Poole Ch.P., Jr. Handbook of Superconductivity
Подождите немного. Документ загружается.

~
o
~ O
L~. L--.. i--.. L--.. L.~. L-~. r-.. c~ oo oo oO ~h ~,~ ~,~
oO L-~ u~ r... L-~.
I
I I I
v
!
r
~o
-S
o~
G
r
~o
76
B. Elements and Alloys 77
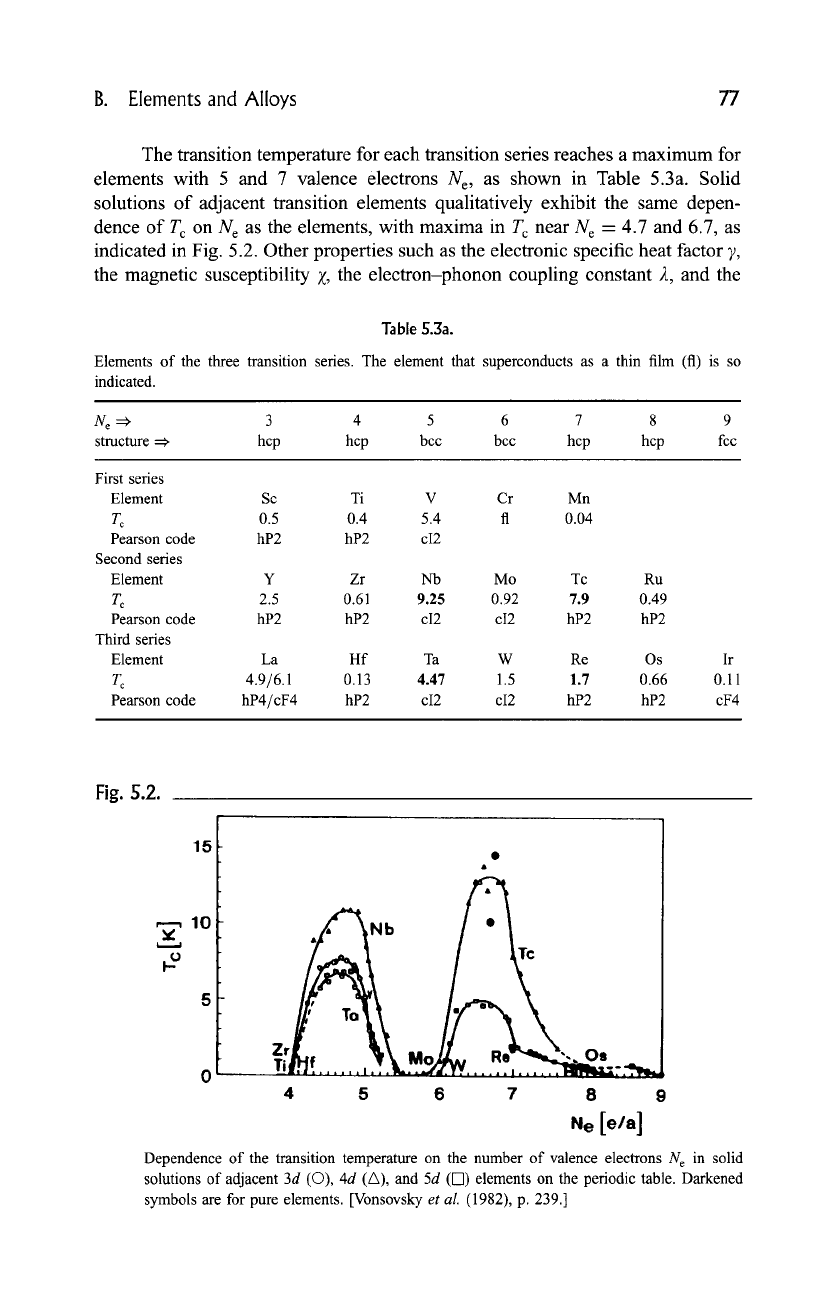
The transition temperature for each transition series reaches a maximum for
elements with 5 and 7 valence electrons Ne, as shown in Table 5.3a. Solid
solutions of adjacent transition elements qualitatively exhibit the same depen-
dence of T c on N e as the elements, with maxima in T c near N e = 4.7 and 6.7, as
indicated in Fig. 5.2. Other properties such as the electronic specific heat factor 7,
the magnetic susceptibility Z, the electron-phonon coupling constant 2, and the
Table 5.3a.
Elements of the three transition series. The element that superconducts as a thin film (fl) is so
indicated.
Ne=* 3 4 5 6 7 8 9
structure =, hcp hcp bcc bcc hcp hcp fcc
First series
Element Sc Ti V Cr Mn
T c 0.5 0.4 5.4 fl 0.04
Pearson code hP2 hP2 ci2
Second series
Element Y Zr Nb Mo Tc
T c 2.5 0.61 9.25 0.92 7.9
Pearson code hP2 hP2 ci2 ci2 hP2
Third series
Element La Hf Ta W Re
T c 4.9/6.1 0.13 4.47 1.5 1.7
Pearson code hP4/cF4 hP2 ci2 ci2 hP2
Ru
0.49
hP2
Os
0.66
hP2
Ir
0.11
cF4
Fig. 5.2.
15
10
8 ~
9 Nb 9 Tc
Zr q
Ti "'" ;--~,~_
4 5 6 7 8 9
Ne [e/a I
Dependence of the transition temperature on the number of valence electrons N e in solid
solutions of adjacent 3d (O), 4d (A), and 5d (71) elements on the periodic table. Darkened
symbols are for pure elements. [Vonsovsky
et al.
(1982), p. 239.]
78 Chapter 5: Superconductor Types
inverse Debye temperature exhibit similar correlations with Ne. Table 5.3b shows
T c of nontransition elements in the format of Table 5.3a. We use the same values
of Ne for the elements that were employed by Phillips (1989).
Miedema (1973, 1974) noted that some physical properties of AB alloys
such as the density of
states D(EF)AB = DAB
at the Fermi level sometimes depend
linearly on the mole fractions f of the constituents,
DAB --fADA(N A)
+fBDB(NB),
(2)
where the individual densities of states
D A
and D B depend on the number of
valence electrons N A and N~ on atoms A and B, respectively. The properties T c,
7, 2, and 0~ 1 mentioned earlier often approximate expressions similar to Eq. (2).
Table 5.3b.
Some non transition series elements. Elements that superconduct only at high pressure (pr) or as thin
films (fl) are so indicated, a
Ne=* 2 3 4 5 6
Second row
Element A1 Si P
Tc 1.2 fl/pr pr
Pearson code cF4 tI4 oS8
Third row
Element Zn Ga Ge As
T c 0.9 1.1/6.2 fl/pr pr
Pearson code hP2 oS8/mS4 tI4
Fourth row
Element Cd In Sn Sb
T c 0.5 3.4 3.7 pr
Pearson code hP2 tI2 tI4
Fifth row
Element Hg T1 Pb Bi
T c 4.15/3.9 2.4/2.4 7.2 fl/pr
Pearson code hR3/tI2 hP2/cI2 cF4
Se
pr
Te
pr
a
Several other superconducting elements are: (symbol, Ne, T c, Pearson code)
Am 3 1.0 cF4 Lu 3 1.0 hP2
r-Ca 2 0.5 hP2 Pa 3 1.4 tI2
),-Ga 3 7.0 oS40 Th 3 1.4 cF4
6-Ga 3 7.9 hR66 7-U 3 0.2 ci2
Transition temperatures at high pressure are:
Ge 4 5.4 tI4 11.5 GPa Si 4 7.1
tI4 13 GPa

C. Description of the Data Tables 79
C
Description of the Data Tables
The transition temperature T c is, perhaps, the most important characteristic of a
superconductor. This is because it is an index of the goodness of a material and of
its suitability for practical applications. For example, we know from simple
theory, confirmed by BCS, that the upper critical field Bc2 and the critical current
density Jc are both proportional to T c. We can also conjecture from the data on
elements mentioned in the previous section that T c might be expected to depend
on the number N e of valence electrons. We have, accordingly, composed a
number of tables that present the transition temperatures of compounds of various
types by arranging their constituent elements in rows and columns according to
the number of their valence electrons. The N e value is given for each element that
is listed. Separate tables are provided for the structures that include many
examples of superconductors, such as the A15 compounds and the Chevrel
phases. Some classes of materials have more than one structure, such as the Laves
phases, and when this occurs an asterisk (*) is used to differentiate them. Some of
the tables display data for several structures of the same chemical formula, such
as AB2, and if this is the case the rows and columns are labeled with the structure
type when it is the same for every element in a particular row or column.
Occasionally, an individual compound has two structural modifications, both of
which superconduct, and sometimes this will be indicated.
The tables are arranged in the order of increasing complexity of their
chemical formulas, with elements A first, binary compounds AmB n second,
ternary compounds AmBnC p third, etc. The tables for binary compounds present
data in the order AB, AB2, AB 3 ..... AraB ~, and analogously for the ternary
compounds. The T c values for additional compounds that remain after presenting
the main structure types, such as for miscellaneous AB 2 compounds, are listed in
separate tables arranged alphabetically by element A and then by element B. Each
table caption provides the compound type and structure(s), and the compounds in
the miscellaneous listings are identified by their structure type. The structures are
designated by abbreviated Pearson codes, such as cF, where the initial lowercase
letter indicates the crystal system and the final capital letter denotes the type of
lattice, in accordance with the following notation:
Crystal system: c = cubic, h = hexagonal, t = tetragonal, o = orthorhombic,
m = monoclinic; Lattice type: P = primitive, S -- side-centered, I = body-
centered, F = face-centered, R = rhombohedral
The Pearson code ends with a number, such as cF8, where 8 denotes the number
of atoms in the unit cell. The abbreviations bcc, fcc, and hcp are occasionally
used for body-centered cubic, face-centered cubic, and hexagonal close-packed,
respectively. Chapter 6 provides a more detailed explanation of Pearson codes.
Phillips (1989) uses a Pearson code classification for the arrangement of the T c
80 Chapter 5:
Superconductor Types
tables in the appendix of his book. Sometimes the Strukturbericht symbol (e.g.,
A15), compound designation (e.g., NaC1) or common name (e.g., Chevrel) are
given in table captions.
D
Classical Compounds
Many types of compounds have been found to superconduct, and the main ones
were summarized in Table 3.2 of our earlier work (Poole
et al.,
1995). In this
section we will make some comments on the principal classes that were widely
studied before the advent of high T c. Motivated by the results mentioned earlier
for the elements, we will examine how the transition temperature T c correlates
with the number N e of valence electrons in the compounds within each structure
type.
Tables 5.4 to 5.7 show that the highest T c values for four types of
AB
compounds come at the following electron counts:
fcc
NaC1 type N e = 4.5 and 5
bcc
CsC1 type N e = 6.5
Hexagonal
NiAs type N e = 7.5 and 8
Orthorhombic
FeAs type N e -- 6.5
These were obtained from the expression
N e --lANe A -+-fBNBe,
(3)
Table 5.4.
AB compound, NaC1 type
(rock salt),
cF8.
B =~ B Y C Ge N O
A 3 3 4 4 5 6
Se
6
La 3
Th
3
Ti4
Zr 4
Hf 4
V5
Nb5
Ta5
Te6
Mo6
W6
Re 7
3.4
3.1
1.5
1.4
3.4 5.6 0.14
0.25 10.7
0.25 8.8
8.5
11.1 16.6 1.4
10.2 14.0
0.4
14.3
10.0
3.4
LaTe
1.5, PdD 10.7, PdH 9.6, NbW 13.6.
14.8
0.9
0.5
1.0
1.7
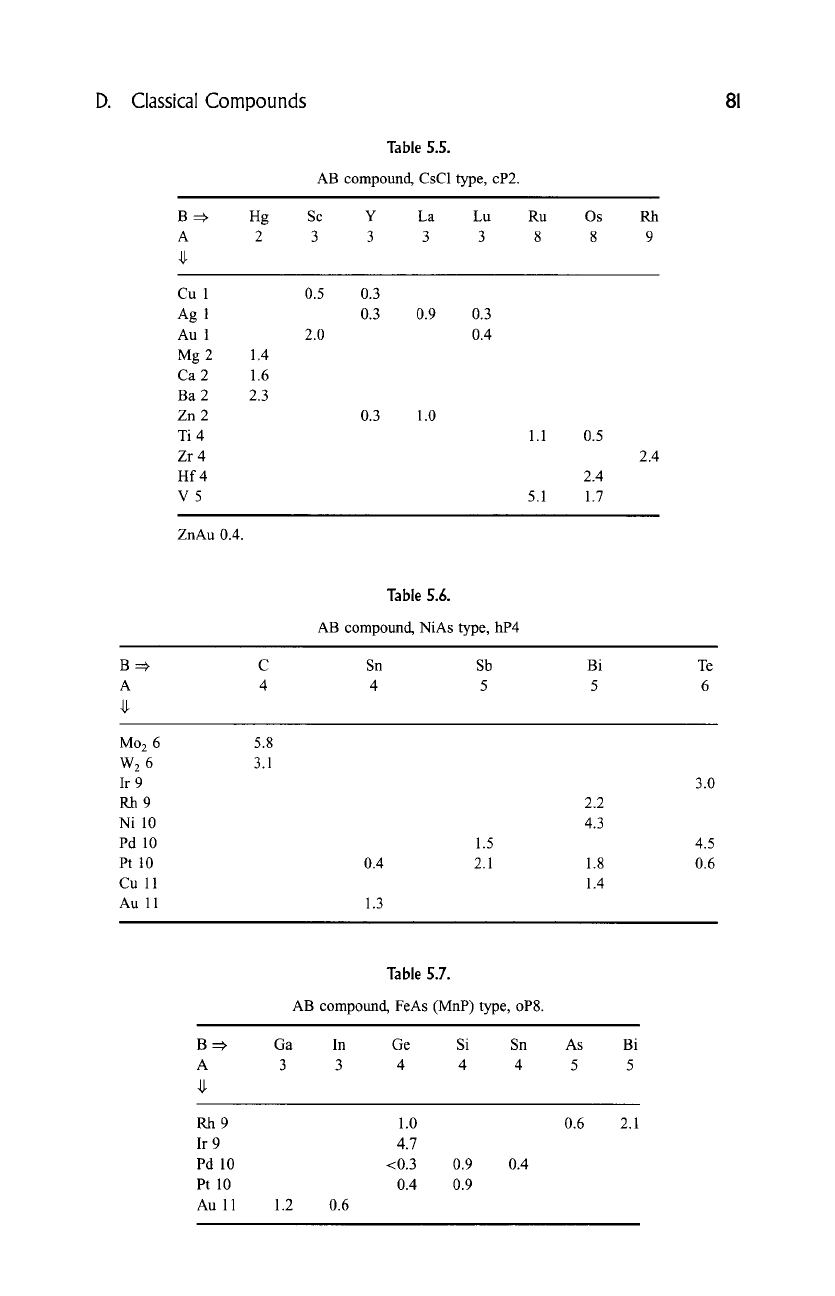
D. Classical Compounds 81
Table 5.5.
AB compound, CsC1 type, cP2.
B =, Hg
A 2
Sc Y La Lu Ru Os Rh
3 3 3 3 8 8 9
Cu 1
Ag 1
Au 1
Mg 2
Ca 2
Ba 2
Zn 2
Ti 4
Zr 4
Hf 4
V5
1.4
1.6
2.3
0.5 0.3
0.3 0.9 0.3
2.0 0.4
0.3 1.0
1.1
5.1
0.5
2.4
1.7
2.4
ZnAu 0.4.
Table
S.6.
AB compound, NiAs type, hP4
B=:~
A
C Sn Sb Bi Te
4 4 5 5 6
Mo 2 6
W 2 6
Ir 9
Rh9
Ni 10
Pd 10
Pt 10
Cu 11
Au 11
5.8
3.1
1.5
0.4 2.1
1.3
2.2
4.3
1.8
1.4
3.0
4.5
0.6
B=:~
A
Rh9
Ir 9
Pd 10
Pt 10
Au 11
Table 5.7.
AB compound, FeAs (MnP) type, oP8.
Ga In Ge
Si Sn As Bi
3 3 4 4 4 5 5
1.2
0.6
1.0
4.7
<0.3
0.4
0.9 0.4
0.9
0.6
2.1
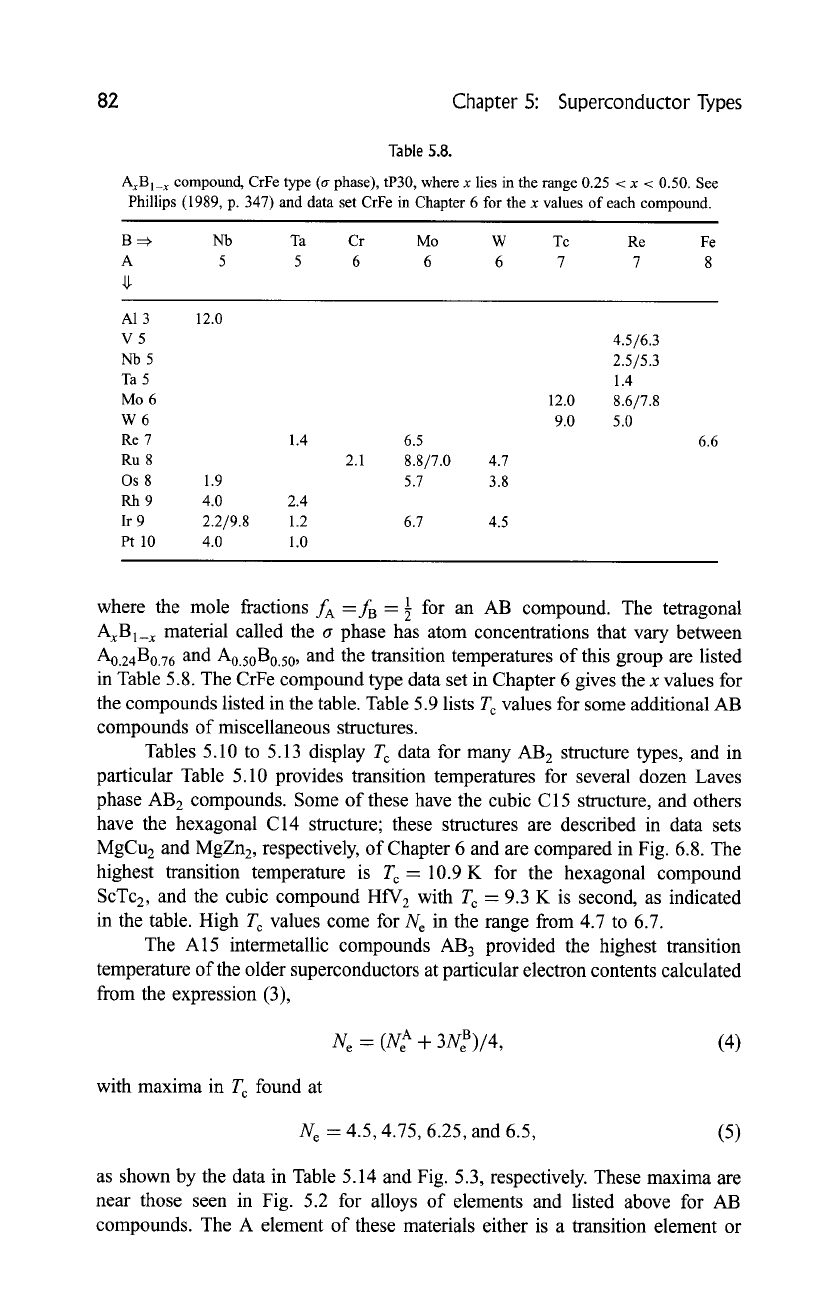
82 Chapter 5: Superconductor Types
Table 5.8.
AxBI_ x compotmd, CrFe type (a phase), tP30, where x lies in the range 0.25 < x < 0.50. See
Phillips (1989, p. 347) and data set CrFe in Chapter 6 for the x values of each compound.
B =~ Nb
Ta Cr Mo W Tc Re Fe
A 5 5 6 6 6 7 7 8
A1 3 12.0
V5
Nb5
Ta5
Mo6
W6
Re 7
Ru 8
Os 8 1.9
Rh9 4.0
Ir 9 2.2/9.8
Pt 10 4.O
1.4
2.4
1.2
1.0
6.5
2.1 8.8/7.0 4.7
5.7 3.8
6.7 4.5
12.0
9.0
4.5/6.3
2.5/5.3
1.4
8.6/7.8
5.0
6.6
where the mole fractions fA--fB--89 for an AB compound. The tetragonal
AxBI_ x material called the a phase has atom concentrations that vary between
A0.24B0.76
and A0.50B0.50, and the transition temperatures of this group are listed
in Table 5.8. The CrFe compound type data set in Chapter 6 gives the x values for
the compounds listed in the table. Table 5.9 lists T c values for some additional AB
compounds of miscellaneous structures.
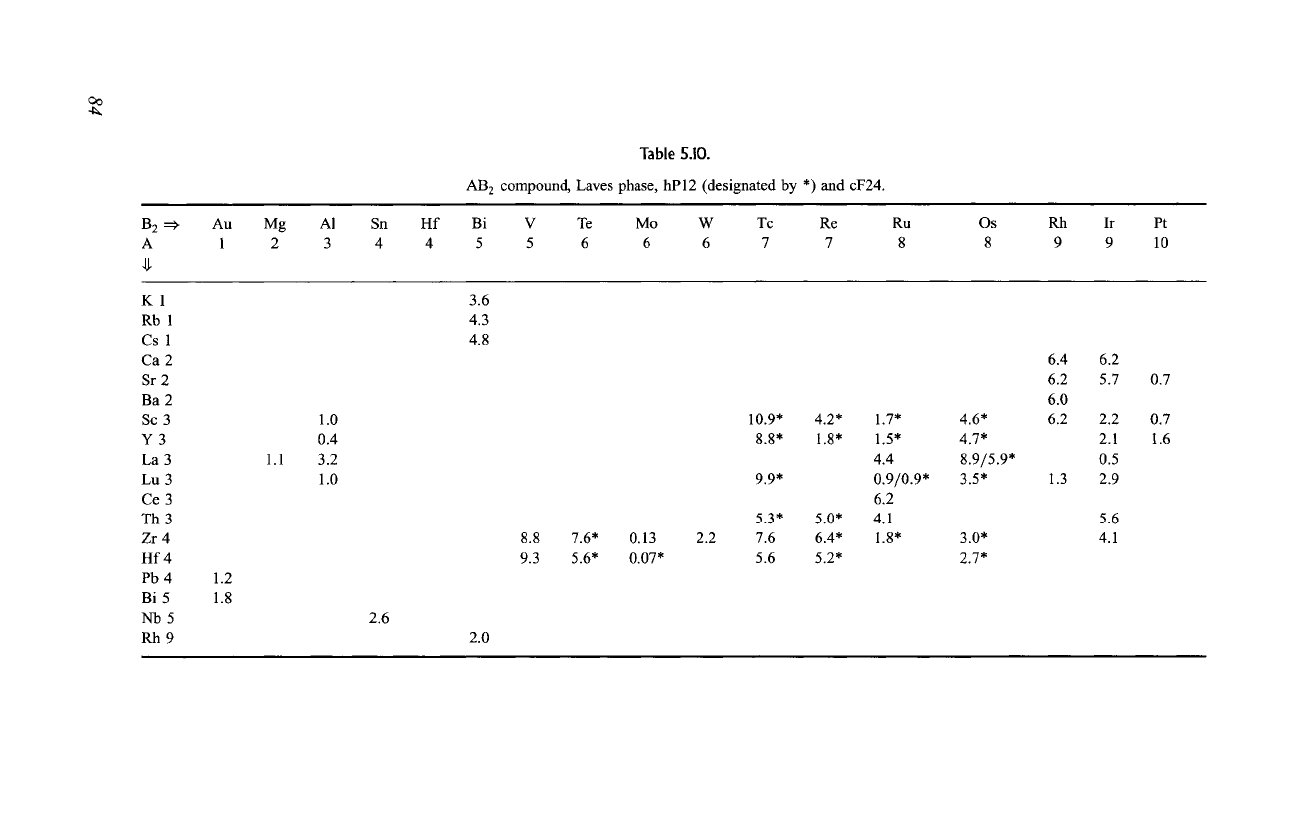
Tables 5.10 to 5.13 display T c data for many AB 2 structure types, and in
particular Table 5.10 provides transition temperatures for several dozen Laves
phase AB 2 compounds. Some of these have the cubic C15 structure, and others
have the hexagonal C14 structure; these structures are described in data sets
MgCu 2 and MgZn2, respectively, of Chapter 6 and are compared in Fig. 6.8. The
highest transition temperature is T c -10.9 K for the hexagonal compound
ScTc2, and the cubic compound HfV 2 with T c = 9.3 K is second, as indicated
in the table. High T c values come for N e in the range from 4.7 to 6.7.
The A15 intermetallic compounds AB 3 provided the highest transition
temperature of the older superconductors at particular electron contents calculated
from the expression (3),
N e - (N A + 3NeB)/4, (4)
with maxima in Tr found at
N e - 4.5, 4.75, 6.25, and 6.5,
(5)
as shown by the data in Table 5.14 and Fig. 5.3, respectively. These maxima are
near those seen in Fig. 5.2 for alloys of elements and listed above for AB
compounds. The A element of these materials either is a transition element or
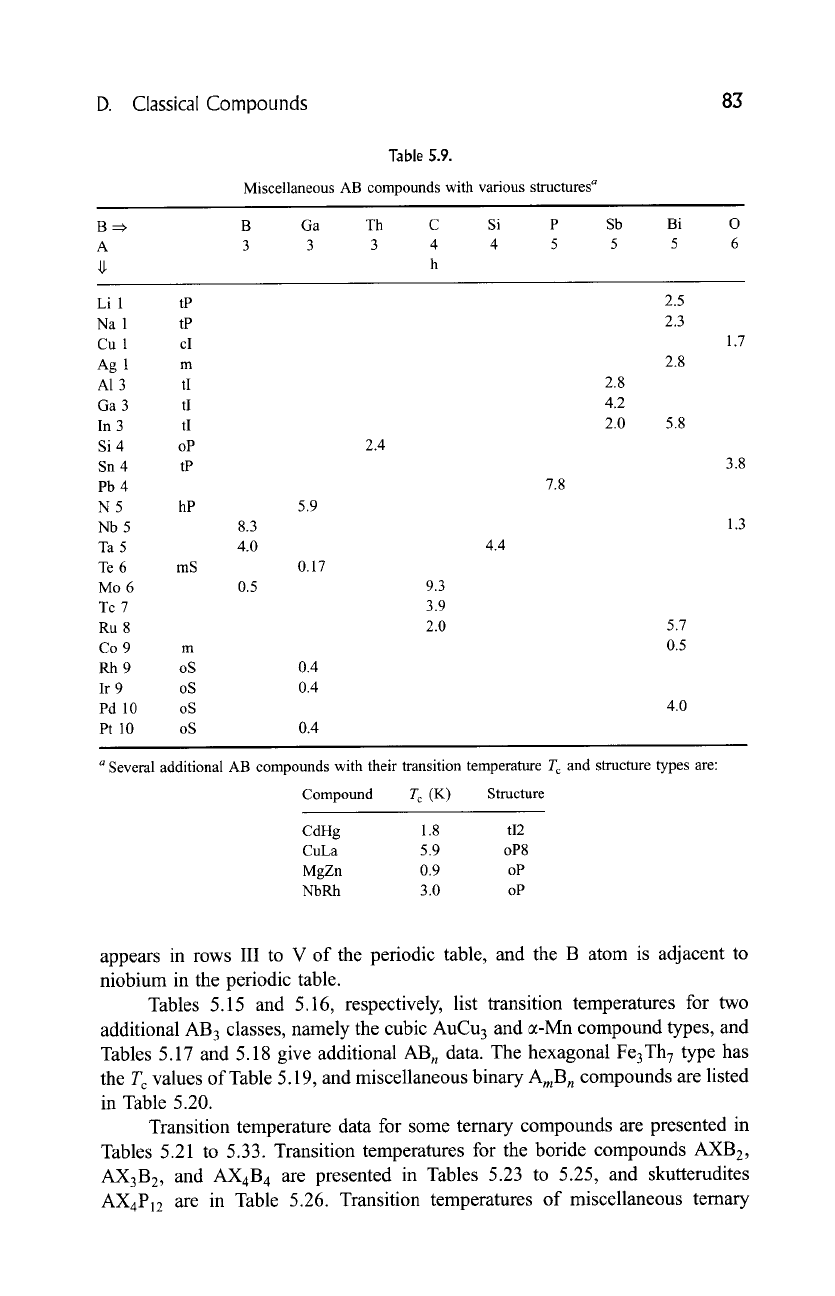
D. Classical Compounds 83
Table
5.9.
Miscellaneous AB compounds with various structures a
B =~ B Ga Th C Si P
A 3 3 3 4 4 5
h
Sb
5
Bi
5
Li 1 tP
Na 1 tP
Cu 1 cI
Ag 1 m
A1
3 tI
Ga 3 tI
In 3 tI
Si 4 oP
Sn 4 tP
Pb 4
N 5 hP
Nb5
Ta5
Te
6 mS
Mo6
Tc
7
Ru 8
Co 9 m
Rh 9 oS
Ir 9 oS
Pd 10 oS
Pt 10 oS
5.9
2.4
8.3
4.0 4.4
0.17
0.4
0.4
0.4
0.5
9.3
3.9
2.0
7.8
2.8
4.2
2.0
2.5
2.3
2.8
5.8
5.7
0.5
4.0
1.7
3.8
1.3
a Several additional AB compounds with their transition temperature T c and structure types are:
Compound T c (K) Structure
CdHg 1.8 tI2
CuLa 5.9 oP8
MgZn 0.9 oP
NbRh 3.0 oP
appears in rows III to V of the periodic table, and the B atom is adjacent to
niobium in the periodic table.
Tables 5.15 and 5.16, respectively, list transition temperatures for two
additional AB 3 classes, namely the cubic AuCu 3 and a-Mn compound types, and
Tables 5.17 and 5.18 give additional AB n data. The hexagonal Fe3Th 7 type has
the T c values of Table 5.19, and miscellaneous binary
AraB n
compounds are listed
in Table 5.20.
Transition temperature data for some ternary compounds are presented in
Tables 5.21 to 5.33. Transition temperatures for the boride compounds AXB2,
AX3B2, and AX4B 4 are presented in Tables 5.23 to 5.25, and skutterudites
AX4P12 are
in Table 5.26. Transition temperatures of miscellaneous ternary
~k
S~
0
0
0
L~ lt~
84
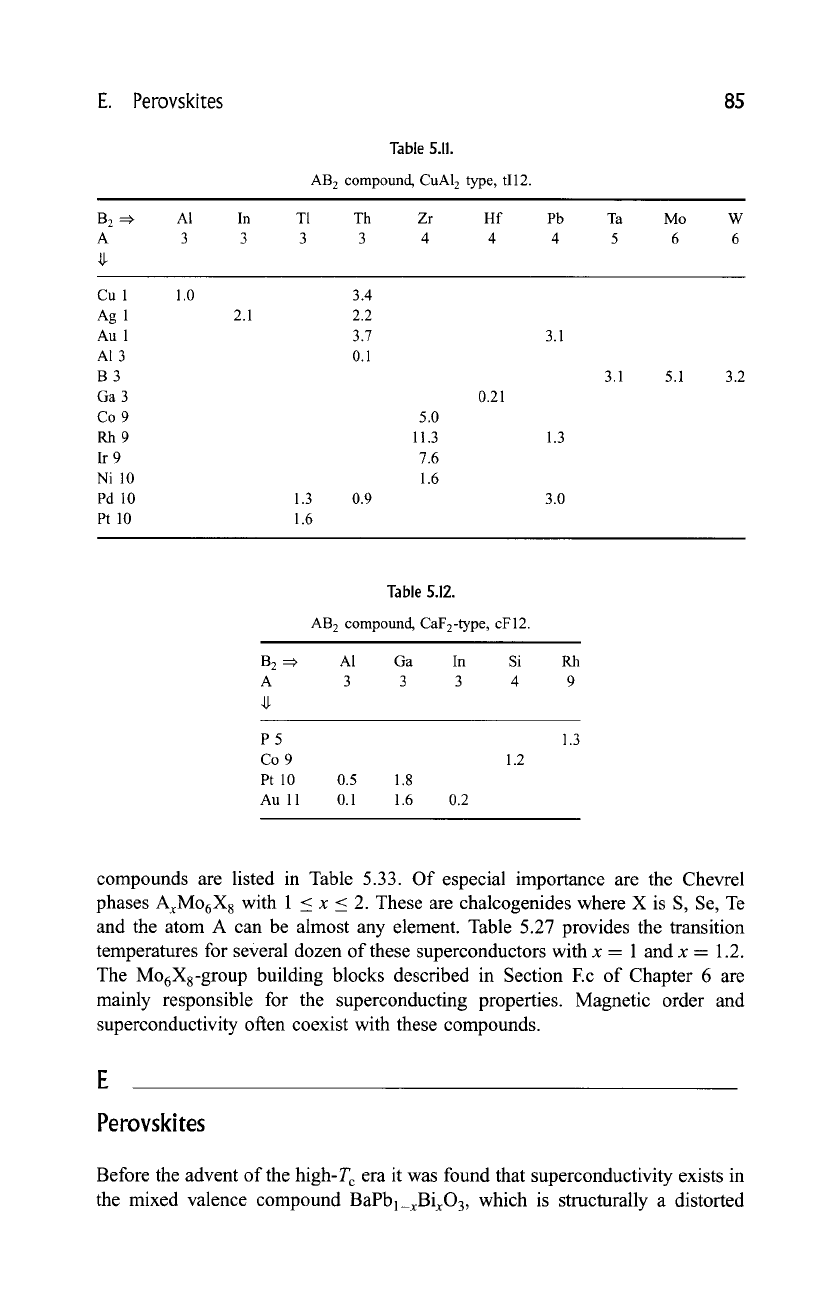
E. Perovskites 85
Table 5.11.
AB 2 compound, CuA12 type, tI12.
B 2 =:~
A
A1 In T1 Th Zr Hf Pb Ta Mo W
3 3 3 3 4 4 4 5 6 6
Cu 1
Ag 1
Au 1
A1 3
B3
Ga 3
Co 9
Rh9
Ir 9
Ni 10
Pd 10
Pt 10
1.0
2.1
1.3
1.6
3.4
2.2
3.7
0.1
5.0
11.3
7.6
1.6
0.21
3.1
1.3
0.9 3.0
3.1
5.1 3.2
B 2 ==~
A
P5
Co 9
Pt 10
Au 11
Table 5.12.
AB 2 compound, CaF2-type , cF 12.
A1 Ga In Si Rh
3 3 3 4 9
0.5 1.8
0.1 1.6 0.2
1.2
1.3
compounds are listed in Table 5.33. Of especial importance are the Chevrel
phases AxMo6X 8 with 1 < x _< 2. These are chalcogenides where X is S, Se, Te
and the atom A can be almost any element. Table 5.27 provides the transition
temperatures for several dozen of these superconductors with x - 1 and x - 1.2.
The Mo6X8-grou p building blocks described in Section Ec of Chapter 6 are
mainly responsible for the superconducting properties. Magnetic order and
superconductivity often coexist with these compounds.
Perovskites
Before the advent of the high-T c era it was found that superconductivity exists in
the mixed valence compound BaPbl_xBixO3, which is structurally a distorted
