. 8.3). The real coordinations are often
distorted to better accommodate a particular cation. The strongest covalent
bonding is generally observed within the DO 2 (CuO2) layers and within the
coordination polyhedra of the A cations.
a. Coordination Polyhedra
Figure 8.11 shows ideal coordination polyhedra represented in high-T c super-
conducting cuprates. The coordination number of the D (Cu) atoms can be 6, 5,
or 4. Six oxygen neighbors form an octahedron (6o), as in perovskite. This
coordination of the D atoms is observed only in cuprates k/01 with a single
294
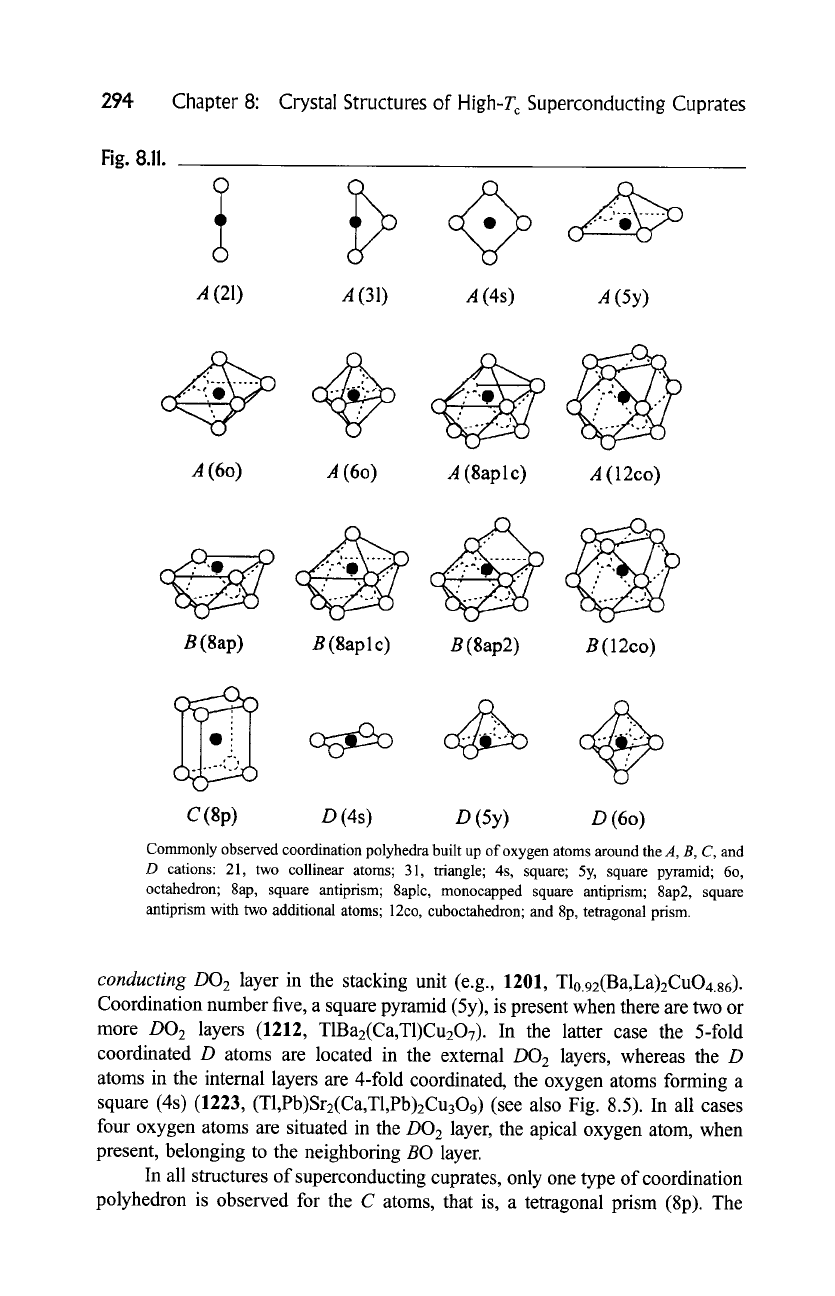
Fig. 8.11.
Chapter 8:
Crystal Structures of High-T~ Superconducting Cuprates
A (21) A (31) A (4s) A (5y)
A (60) A (60) A (8aplc) A (12co)
B (8ap) B(8aplc) B (8ap2) B(12co)
C (8p) D (4s) D (5y) D (60)
Commonly observed coordination polyhedra built up of oxygen atoms around the A, B, C, and
D cations: 21, two collinear atoms; 31, triangle; 4s, square; 5y, square pyramid; 6o,
octahedron; 8ap, square antiprism; 8aplc, monocapped square antiprism; 8ap2, square
antiprism with two additional atoms; 12co, cuboctahedron; and 8p, tetragonal prism.
conducting
DO 2 layer in the stacking unit (e.g., 1201, T10.92(Ba,La)2CuO4.86).
Coordination number five, a square pyramid (5y), is present when there are two or
more DO 2 layers (1212, T1Baz(Ca,T1)Cu207). In the latter case the 5-fold
coordinated D atoms are located in the external DO 2 layers, whereas the D
atoms in the internal layers are 4-fold coordinated, the oxygen atoms forming a
square (4s) (1223, (T1,Pb)Sr2(Ca, T1,Pb)2Cu309) (see also Fig. 8.5). In all cases
four oxygen atoms are situated in the DO 2 layer, the apical oxygen atom, when
present, belonging to the neighboring BO layer.
In all structures of superconducting cuprates, only one type of coordination
polyhedron is observed for the C atoms, that is, a tetragonal prism (8p). The

E Ideal and Real Cation Coordinations 295
oxygen atoms forming the tetragonal prism may belong to two neighboring DO 2
layers (0212, (La, Sr, Ca)z(Ca,La)Cu2Os.91), one DO 2 and one O2 layer (0222,
(]N~d, Sr)2(Nd, Ce)zCuzO7.84), or two O 2
layers (0232, Srz(Ce,Y)3(Cu,Fe)2Olo) (see
also Fig. 8.6).
The coordination number of the B atoms in perovskite and cuprates
containing one
bridging BO
layer in the stacking unit is 12, the surrounding
oxygen atoms forming a cuboctahedron (12co) (0112, BaY(Cu,Fe)2Os). Four
oxygen atoms are located in the BO layer, and the other eight in the two
neighboring DO 2 layers. When a second BO layer is added, the coordination
number of the B atoms is reduced to 9, the nine oxygen atoms forming a
monocapped square antiprism (8aplc) (0212). In the real structures the BO layers
are often puckered so that the B atoms are located closer to the center of the
polyhedron. This coordination is also observed when an AO layer with oxygen
atoms centering all squares of the cation square mesh is present (1212,
T1Baz(Ca, T1)Cu207) (see also Fig. 8.4). Depending on the number and the
arrangement of the oxygen atoms in the neighboring
additional
layer, the
coordination number of the B cation varies: 8 (square antiprism, 8ap) for an A
layer (1212,
CuBa2YCu206.26),
10 (two capping atoms on one side, 8ap2) for an
AO' layer (1212, CuBazYCu207), and 12 (cuboctahedron) for an AO 2 layer
(4212, (Ti,Gd, Ca)4Baz(Gd, Ca)Cu2Ol2). The B sites are occupied by Ba, Sr, or
rare-earth elements in ionized states, with little localized bonding and conse-
quently few requirements on the shape of the coordination polyhedron.
Depending on the exact type of
additional
layers present (A, AO, AO', AO',
or AO2) , a wide range of different coordinations are found. In contrast to the B
metal atoms, the elements found on the A sites, generally nonmetals, have
characteristic coordination polyhedra, with a large proportion of covalent bond-
ing. Five types of ideal coordination polyhedra are observed: two collinear atoms
(21), a triangle (31), a square (4s), a square pyramid (5y), and an octahedron (6o).
Linear coordination is typical for Hg or Cu. In this case the
additional
layer
contains no oxygen atoms (type A), and the neighboring layers are either BO
(1201, HgBazCuO4.18) or AO (3212, (Pb,Cu)3SrzYCu208). Trigonal coordination
is characteristic of carbon atoms (1201, C(Ba, Sr)zCuOs.05), the structures
containing planar carbonate units, perpendicular to the AO" layers. Square
coordination is observed for copper atoms in A O' layers, the two neighboring
layers being AO and/or BO (1212, CuBazYCu207). To achieve square pyramidal
coordination, sometimes found for lead atoms (3212, (Pb,Cu)3SrzYCu208), one
of the surrounding layers must contain no oxygen atoms (type A). Bi, Pb, and T1
preferentially form AO layers, which would lead to 6-fold, octahedral coordina-
tion. The real coordination polyhedra are, however, often strongly distorted
because of the presence of lone-electron pairs, and the cations displaced from
the centers of the original octahedra (2212, (Bi,Pb)zSrz(Y, Ca)Cu2Os). The
presence of AO 2 layers will give rise to higher coordination numbers, suit-
able for metal atoms. When the structure contains four
additional
layers in
the stacking sequence
-AOz-AO-AO-AO2- ,
for instance, the cations of the
