Poole Ch.P., Jr. Handbook of Superconductivity
Подождите немного. Документ загружается.


296
Chapter 8: Crystal Structures of High-To Superconducting Cuprates
internal layers have monocapped square antiprismatic coordination (8ap 1 c, 4212,
(Ti,Gd, Ca)4Baz(Gd, Ca)Cu2012). In (Ti,Sm, Ca)sBaz(Ca, Sm)Cu2014 (5212), where
the number of additional layers is five, AO 2 layers alternating with AO layers, the
cations from the AO layers are 12-fold coordinated (cuboctahedron, 12co),
yielding a local perovskite-like atom arrangement.
b. Interatomic Distances
Displacements of the atoms from their ideal positions distort the coordination
polyhedra, and the interatomic distances from the cations to the surrounding
oxygen atoms exhibit a wide range of values.
The atoms in the conducting DO 2 layers are tightly bonded, and the in-
plane Cu-O distances vary little from one superconducting cuprate to another.
Usual distances range from 1.92 to 1.94 A, the shortest distance (1.89 A) being
reported for 0201 (La, Sr)2CuO4) and the longest one (1.98A) for 1201
(C(Ba, Sr)2CuOs.05) and 0011 ((Sr, La)CuO2). This relatively rigid atom layer
determines the values of the cell parameters a and b, a slight puckering being
possible. When there are two or more DO 2 layers, the copper atoms from the
outside DO 2 layers are slightly moved from the center of the square base toward
the center of the pyramid. The distances from the copper atoms to the oxygen
atoms located in the BO layers, that is, the apical atoms of the square pyramid or
octahedron, are considerably longer (2.10-2.82 A), in agreement with the Jahn-
Teller effect. The large variations observed for this Cu-O distance depend on the
size of the B atom and the chemical nature of the A cations. For structures with
bridging SrO layers, the distances from the copper atoms to the apical oxygen
atoms are in general shorter than for structures containing BaO layers. When
additional layers are present, the same apical oxygen atoms are usually tightly
bonded to the A cations, situated on the other side of the BO layer. For example,
in structures of Hg-based compounds, because of the bonding between the
oxygen atoms from the BO layers and the mercury atoms (and because of the
presence of barium atoms in the BO layers), the apical Cu-O distances are very
long (2.70-2.82 A).
For structures with only one separating C layer between two consecutive
DO 2 layers, the tetragonal prisms around the C atoms are almost regular. The
C-O interatomic distances range from 2.39 to 2.61 A. In general, yttrium atoms
have shorter C-O distances than calcium atoms. For structures with two or more
C layers between consecutive DO 2 layers, the distances from the C atom to the
oxygen atoms in the O2 layers are slightly shorter (2.26-2.39 A).
For distorted perovskite-type structures (one BO layer) the interatomic
distances from the B atoms to the vertices of the surrounding cuboctahedra range
from 2.50 to 3.01 A. For structures with two bridging BO layers, where only half
of the cuboctahedron (square antiprism) remains, there are four distances of
similar length to the oxygen atoms in the DO 2 layer (2.58-2.97 A, depending on

G.
Chemical Families
297
the B cation) and four distances of similar length to the oxygen atoms within the
BO layer (2.73-2.88 A). The distances to the capping oxygen atoms in the
neighboring bridging or additional layer vary from 2.28 to 2.37 A and from 2.61
to 3.19 A, respectively.
The A-O interatomic distances, like the coordination polyhedra of the A
atoms, depend on the chemical nature of the cation. As in the previous cases, they
are of the same magnitude as those commonly observed for the corresponding
cations (e.g., Hg 2+, Cu 2+, C 4+, Bi 3+, Pb 2+, T13+) in other oxides. The distances
between the mercury and oxygen atoms along the stacking direction vary from
1.92 to 2.01 A. The corresponding distances for copper atoms in the same 2-fold
linear coordination range from 1.81 to 1.89 A. Copper atoms in 4-fold coordina-
tion have two distances of 1.94 A to oxygen atoms within the AO' layer and two
shorter distances (~ 1.84 A) to oxygen atoms from neighboring AO' and/or BO
layers. Carbon atoms usually show three similar A-O distances of 1.23-1.29 A.
The displacement of the atoms in the AO layers containing Pb are such that the
square pyramids are strongly distorted with three short distances (including one to
an oxygen atom in the neighboring BO layer) of 2.14-2.48 A and two long
distances of 2.95-3.23 A. The resulting coordination polyhedron can be described
as a tetrahedron with one of the vertices occupied by a lone electron pair (~-
tetrahedron). In a similar way, the ideal octahedra around thallium and bismuth
atoms are transformed into tetrahedra and ~p-tetrahedra, respectively. The intera-
tomic distances from thallium atoms to the two oxygen atoms in the neighboring
AO and/or BO layers vary from 1.82 to 2.20 A, whereas the distances to the four
oxygen atoms within AO layer range from 2.30 to 2.56 (two short) and from 2.77
to 3.16 A (two long distances). Bismuth atoms are not bonded to the oxygen
atoms in the neighboring AO (BiO) layer, the distance to the oxygen atoms in BO
layer being 1.96-2.08 A. Within the AO layer there are usually two short (2.02-
2.61 A) and two long interatomic distances, or three short (2.10-2.70 A) and one
long distance.
G
Chemical Families
Chemical families of high-T c superconducting cuprates are usually denoted by
the cation in the additional layers. This allows one to emphasize structural
features (including distortions) common to a particular group of compounds.
Cuprates containing halogens and so-called ladder compounds will also be briefly
discussed. References are specified for first reports, whereas other references for
compounds mentioned here can be found with the data sets in Section H,b.
Compounds with structures containing four and more additional layers and
mainly metal atoms, such as Ti, on the A sites are known, but so far no
superconductivity has been reported.

298
Chapter 8: Crystal Structures of High-Tc Superconducting Cuprates
a. Rare-Earth-Alkaline-Earth Cuprates
The structures of the compounds considered in this section contain no
additional
layers, whereas rate-earth-alkaline-earth superconducting cuprates with A or A O'
layers are treated in the next section.
The first 0101 cuprate, LaCuO3, was prepared by Demazeau
et al.
(1972) at
high pressure. It crystallizes with a trigonal structure based on an R Bravais
lattice, a distorted variant of the cubic perovskite-type structure. Copper is in the
oxidation state Cu 3+ and the compound is not superconducting. Decreasing the
oxygen content produces vacancies in the CuO2 layers. Other members of the
structure series with the general formula
BCmDnO2m+3
(pervoskite is the first
member with n = 1 and m = 0), reported so far, were prepared with a mixture of
Cu and Fe or Co on the D site, none of them being superconducting.
The structure series with the general formula
B2Cn_lCUnO2n+2
includes
structures with two
bridging
layers in the stacking unit.
La2CuO4
(Longo and
Raccah, 1973; Grande
et al.,
1977), LaSrCuO4 (Goodenough
et al.,
1973), and
La2_xBaxCuO4 (x--0-0.2) (Michel and Raveau, 1984) crystallize with a body-
centered tetragonal K2NiF4-type structure (Balz, 1953) or with an orthorhombi-
cally distorted variant (n = 1, 0201). The discovery of superconductivity in the
La-Ba-Cu-O system (T c = 30 K) by Bednorz and Mfiller (1986) started the era
of high-temperature superconductivity. Superconductivity for 0201 compounds
can be achieved not only by partial substitution of trivalent La by a divalent
alkaline-earth element, but also by insertion of additional oxygen. At low
temperature and/or for a low content of alkaline-earth element, the crystal
structures of these compounds are distorted (primitive tetragonal or orthorhom-
bic), while at high temperature and/or for a higher Ba(Sr) content the structures
remain of the K2NiF4 type.
The second member of the structure series (n = 2, 0212) was first prepared
by Nguyen
et al.
(1980) for
La2_xMl+xCu206_6 (M ~
Sr or Ca, x = 0-0.14).
Superconductivity is known only for Ca-containing compounds and was discov-
ered by Cava
et al. (1990a)
for Lal.6Sr0.4CaCu206 (T c -- 60 K).
Members with n = 3 (0223) and n = 4 (0234), with superconducting
transition temperatures of about 90 and 70 K for the nominal compositions
Sro.6Ca0.33CuO2.1o
and
Sro.65Cao.3CuO2.10
(synthesized at high pressure), respec-
tively, were first reported by Adachi
et al.
(1993). Later on it was shown that 0223
compounds may also be prepared without calcium.
A superconducting (Tr = 28 K) 0222 compound was reported by Akimitsu
et al.
(1988) in the Nd-Sr-Ce-Cu-O system (nominal composition
Nd2Sro.sCeo.sCUl.2Oy).
In contrast to the structures belonging to the series
mentioned above, where the CuO2 layers are separated by a single C layer
with mainly alkaline-earth metal atoms, here the CuO2 layers are separated by
double C layers with La or rare-earth metal atoms. A 0232 compound with three
separating
layers between consecutive DO 2 layers has also been reported.

G. Chemical Families 299
An infinite-layer compound (0011) was first prepared by Siegrist
et al.
(1988b) by stabilizing calcium cuprate by Sr
(Ca0.86Sro.14CuO2) ,
whereas the first
superconductor, Sro.s6Ndo.14CuO 2 (T c --40K),
was reported by Smith
et al.
(1991). A nonsuperconducting 0021 compound, Nd2CuO4, was prepared by
Miiller-Buschbaum (and Wollschl~iger, 1975), superconductivity (T c = 24K)
being reported by Tokura
et al.
(1989b) for the partly substituted derivative
Nd1.84Ce0.16Cu03.93
9
b. Ba-Y Cuprates
Superconductivity in the Ba-Y-Cu-O system was discovered by Wu
et al.
(1987)
for Ba0.8Yl.zCuO4_~, the first compound that showed superconductivity above
the temperature of liquid nitrogen and later identified as BazYCu307_ ~ (1212)
(T c = 93 K for 6 = 0.07). For 6 = 0-0.6, the compound crystallizes with an
orthorhombic structure. At 6 = 0.6, the structure becomes tetragonal and super-
conductivity is suppressed. On decreasing the oxygen content, the arrangement of
the oxygen atoms in the
additional
CuO layer becomes disordered. The square
planar coordination of the copper atoms (chains of corner-linked CuO4 squares)
is transformed to defect octahedral (random vacancies in the basal plane) and then
reduced to 2-fold linear.
Another compound in the Ba-Y-Cu-O system, BazYCu408 (2212), was
identified as planar defects in BazYCu307_ ~ by Zandbergen
et al.
(1988b) and
was prepared as bulk material (T c = 81 K) by Karpinski
et al.
(1988b). The 1212
and 2212 compounds can be described by the general formula CUkBazYCUzOk+ 6
(k = 1 and 2, respectively). In the structure of the latter, edge-linked CuO4
squares form zigzag chains and, in contrast to the 1212 compounds, the oxygen
content is fixed. A third superconducting compound in the same system,
Ba4YzCu7015_ ~ (T c = 92K), was observed by Zandbergen
et al.
(1988b) and
further studied by Karpinski
et al.
(1988a). The structure consists of an inter-
growth of 1212 and 2212 stacking units. Cu-1222 compounds with a mixture of
rare-earth elements (one of which is Ce) in the
separating
layers have also been
reported.
c. Bi-Based Cuprates
A superconducting Bi-based material was found for the first time in the Bi-Sr-
Cu-O system by Michel
et al.
(1987). A sample of nominal composition
BizSr2Cu207+ 6 was
reported to have a transition temperature of up to 22 K,
the superconducting compound being later identified as 2201. Maeda
et al.
(1988) obtained a critical temperature above 105K in the Bi-Sr-Ca-Cu-O
system for the nominal composition BiSrCaCuzOy. The structures of Bi-
based superconducting cuprates form a series with the general formula
300
Chapter 8: Crystal Structures of High-T~ Superconducting Cuprates
Bi2B2Cn_lCUnO2n+4
with mainly Sr and Ca on the B and C sites, respectively.
The 2201 (n = 1) and 2212 (n = 2) compounds have considerable homogeneity
ranges, and the 2223 (n = 3) compound is generally prepared with a partial
substitution of Bi by Pb. The superconducting transition temperature increases
with an increasing number of conducting CuO2 layers up to 110 K (2223). Bi-
2222, which is not a member of this structure series, has also been reported.
In all structures of Bi-based superconductors, the bismuth and oxygen
atoms in the additional BiO layers are displaced from the ideal positions on the 4-
fold rotation axes, along [1 1 0] of the tetragonal cell. The displacements occur
in a progressive manner, resulting in the formation of voids large enough to
accommodate extra oxygen atoms and incommensurate modulations. The trans-
lation period of the modulation depends on partial substitutions and on the
oxygen content, but for particular compositions the structures can be conveni-
ently described in large supercells. The Bi sites have ff-tetrahedral coordination,
typical for Bi 3+, the lone electron pairs being located between consecutive BiO
layers that are hence only weakly bonded. The asymmetry of the BiO layer is
probably the reason that phases with a single "pure" BiO layer are not known. A
1232 compound, containing a mixture of Bi and Cu in the additional AO layer
and three separating C layers, has also been reported.
d. TI-and Ga-Based Cuprates
The first Tl-based superconducting material was reported in the T1-Ba-Cu-O
system by Kondoh et al. (1988). A critical temperature of 19 K was measured for
a sample of nominal composition Tll.zBao.sCuOy. Independently, Sheng and
Hermann (1988a) reported critical temperatures up to 90 K for the nominal
compositions TlzBazCu308+~, T1BaCu305.5+6, and T11.5BazCu307.3+6, the super-
conducting compound being later identified as 2201. A short time later, the same
authors (Sheng and Hermann, 1988b) succeeded in preparing superconductors
with T c = 120 K in the T1-Ba-Ca-Cu-O system for the nominal compositions
T12BaCal.5Cu308.5+ ~ and Tll.86BaCaCu307.8+ ~. At present, Tl-based cuprates
constitute one of the largest chemical families of high-T c superconductors,
forming two distinct structure series with the general formulas
T1B2Cn_lCUnO2n+3
and
T12B2Cn_lCUnO2n+4 ,
respectively. The B atoms in
compounds with single additional T10 layers are usually Ba and/or Sr (T1
being partly substituted by Pb or Bi in Sr-containing cuprates), whereas Sr-
containing compounds with two T10 layers have not been reported so far. For
both structure series, the C sites are mainly occupied by Ca, with small amounts
of T1. With an increasing number of conducting CuO2 layers, the superconducting
transition temperature progressively increases, reaching a maximum value for
1223, 1234 (~ 120K) and 2223 (125K), and then decreases. Apart from the
compounds that are members of these two structure series, other compounds,
such as 1222 and 2222, have also been reported.

G. Chemical Families 301
A common feature of the structures of Tl-based superconductors is the
displacement of the thallium and oxygen atoms in the
additional
T10 layers from
the ideal positions on the 4-fold rotation axes. The resulting tetrahedral coordina-
tion of the thallium atoms is typical for T13+. Vacancies may occur on the O sites
in the
additional
layers.
Ga-based compounds were first reported by Vaughey
et al.
(1991)
(GaLaSrCuOs, 1201) and Roth
et al.
(1991) (Gao.97Sr2YCu207, 1212), whereas
the first superconductor (T c = 35 K), a 1212 compound (GaSrzEr0.6Cao.4Cu207),
was prepared by Cava
et al.
(1991). The structures of the Ga-based cuprates
known so far contain a single
additional
GaO layer and are described by the
general formula
GaB2Cn_lCUnOzn+3.
The B sites are occupied by Sr (or a mixture
of Sr and La), superconductivity occurring when Ca is present on the C sites. As
in the corresponding structures of Tl-based cuprates, the A cations are surrounded
by oxygen atoms forming tetrahedra; however, the GaO4 tetrahedra are arranged
in chains along [1 1 0] of the tetragonal cell, lowering the symmetry to
orthorhombic.
e. Pb-Based Cuprates
Bi- and Tl-based superconductors are often prepared with a partial substitution of
Bi and T1 by Pb. In the latter case, the substitution can reach 50 at.%. Structures
containing only Pb on the A sites in the
additional
layers are not known so far;
however, a large number of compounds where Pb is the majority element on the A
site are known and form a distinct family of Pb-based superconducting cuprates.
Superconductivity was discovered in the Pb-Sr-Y-Ca-Cu-O system by Cava
et
al.
(1988), a critical temperature of 68 K being measured for a sample of
nominal composition Pb2Sr2Y0.sCa0.sCu308 (3212). For structures with a
single
separating C
layer between consecutive DO 2 layers, the series with one,
two, or three
additional
layers are described by the general formulas
(Pb,M)kB2Cn_lCunOk+2n+2
(k = 1, 2 or 3). The majority of the compounds
contain tfivalent cations (e.g., y3+) in the
separating C
layers and become
superconducting when part of them are substituted by divalent cations (Ca2+).
The structures with two
separating C
layers between the DO 2 layers can be
grouped into a series with the general formula
(Pb,M)kB2C2Cu2Ok+ 8.
Note that
the general formulas given here consider AO layers but the total number of
oxygen atoms in the
additional
layers may differ from k.
In most Pb-based cuprates, copper atoms are also present in the
additional
layers, whereas for compounds with one
additional AO
layer, mixtures of lead
(Pb 4+) and other elements (alkaline-earth, Sc, Cd) on the A site have also been
reported. In compounds with three
additional
layers, the lead and copper atoms
are always ordered, giving the stacking sequence PbO-Cu-PbO (oxidation states
Pb 2+ and Cu+). Ordering can also occur in compounds with two
additional
layers.
302
Chapter 8: Crystal Structures of High-T~ Superconducting Cuprates
f. Hg-Based Cuprates
Superconductivity for Hg-based materials was discovered in the Hg-Ba-Cu-O
system (HgBazCuO4.10, 1201) by Putilin
et al.
(1993a), the critical temperature
reaching 94 K. Later, Schilling
et al.
(1993) reported two high-T c superconduct-
ing compounds in the Hg-Ba-Ca-Cu-O system, HgBazCaCu206+ 6 (1212) and
HgBazCazCu308+ 6 (1223). The critical temperature of 133 K, attributed to Hg-
1223, is the highest value reported so far at ambient pressure and can be increased
to 157K by applying high pressure (23.5 GPa). Hg-based superconducting
cuprates form a series with the general formula
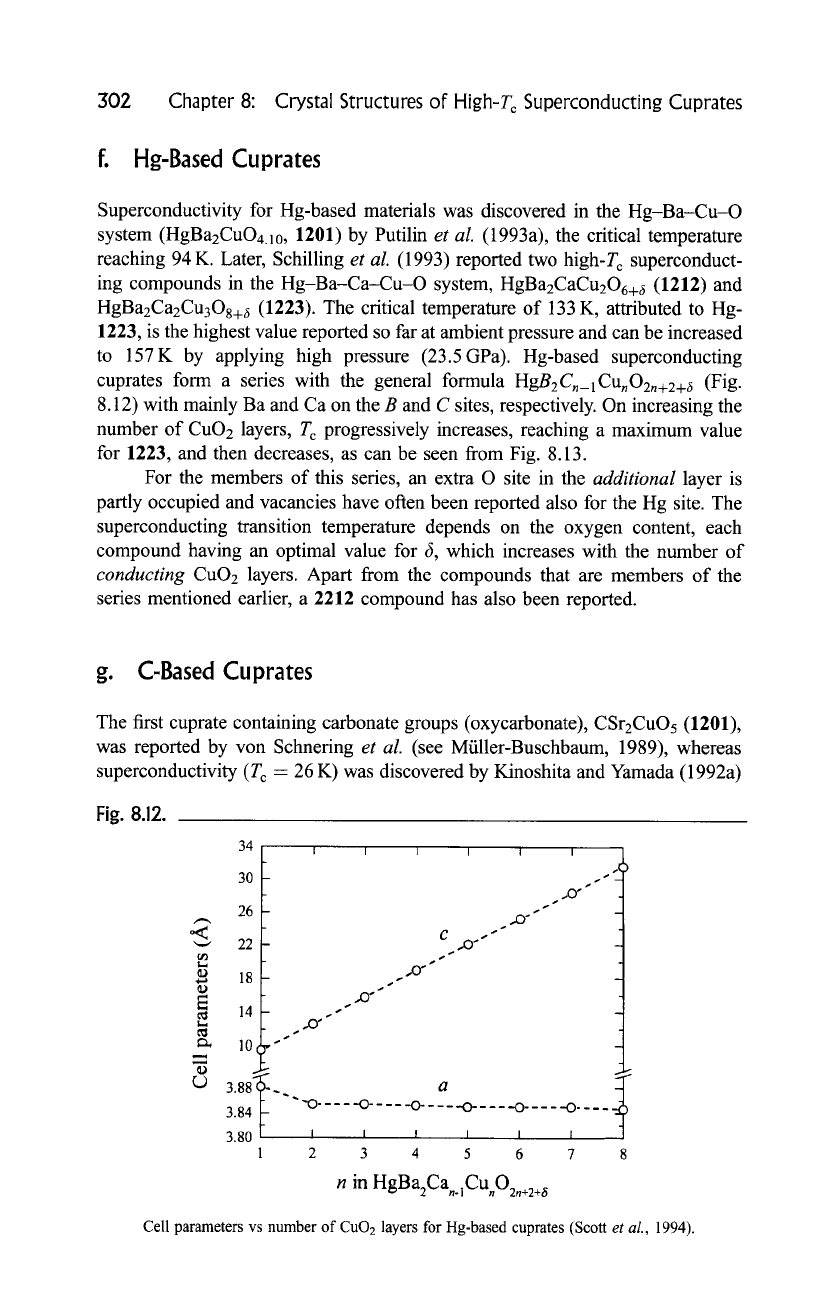
HgBzCn_lCUnO2n+2+6
(Fig.
8.12) with mainly Ba and Ca on the B and C sites, respectively. On increasing the
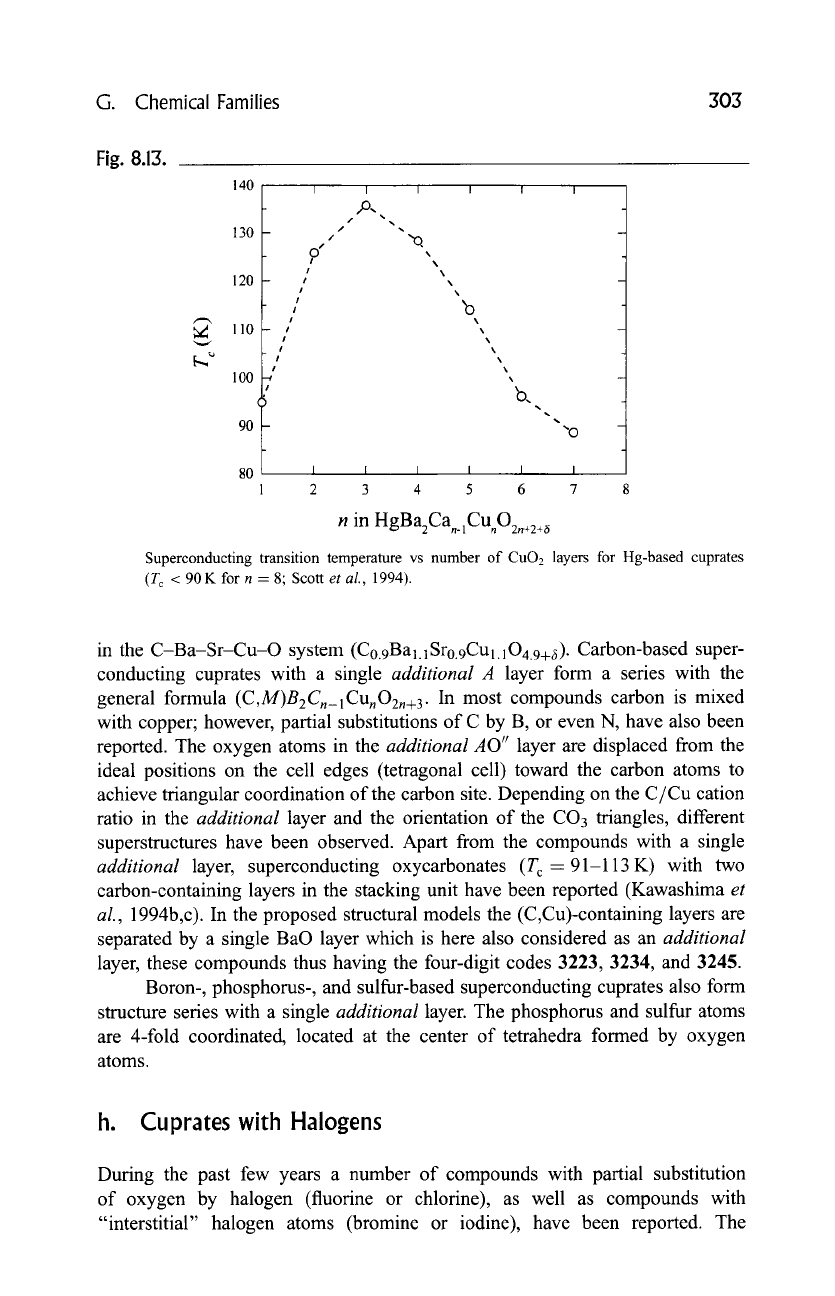
number of CuO2 layers, T c progressively increases, reaching a maximum value
for 1223, and then decreases, as can be seen from Fig. 8.13.
For the members of this series, an extra O site in the
additional
layer is
partly occupied and vacancies have often been reported also for the Hg site. The
superconducting transition temperature depends on the oxygen content, each
compound having an optimal value for 5, which increases with the number of
conducting
CuO2 layers. Apart from the compounds that are members of the
series mentioned earlier, a 2212 compound has also been reported.
g. C-Based Cuprates
The first cuprate containing carbonate groups (oxycarbonate), CSr2CuO5 (1201),
was reported by von Schnering
et al.
(see M/iller-Buschbaum, 1989), whereas
superconductivity (T c - 26 K) was discovered by Kinoshita and Yamada (1992a)
Fig. 8.12.
34 l
30
26
o<
,~ 22
~) 18
..,
E
r 14
tD
r,.)
s
.s
I I I I .... I
,X)"
s
s
CO.,,,"
.,. s ~
,,0"
.2)"
a.
a.
3.84t-- " " "0 .... -0-----0-----0---- -0 .... --0 ....
3.80 ~ I I J I I I
1 2 3 4 5 6 7 8
n in HgBa2Ca .]Cu O2,+2+~
Cell parameters vs number of
CuO 2
layers for Hg-based cuprates (Scott
et al.,
1994).
G. Chemical Families
303
Fig. 8.13.
140
130
120
110
100
90
I I I I I I
/ %
/
0 /
I %
%
!
, 'o
i I ~
I
I ~,
I
' b.
'%.
%
"o
80 I I I I I _. !
2 3 4 5 6 7 8
n in HgBa2Can.lCUnO2.+2+~
Superconducting transition temperature vs number of CuO2 layers for Hg-based cuprates
(T c < 90 K for n = 8" Scott
et al.,
1994).
in the C-Ba-Sr-Cu-O system
(C0.9Bal.lSr0.9CUl.lO4.9+6).
Carbon-based super-
conducting cuprates with a single
additional A
layer form a series with the
general formula
(C,M)B2Cn_lCUnO2n+3.
In most compounds carbon is mixed
with copper; however, partial substitutions of C by B, or even N, have also been
reported. The oxygen atoms in the
additional AO"
layer are displaced from the
ideal positions on the cell edges (tetragonal cell) toward the carbon atoms to
achieve triangular coordination of the carbon site. Depending on the C/Cu cation
ratio in the
additional
layer and the orientation of the CO3 triangles, different
superstructures have been observed. Apart from the compounds with a single
additional
layer, superconducting oxycarbonates (T c =91-113K) with two
carbon-containing layers in the stacking unit have been reported (Kawashima
et
al.,
1994b,c). In the proposed structural models the (C,Cu)-containing layers are
separated by a single BaO layer which is here also considered as an
additional
layer, these compounds thus having the four-digit codes 3223, 3234, and 3245.
Boron-, phosphorus-, and sulfur-based superconducting cuprates also form
structure series with a single
additional
layer. The phosphorus and sulfur atoms
are 4-fold coordinated, located at the center of tetrahedra formed by oxygen
atoms.
h. Cuprates with Halogens
During the past few years a number of compounds with partial substitution
of oxygen by halogen (fluorine or chlorine), as well as compounds with
"interstitial" halogen atoms (bromine or iodine), have been reported. The

304
Chapter 8: Crystal Structures of High-Tc Superconducting Cuprates
majority of these compounds were prepared by high-pressure synthesis (4-6 GPa)
giving only small amount of the final product, generally insufficient for a
complete structure determination. In the present work, crystallographic para-
meters for such compounds are given only when the classification has been
deduced.
Substitution of oxygen has been intensively studied for cuprates without
additional layers, such as 0201 and 0212. Substitution of 02- by F- (Cl-) takes
place in the bridging BO layers, making it possible to decrease the average
oxidation state of the B cation and in some cases significantly increasing the
critical temperature [e.g., Cal.9Nao.lCuO2C12, T c = 26 K (Argyriou et aL, 1995)
and
Sr2.3Ca0.TCU204.76Cll.24 , r c =
80K (Jin et aL, 1995)]. In the case where
complete substitution has taken place in the bridging layers, an extra layer of
halogen atoms can be inserted between consecutive BO layers, without changing
the relative shifts of these, leading to a local fluorite-type atom arrangement
[Sr2CuO2F2.57 , T c =
46K (A1-Mamouri et aL, 1994) and
Sr2gaCu204.6F2.0,
T c = 99 K (Kawashima et aL, 1994a)].
According to some reports, bromine and iodine atoms can be incorporated
into basic structures, forming an extra additional layer [(Bi2I)Sr2Ca2Cu3Oy
(3223), T c = 100 K (Xiang et al., 1991)].
i. Ladder Compounds
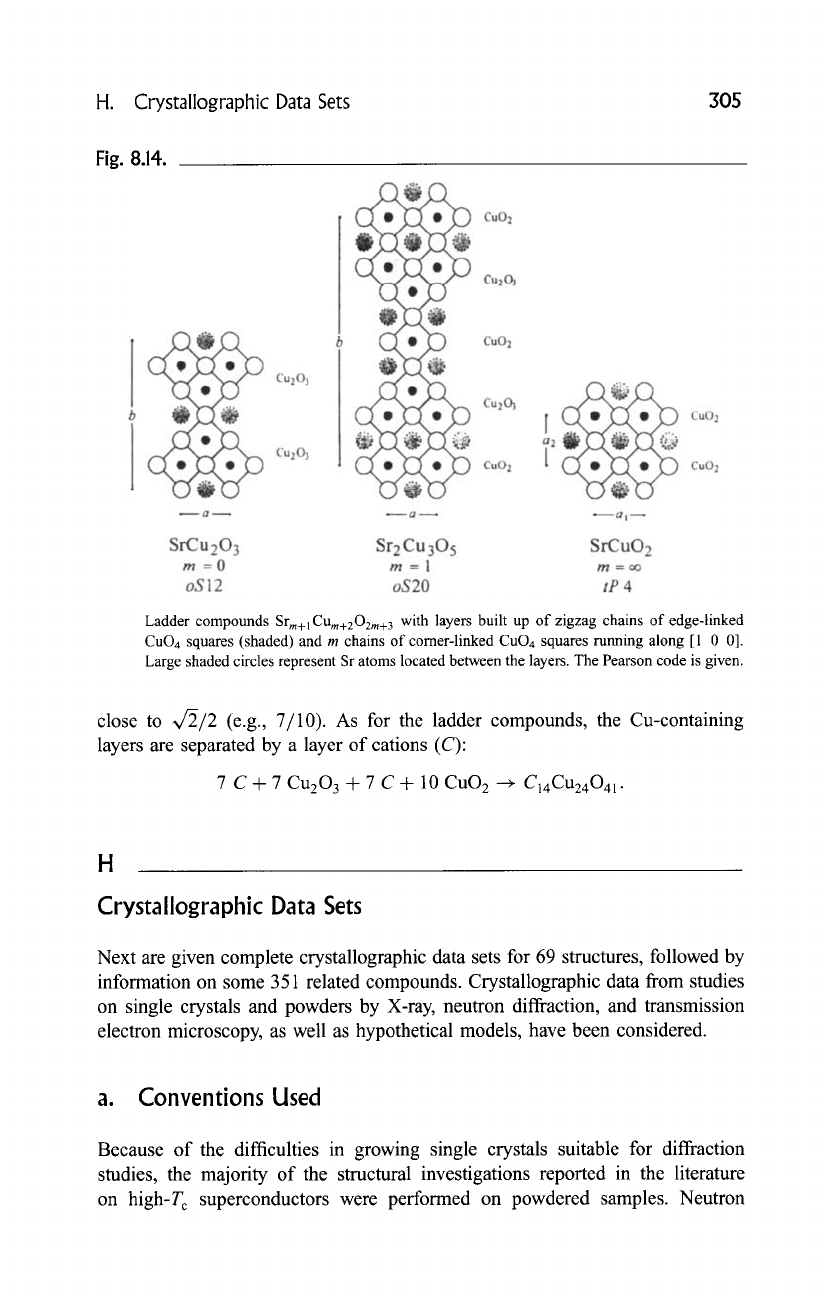
Compounds with the general formula
Srn_lCUn+lO2n
(n = 3, 5, 7 ..... oo) were
first reported by Hiroi et al. (1991). They are generally referred to as ladder
compounds and can only be prepared at high pressure. Their structures contain
CUn+lO2n
layers separated by charge-compensating layers of strontium atoms
(Fig. 8.14). In the former, zigzag chains of edge-linked CuO4 squares share
comers with m chains of corner-linked CuO4 squares, leading to an alternative
general formula:
(m + 1)Sr +
Cu203
-~-
m
CuO 2
+
Srm+lCUm+202m+3
(m = 0, 1,2 ..... oo).
In the limiting case where m is infinitely large, square-mesh DO 2 layers are
formed and the infinite-layer compound (0011) is obtained.
Superconductivity at high pressure (T c = 12 K at 3 GPa) has been observed
by Uehara et al. (1996) for
Ca13.6Sro.4Cuz4041.s4,
which crystallizes with an
orthorhombic, (SrgCa6)Cu24041 (McCarron et aL, 1988) or (Casga6)Cu24041
(Siegrist et aL, 1988a) type structure. In this structure Cu203 layers, similar to
those found in the ladder compounds (m = 0), alternate with a new kind of CuO2
layer, consisting of single straight chains of edge-linked CuO4 squares. The
squares of the Cu203 layer are rotated by 45 ~ with respect to those of the CuO2
layer and the translation periods approximately coincide for a ratio of multiples
H. Crystallographic Data Sets 305
Fig. 8.14.
Ladder compounds
Srm+lCUm+202m+3
with layers built up of zigzag chains of edge-linked
CuO4 squares (shaded) and m chains of corner-linked CuO4 squares running along [1 0 0].
Large shaded circles represent Sr atoms located between the layers. The Pearson code is given.
close to ~/2/2 (e.g., 7/10). As for the ladder compounds, the Cu-containing
layers are separated by a layer of cations (C):
7 C + 7
Cu203
-~- 7 C + 10
CuO 2 ~ C14Cu24041.
H
Crystallographic Data Sets
Next are given complete crystallographic data sets for 69 structures, followed by
information on some 351 related compounds. Crystallographic data from studies
on single crystals and powders by X-ray, neutron diffraction, and transmission
electron microscopy, as well as hypothetical models, have been considered.
a. Conventions Used
Because of the difficulties in growing single crystals suitable for diffraction
studies, the majority of the structural investigations reported in the literature
on high-Tr superconductors were performed on powdered samples. Neutron
