Poole Ch.P., Jr. Handbook of Superconductivity
Подождите немного. Документ загружается.

96 Chapter 5: Superconductor Types
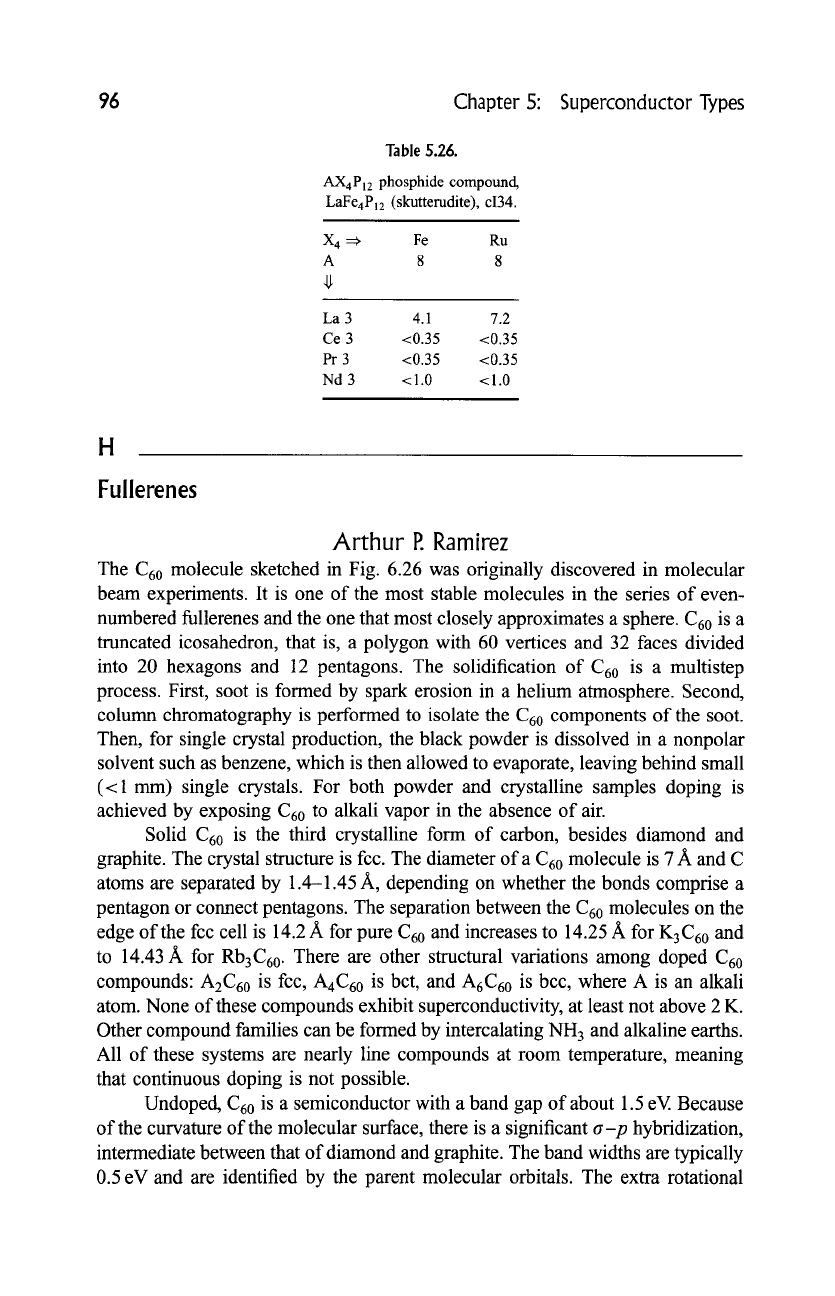
Table 5.26.
AX4P12 phosphide compound,
LaFe4P12 (skutterudite), ci34.
X 4 =~ Fe Ru
A 8 8
La 3 4.1 7.2
Ce 3 <0.35 <0.35
Pr 3 <0.35 <0.35
Nd 3 < 1.0 < 1.0
H
Fullerenes
Arthur
P. Ramirez
The C60 molecule sketched in Fig. 6.26 was originally discovered in molecular
beam experiments. It is one of the most stable molecules in the series of even-
numbered fullerenes and the one that most closely approximates a sphere. C60 is a
truncated icosahedron, that is, a polygon with 60 vertices and 32 faces divided
into 20 hexagons and 12 pentagons. The solidification of C60 is a multistep
process. First, soot is formed by spark erosion in a helium atmosphere. Second,
column chromatography is performed to isolate the C60 components of the soot.
Then, for single crystal production, the black powder is dissolved in a nonpolar
solvent such as benzene, which is then allowed to evaporate, leaving behind small
(<1 mm) single crystals. For both powder and crystalline samples doping is
achieved by exposing C60 to alkali vapor in the absence of air.
Solid C60 is the third crystalline form of carbon, besides diamond and
graphite. The crystal structure is fcc. The diameter of a C60 molecule is 7 A and C
atoms are separated by 1.4-1.45 A, depending on whether the bonds comprise a
pentagon or connect pentagons. The separation between the C60 molecules on the
edge of the fcc cell is 14.2 A for pure C60 and increases to 14.25 A for K 3 C60 and
to 14.43 A for Rb3C60. There are other structural variations among doped C60
compounds: A2C60 is fcc, A4C60
is bct, and
A6C60 is bcc,
where A is an alkali
atom. None of these compounds exhibit superconductivity, at least not above 2 K.
Other compound families can be formed by intercalating NH 3 and alkaline earths.
All of these systems are nearly line compounds at room temperature, meaning
that continuous doping is not possible.
Undoped, C60 is a semiconductor with a band gap of about 1.5 eV. Because
of the curvature of the molecular surface, there is a significant ~-p hybridization,
intermediate between that of diamond and graphite. The band widths are typically
0.5 eV and are identified by the parent molecular orbitals. The extra rotational
H. Fullerenes 97
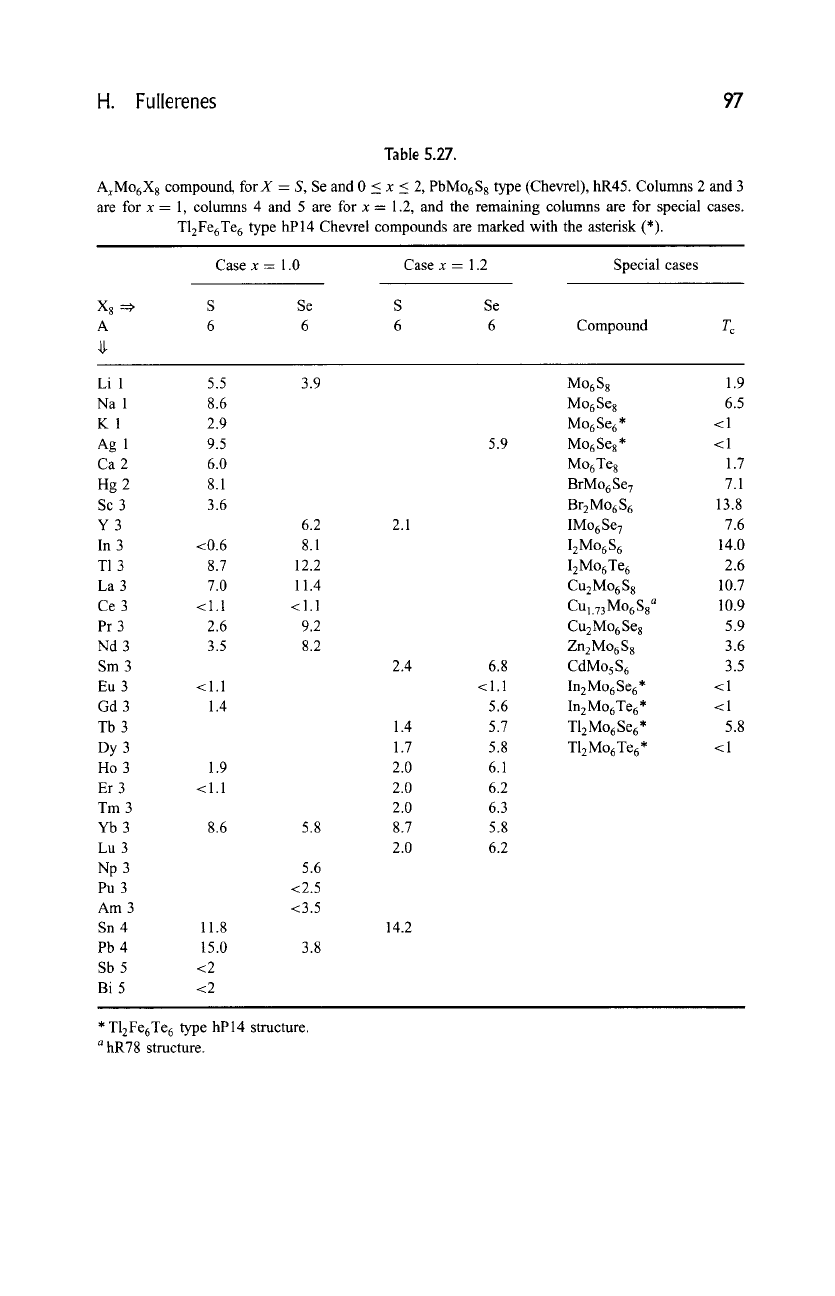
Table 5.27.
AxMo6X8 compound, for X = S, Se and 0 < x < 2, PbMo6S 8 type (Chevrel), hR45. Columns 2 and 3
are for x- 1, columns 4 and 5 are for x = 1.2, and the remaining columns are for special cases.
T12Fe6Te 6 type hP14 Chevrel compounds are marked with the asterisk (*).
Case x = 1.0 Case x = 1.2 Special cases
X 8 =~ S Se S Se
A 6 6 6 6 Compound T c
Li 1 5.5 3.9
Na 1 8.6
K 1 2.9
Ag 1 9.5
Ca 2 6.0
Hg 2 8.1
Sc 3 3.6
Y 3 6.2 2.1
In 3 <0.6 8.1
T1 3 8.7 12.2
La 3 7.0 11.4
Ce 3 <1.1 <1.1
Pr 3 2.6 9.2
Nd 3 3.5 8.2
Sm 3 2.4
Eu 3 <1.1
Gd 3 1.4
Tb 3 1.4
Dy 3 1.7
Ho 3 1.9 2.0
Er 3 <1.1 2.0
Tm 3 2.0
Yb 3 8.6 5.8 8.7
Lu 3 2.0
Np 3 5.6
Pu 3 <2.5
Am 3 <3.5
Sn 4 11.8 14.2
Pb 4 15.0 3.8
Sb 5 <2
Bi 5 <2
5.9
6.8
<1.1
5.6
5.7
5.8
6.1
6.2
6.3
5.8
6.2
Mo6S 8 1.9
Mo6Se 8 6.5
Mo6Se6* <1
Mo6Se8* <1
Mo6Te 8 1.7
BrMo 6 Se 7 7.1
Br2Mo6S 6 13.8
IMo6Se 7 7.6
IzMo6S 6 14.0
IzMo6Te 6 2.6
CuzMo6S 8 10.7
CUl.73Mo688 a 10.9
Cu2Mo6Se 8 5.9
ZnzMo6S 8 3.6
CdMo5S 6 3.5
InzMo 6 Se 6. < 1
In 2 Mo 6 Te 6 * < 1
TlzMo6Se6* 5.8
TlzMo6Te6 * <1
* T12Fe6Te 6 type hP14 structure.
a
hR78 structure.
98
Chapter S: Superconductor Types
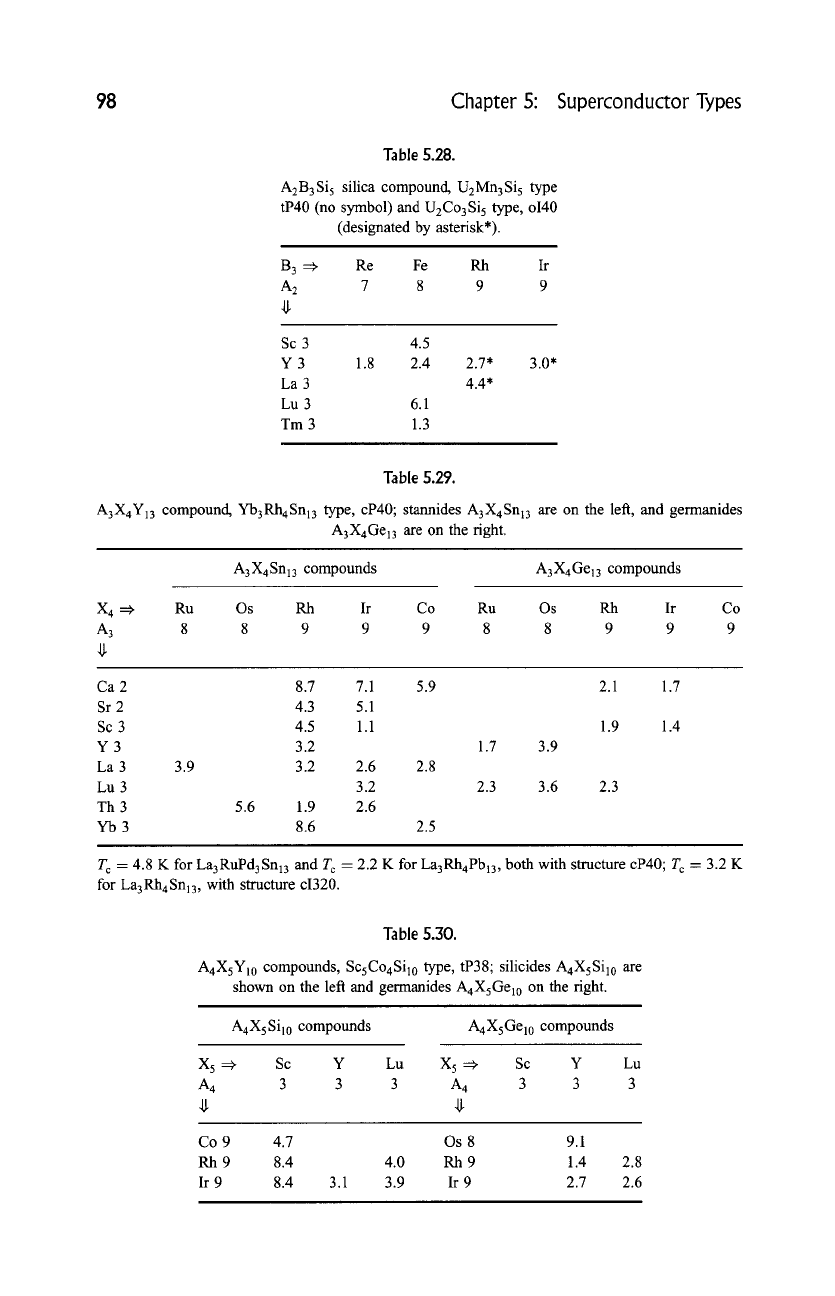
Table 5.28.
A2B3Si 5 silica compound, U2Mn3Si 5 type
tP40 (no symbol) and U2Co3Si 5 type, o140
(designated by asterisk*).
B 3 =:~ Re Fe Rh Ir
A 2 7 8 9 9
Sc 3
Y3
La 3
Lu 3
Tm 3
4.5
1.8 2.4 2.7* 3.0*
4.4*
6.1
1.3
Table 5.29.
A3X4Y13 compound, Yb3Rh4Snl3 type, cP40; stannides A3X4Snl3 are on the left, and germanides
A 3 X 4 Gel3 are on the right.
A3X4Snl3 compounds A3X4Gel3 compounds
X 4 :=~
Ru Os Rh Ir Co Ru Os Rh Ir Co
A 3 8 8 9 9 9 8 8 9 9 9
Ca 2 8.7 7.1
Sr 2 4.3 5.1
Sc 3 4.5 1.1
Y 3 3.2
La 3 3.9 3.2 2.6 2.8
Lu 3 3.2
Th 3 5.6 1.9 2.6
Yb 3 8.6 2.5
5.9 2.1 1.7
1.7 3.9
2.3 3.6 2.3
1.9 1.4
T c = 4.8 K for La3RuPd3Sn13 and Tc = 2.2 K for La3Rh4Pbl3 , both with structure cP40; T~ = 3.2 K
for La3Rh4Snl3, with structure ci320.
Table
5.30.
A4XsY10 compounds, Sc5Co4Si10 type, tP38; silicides A4XsSil0 are
shown on the left and germanides AaX5Gel0 on the right.
A4XsSil0 compounds
A4XsGel0 compounds
X 5 =, Sc Y Lu X 5 =, Sc Y Lu
A 4 3 3 3 A 4 3 3 3
Co 9
Rh9
Ir 9
4.7 Os 8 9.1
8.4 4.0 Rh 9 1.4 2.8
8.4 3.1 3.9 Ir 9 2.7 2.6
H. Fullerenes 99
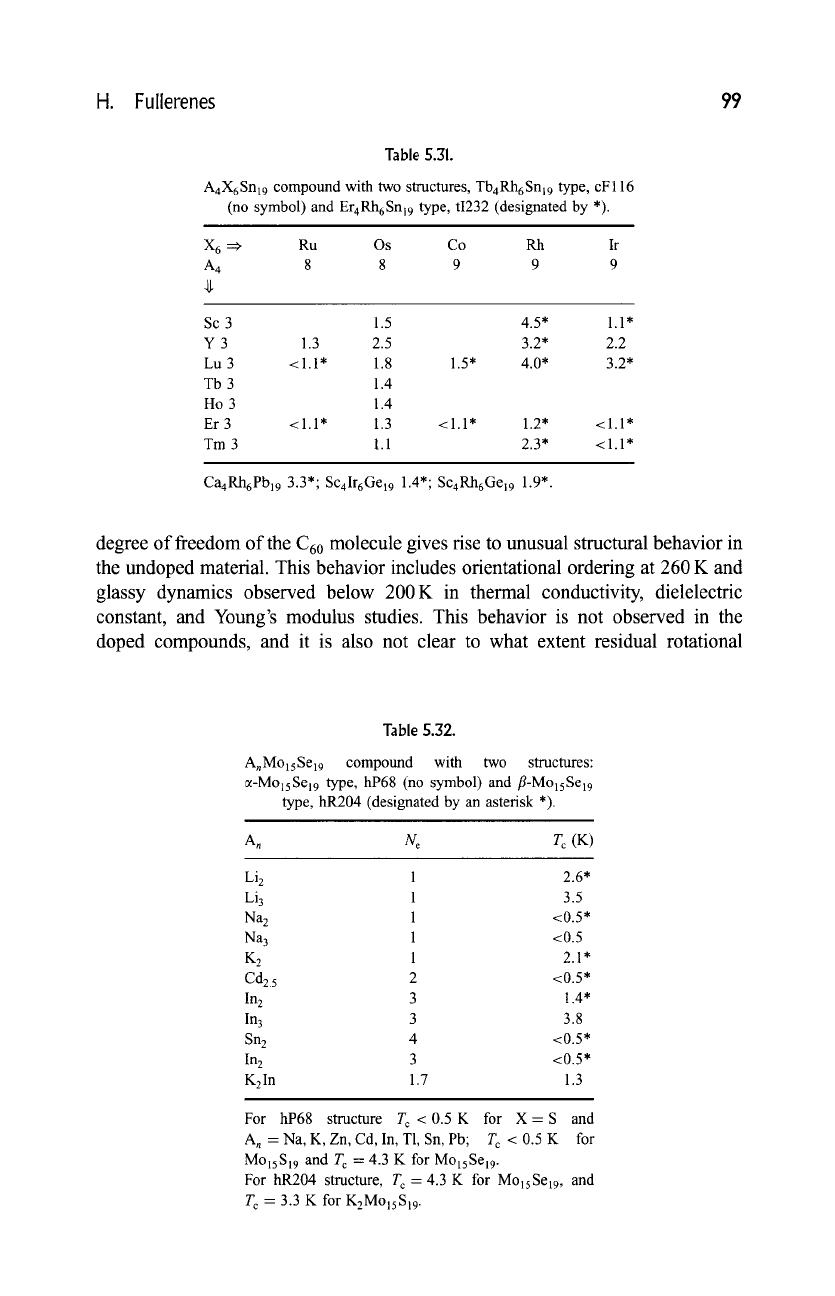
Table 5.31.
A4X6Snl9 compound with
two structures,
Tb4Rh6Snl9 type, cF116
(no
symbol) and
Er4Rh6Snl9 type, ti232 (designated by *).
X 6 ==} Ru Os Co Rh Ir
A 4
8 8 9 9 9
Sc 3 1.5
Y 3 1.3 2.5
Lu 3 <1.1" 1.8
Tb 3 1.4
Ho 3 1.4
Er 3 <1.1" 1.3
Tm 3
1.1
1.5*
4.5* 1.1"
3.2* 2.2
4.0* 3.2*
<1.1" 1.2" <1.1"
2.3* <1.1"
Ca4Rh6Pbl9 3.3*; Sc4Ir6Gel9 1.4"; Sc4Rh6Gel9 1.9".
degree of freedom of the
C60
molecule gives rise to unusual structural behavior in
the undoped material. This behavior includes orientational ordering at 260 K and
glassy dynamics observed below 200 K in thermal conductivity, dielelectric
constant, and Young's modulus studies. This behavior is not observed in the
doped compounds, and it is also not clear to what extent residual rotational
Table
5.32.
A nMO15 Se19 compound with
two structures:
~-MolsSel9 type, hP68 (no
symbol) and
fl-Mo15Se19
type, hR204 (designated by an asterisk *).
A~ N e Tr (K)
Li 2 1 2.6*
Li 3 1 3.5
Na 2 1 <0.5*
Na 3 1 <0.5
K2 1 2.1"
Cd2. 5 2 <0.5*
In 2 3 1.4"
In 3 3 3.8
Sn 2 4 <0.5*
In 2 3 <0.5*
K2In 1.7 1.3
For hP68 structure
T c <0.5K for X=S
and
A~ = Na, K, Zn, Cd, In, T1, Sn, Pb; T c < 0.5 K
for
MolsS19 and T c = 4.3 K for MOlsSel9.
For hR204 structure,
T c = 4.3 K for MOlsSel9 ,
and
T c = 3.3 K for K2Mo15S19.
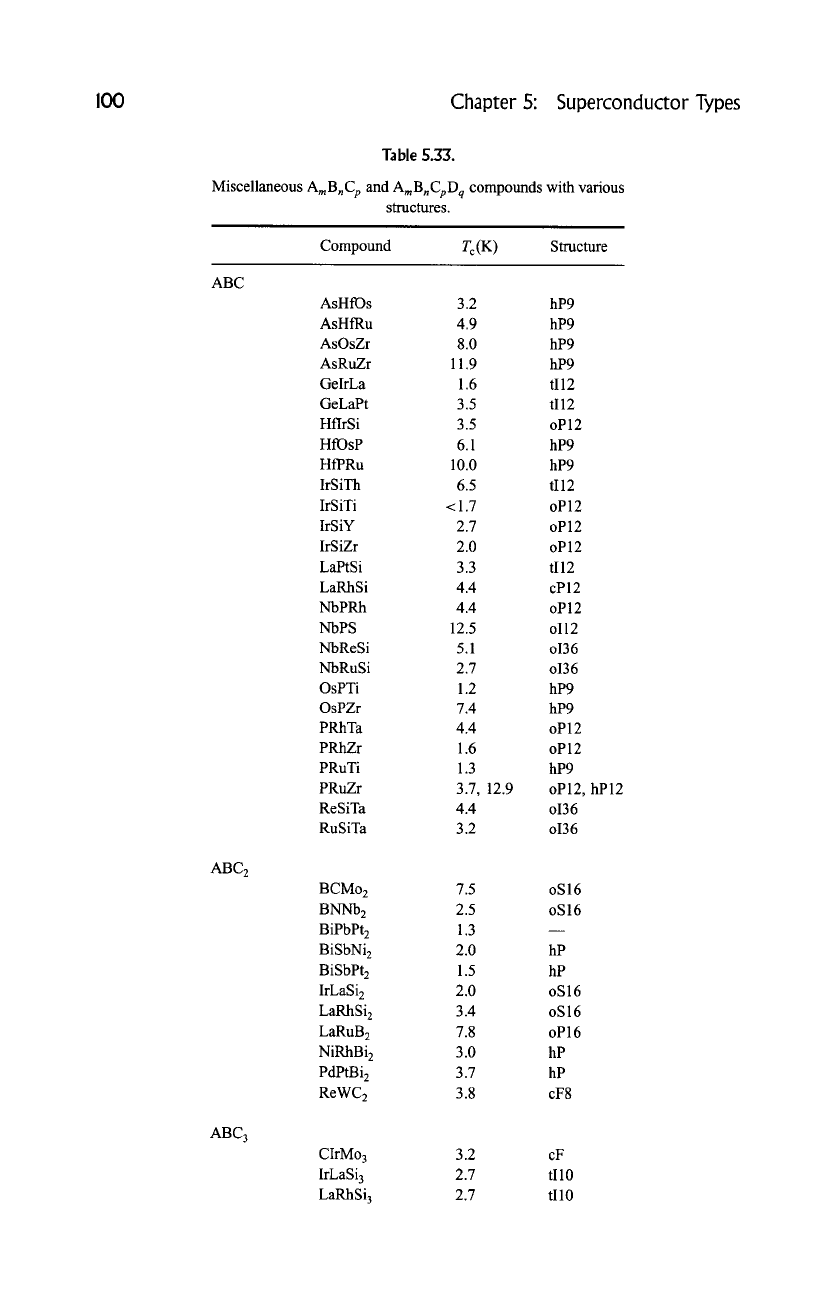
I00
Chapter 5: Superconductor Types
Table 5.33.
Miscellaneous AmBnC p and AmBnCpD q compounds with various
structures.
Compound Tc(K ) Structure
ABC
ABC2
ABC 3
AsHfOs 3.2 hP9
AsHfRu 4.9 hP9
AsOsZr 8.0 hP9
AsRuZr 11.9 hP9
GelrLa 1.6 tI12
GeLaPt 3.5 tI12
HflrSi 3.5 oP 12
HfOsP 6.1 hP9
HfPRu 10.0 hP9
IrSiTh 6.5 tI12
IrSiTi <1.7 oP12
IrS iY 2.7 o P 12
IrSiZr 2.0 oP12
LaPtSi 3.3 tI12
LaRhSi 4.4 cP 12
NbPRh 4.4 oP12
NbPS 12.5 oi12
NbReSi 5.1 oi36
NbRuSi 2.7 o136
OsPTi 1.2 hP9
OsPZr 7.4 hP9
PRhTa 4.4 oP12
PRhZr 1.6 oP12
PRuTi 1.3 hP9
PRuZr 3.7, 12.9 oP12, hP12
ReSiTa 4.4 o136
RuSiTa 3.2 o136
BCMo 2 7.5 oS 16
BNNb 2 2.5 oS 16
BiPbPt2 1.3
BiSbNi 2 2.0 hP
BiSbPt 2 1.5 hP
IrLaSi 2 2.0 oS 16
LaRhSi 2 3.4 oS 16
LaRuB 2 7.8 oP 16
NiRhBi 2 3.0 hP
PdPtBi 2 3.7 hP
ReWC 2 3.8 oF8
CIrMo 3 3.2 cF
IrLaSi 3 2.7 tI10
LaRhSi 3 2.7 tI10
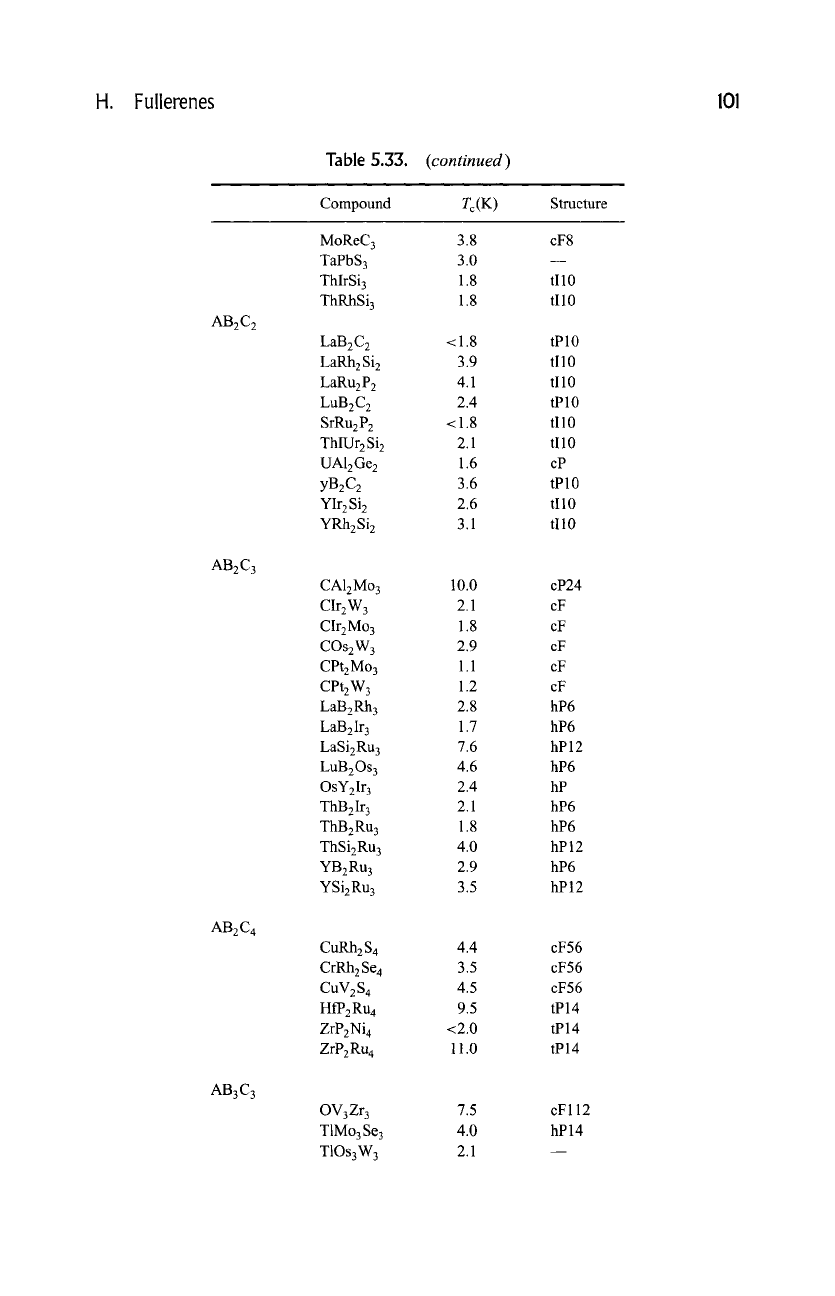
H. Fullerenes 101
AB2C 2
AB2C3
AB2C4
AB3C3
Table 5.33.
Compound
(continued)
Tc(K)
Structure
MoReC 3
TaPbS 3
ThlrSi 3
ThRhSi 3
LaB2C 2
LaRhzSi 2
LaRu2P 2
LuB2C 2
SrRu2P 2
ThlUr2Si 2
UAlzGe z
yBzC2
YIrzSi 2
YRhzSi 2
3.8
3.0
1.8
1.8
<1.8
3.9
4.1
2.4
<1.8
2.1
1.6
3.6
2.6
3.1
cF8
tI10
tI10
tPl0
tI10
tI10
tPl0
tI10
tI10
cP
tP10
tI10
tI10
CA12Mo 3
CIr2W 3
CIr2Mo 3
COs2W 3
CPt2Mo 3
CPt2W 3
LaB2Rh 3
LaB2Ir 3
LaSi2Ru 3
LuB2Os 3
OsY2Ir 3
ThB2Ir 3
ThB2Ru 3
ThSi2Ru 3
YB2Ru 3
YSi2Ru 3
10.0
2.1
1.8
2.9
1.1
1.2
2.8
1.7
7.6
4.6
2.4
2.1
1.8
4.0
2.9
3.5
cP24
cF
cF
cF
cF
cF
hP6
hP6
hP12
hP6
hP
hP6
hP6
hP12
hP6
hP12
CuRh2 S 4
CrRh2 Se 4
CHV2S 4
HfP2Ru 4
ZrP2Ni 4
ZrP2Ru 4
4.4
3.5
4.5
9.5
<2.0
11.0
cF56
cF56
cF56
tP14
tP14
tP14
OV3Zr 3
T1Mo3Se 3
T1Os3W 3
7.5
4.0
2.1
cFll2
hP14
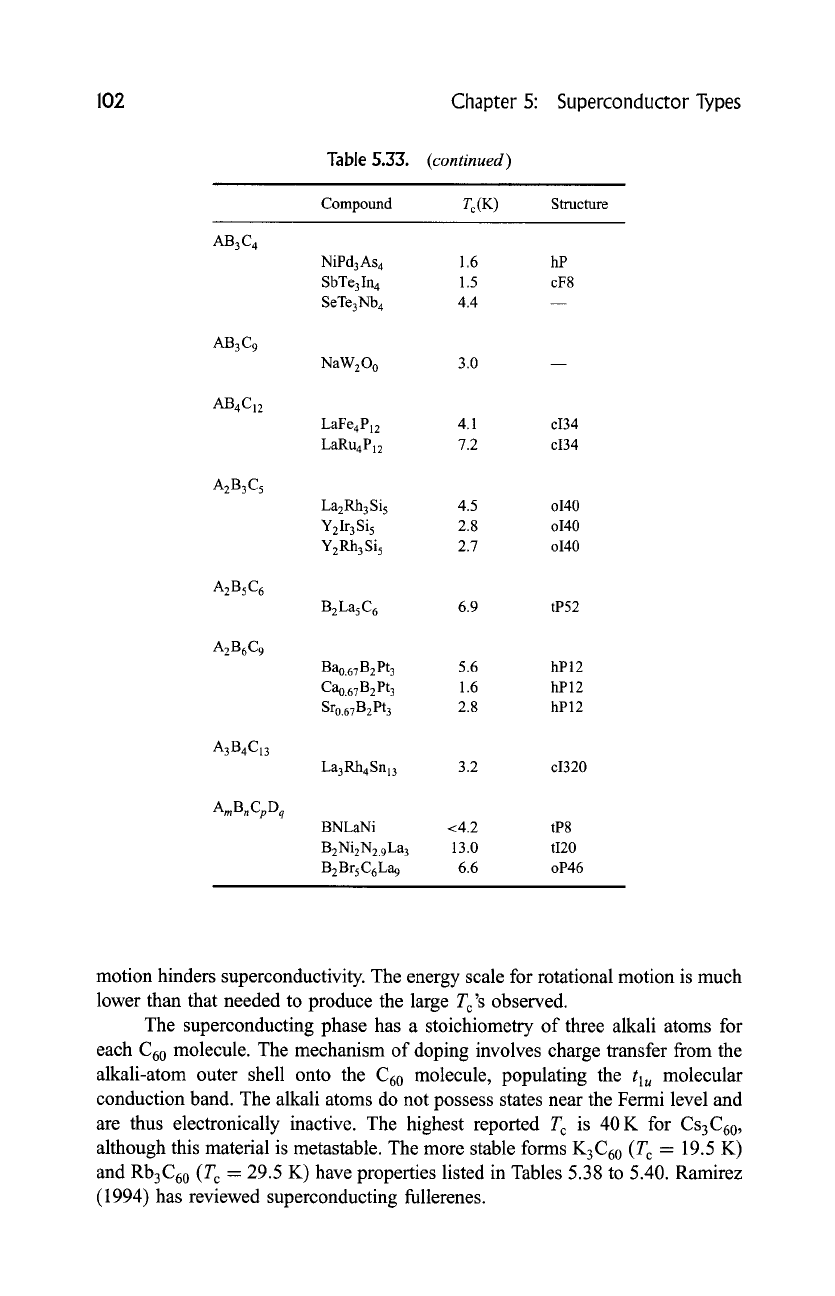
102 Chapter 5: Superconductor Types
AB3C 4
AB3C9
AB4C12
A2B3C5
A2BsC6
A2B6C9
A3B4C13
AmBnCpDq
Table S.33.
(continued)
Compound Tc(K )
Structure
NiPd3As 4 1.6 hP
SbTe3In 4 1.5 cF8
SeTe3Nb 4 4.4
NaW200 3.0
LaFe4P12 4.1 ci34
LaRu4P12 7.2 ci34
La2Rh3 Si 5 4.5 o140
Y2Ir3Si5 2.8 o140
Y2Rh3Si5 2.7 o140
B2La5C 6 6.9 tP52
Bao.67 B 2 Pt 3 5.6 hP 12
Cao.67B 2 Pt 3 1.6 hP 12
Sro.67B2Pt 3 2.8 hP12
La3Rh4Snl3 3.2 ci320
BNLaNi <4.2 tP8
B2Ni2N2.9La 3 13.0 tI20
B2BrsC6La 9 6.6 oP46
motion hinders superconductivity. The energy scale for rotational motion is much
lower than that needed to produce the large T c's observed.
The superconducting phase has a stoichiometry of three alkali atoms for
each C60 molecule. The mechanism of doping involves charge transfer from the
alkali-atom outer shell onto the C60 molecule, populating the
tau
molecular
conduction band. The alkali atoms do not possess states near the Fermi level and
are thus electronically inactive. The highest reported T c is 40 K for Cs3C60,
although this material is metastable. The more stable forms K3C60 (T c -- 19.5 K)
and Rb3C60 (T c = 29.5 K) have properties listed in Tables 5.38 to 5.40. Ramirez
(1994) has reviewed superconducting fullerenes.
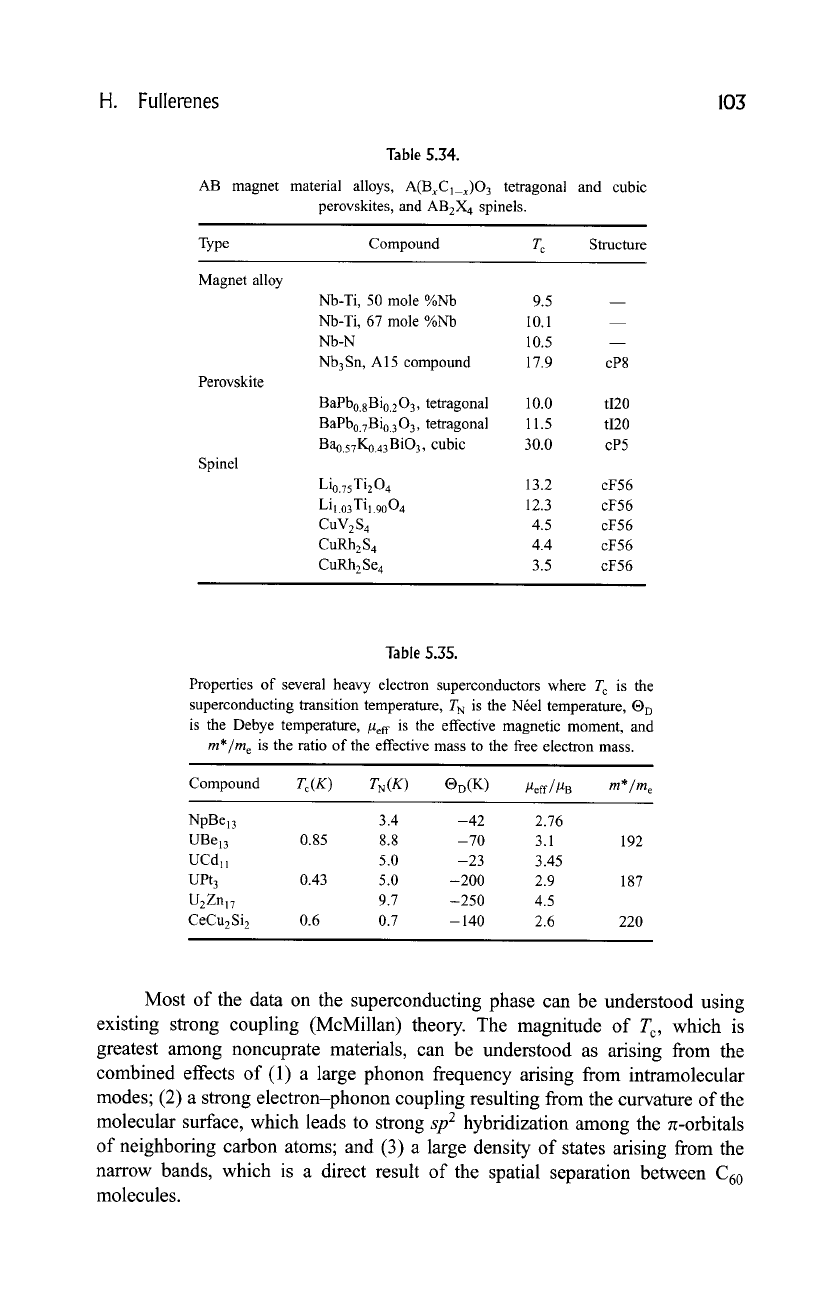
H. Fullerenes
103
Table 5.34.
AB magnet material alloys, A(BxCx_x)O 3 tetragonal and cubic
perovskites, and AB2X 4 spinels.
Type Compound T c Structure
Magnet alloy
Perovskite
Spinel
Nb-Ti, 50 mole %Nb 9.5
Nb-Ti, 67 mole %Nb 10.1
Nb-N 1 O.5
Nb3Sn, A15 compound 17.9
cP8
BaPb0.8Bi0203, tetragonal 10.0 tI20
BaPb0.7Bi0. 3 03, tetragonal 11.5 tI20
Ba0.57K0.43 BiO3, cubic 30.0 cP5
Li0.75Ti204 13.2 cF56
Li 1.03 Ti1.90
04
12.3 cF 56
CuV2S 4 4.5 cF56
CuRh2S 4 4.4 cF56
CuRh2Se 4 3.5 cF56
Table 5.35.
Properties of several heavy electron superconductors where T c is the
superconducting transition temperature, T N is the Nrel temperature, |
is the Debye temperature, /2ef f is the effective magnetic moment, and
m*/m e
is the ratio of the effective mass to the free electron mass.
Compound
Tc(K )
TN(K ) OD(K ) ]2eff/]2 B
m*/m e
NpBel3 3.4 -42 2.76
UBe13 0.85 8.8 -70 3.1 192
UCdll 5.0 -23 3.45
UPt 3 0.43 5.0 -200 2.9 187
U2Znl7 9.7 -250 4.5
CeCu2 Si 2 0.6 0.7 - 140 2.6 220
Most of the data on the superconducting phase can be understood using
existing strong coupling (McMillan) theory. The magnitude of T c, which is
greatest among noncuprate materials, can be understood as arising from the
combined effects of (1) a large phonon frequency arising from intramolecular
modes; (2) a strong electron-phonon coupling resulting from the curvature of the
molecular surface, which leads to strong
sp 2
hybridization among the n-orbitals
of neighboring carbon atoms; and (3) a large density of states arising from the
narrow bands, which is a direct result of the spatial separation between C60
molecules.
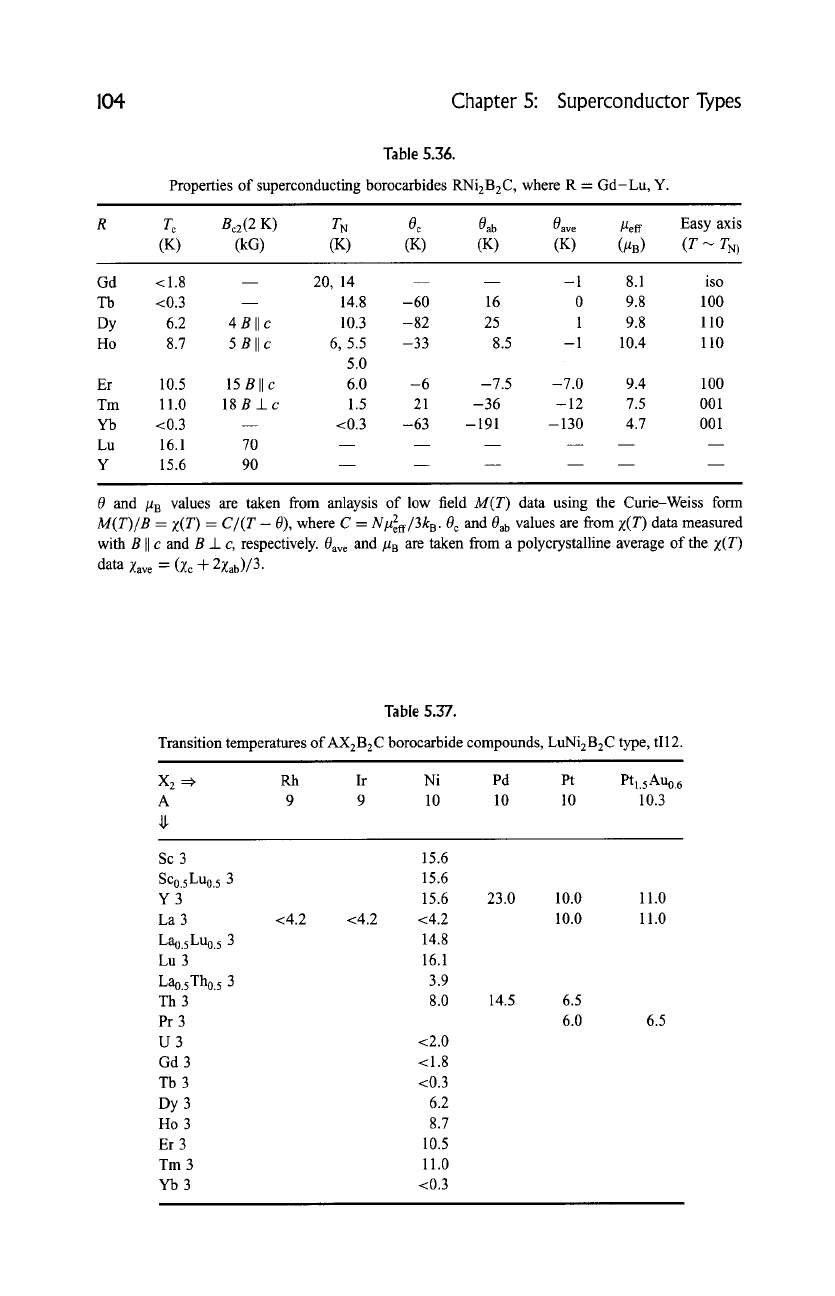
104 Chapter S: Superconductor Types
Table
5.36.
Properties of superconducting borocarbides RNi2B2C , where R = Gd-Lu, Y.
T c Bc2(2 K) T N 0 c 0ab
0ave
lAeff Easy axis
(K) (kG) (K) (K) (K) (K) (lAB) (T-~ TN)
Gd <1.8 -- 20, 14 -- -1 8.1 iso
Tb <0.3 14.8 -60 16 0 9.8 100
Dy 6.2 4 B II e 10.3 -82 25 1 9.8 110
Ho 8.7 5
Bile
6, 5.5 -33 8.5 -1 10.4 110
5.0
Er 10.5 15 B II c 6.0 -6 -7.5 -7.0 9.4 100
Tm 11.0 18 B _1_ c 1.5 21 -36 -12 7.5 001
Yb <0.3 -- <0.3 -63 -191 -130 4.7 001
Lu 16.1 70
Y 15.6 90
0 and IAB values are taken from anlaysis of low field
M(T)
data using the Curie-Weiss form
M(T)/B = z(T) = C/(T - 0),
where C =
NlA2ff/3kB .
0 c and 0ab values are from
z(T)
data measured
with B
II
c and B A_ c, respectively.
0av e
and lAB are taken from a polycrystalline average of the z(T)
data Zave
= (Xc q-
2Zab)/3.
Table 5.37.
Transition temperatures of AX2B2C borocarbide compounds, LuNi2B2C type, tI12.
X 2 =:~
Rh Ir Ni Pd Pt Ptl.sAu0. 6
A 9 9 10 10 10 10.3
Sc 3
Sco.sLuo. 5 3
Y3
La 3
Lao.sLuo. 5 3
Lu 3
Lao. 5Tho. 5 3
Th 3
Pr 3
U3
Gd 3
Tb3
Dy 3
Ho 3
Er 3
Tm 3
Yb3
15.6
15.6
15.6 23.0 10.0 11.0
<4.2 <4.2 <4.2 10.0 11.0
14.8
16.1
3.9
8.0 14.5 6.5
6.0
<2.0
<1.8
<0.3
6.2
8.7
10.5
11.0
<0.3
6.5
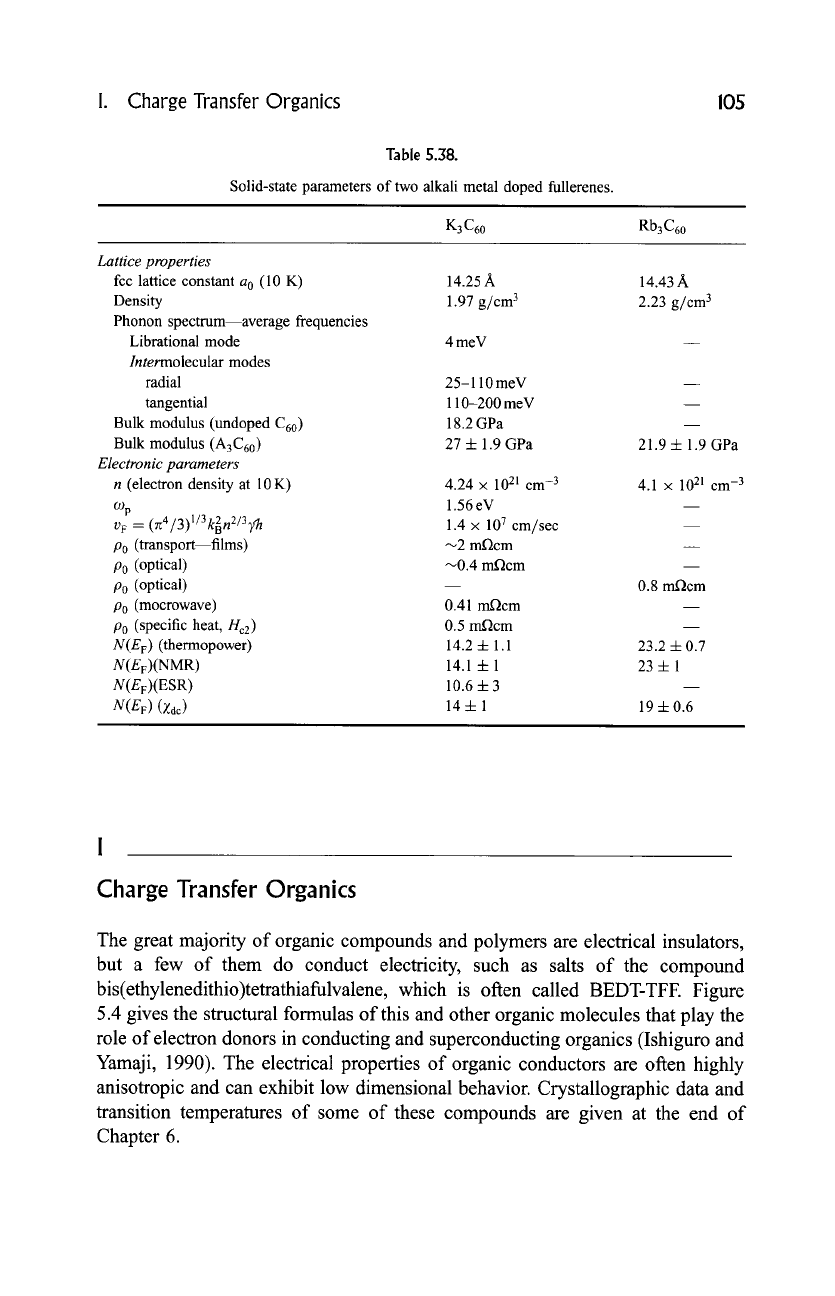
I. Charge Transfer Organics 105
Table 5.38.
Solid-state parameters of two alkali metal doped fullerenes.
K3 C60 Rb3 C60
Lattice properties
fcc lattice constant a 0 (10 K) 14.25 A 14.43 A
Density 1.97 g/cm 3 2.23 g/cm 3
Phonon spectrum--average frequencies
Librational mode 4 meV
Intermolecular
modes
radial 25-110 meV
tangential 110-200 meV
Bulk modulus (undoped C60 ) 18.2 GPa
Bulk modulus (A3C60) 27 4- 1.9 GPa 21.9 + 1.9 GPa
Electronic parameters
n (electron density at 10 K) 4.24 x 1021 cm -3 4.1 x 1021 cm -3
% 1.56
eV
--
v F
= (zt4/3)l/3k2nZ/3]fh
1.4 x 107 cm/sec --
P0 (transport--films) ~2 m.Qcm
P0 (optical) ~0.4 m-Qcm
P0 (optical) 0.8 m,Qcm
P0 (mocrowave) 0.41 m,Qcm
P0 (specific heat, Hc2 ) 0.5 m.Qcm
N(Ev)
(thermopower) 14.2 4- 1.1 23.2 4- 0.7
N(E v)(NMR)
14.1 4- 1 23 -1- 1
N(Ev)(ESR) 10.6 4- 3
N(Ev)
(Zdc) 14 4- 1 19 4- 0.6
Charge Transfer Organics
The great majority of organic compounds and polymers are electrical insulators,
but a few of them do conduct electricity, such as salts of the compound
bis(ethylenedithio)tetrathiafulvalene, which is often called BEDT-TFE Figure
5.4 gives the structural formulas of this and other organic molecules that play the
role of electron donors in conducting and superconducting organics (Ishiguro and
Yamaji, 1990). The electrical properties of organic conductors are often highly
anisotropic and can exhibit low dimensional behavior. Crystallographic data and
transition temperatures of some of these compounds are given at the end of
Chapter 6.
