Polaina J., MacCabe A.P. (ed.). Industrial Enzymes. Structure, Function and Applications
Подождите немного. Документ загружается.

442 ADÁNYI ET AL.
O
OH
OH
HO
HO
OH
H
O
OH
HO
HO
OH
O
FAD
FADH
2
O
2
H
2
O
2
Glucose oxidase
reductive half-reaction
oxidative half-reaction
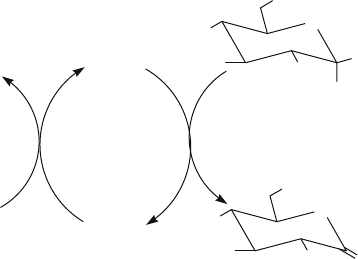
Figure 1. The reaction scheme for the oxidation of -D-glucose catalysed by glucose oxidase
P. purpurogenum, P. variabile, P. chrysogenum and A. fumaricus are also good
candidates for industrial applications (Crueger and Crueger, 1990; Petruccioli
et al., 1993; Leiter et al., 2004). The A. niger and P. amagasakiense enzymes
are homodimeric glycoproteins of 160 kDa molecular mass with one molecule of
non-covalently bound FAD cofactor per subunit (Fig. 2; Wohlfahrt et al., 1999).
Dissociation of subunits is only possible under denaturing conditions and is accom-
panied by the loss of the coenzyme FAD (Gouda et al., 2003). A. niger GOx has
10–16% mannose-type carbohydrate content, the carbohydrate moieties being N -
or O-glycosidically linked to the protein (Takegawa et al., 1989). The two GOxs
share 81% sequence similarity and exhibit similar glucose oxidation kinetics.
GOxs function by a Ping-Pong Bi-Bi mechanism which is divided into a reductive
half-reaction and an oxidative half-reaction (Fig. 1). In the reductive half-reaction
the substrate reduces FAD by hydride transfer forming dihydro-FAD FADH
2
and the oxidized product. Numerous sugars and derivatives of -D-glucose are
the substrates of GOx but the enzyme shows far greater activity with -D-glucose
(Pazur and Kleppe, 1964). In the oxidative half reaction, the reduced flavin is
regenerated by O
2
that undergoes reduction to H
2
O
2
. Besides O
2
, both one-electron
acceptors (such as transition metal complexes, ferrocene derivatives, and nitroxide
radicals) and two-electron acceptors (benzoquinone derivatives) are good substrates
for the reduced enzyme (Chan and Bruice, 1977; Bourdillon et al., 1993; Ryabov
et al., 1999).
One-electron acceptors are generally used as mediators between GOx and
the metal or graphite electrode. The largest mediator groups are derivatives of
ferrocene that are small enough to penetrate to the active centre of GOx and
exchange electrons (Alvarez-Icaza et al., 1995; Forrow et al., 2002). When the
mediator cannot penetrate to the active site due to its bulkiness, a long-range
electron transfer event takes place. Theoretical studies have predicted the length
and path for electron transfer from the flavin cofactor to the surface of the protein
(Alvarez-Icaza et al., 1995).
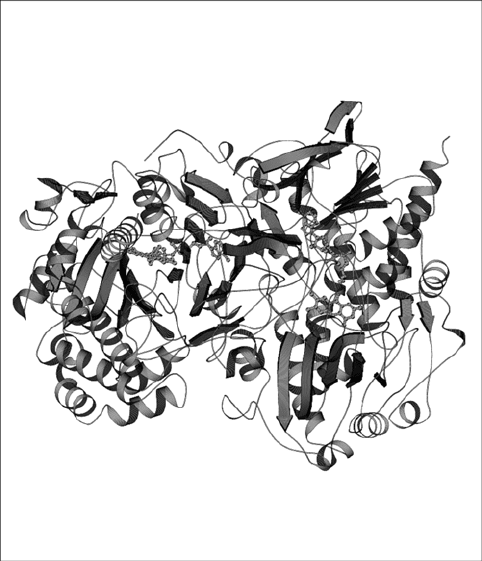
H
2
O
2
PRODUCING AND DECOMPOSING ENZYMES 443
Figure 2. The homodimeric structure of glucose oxidase from Penicillium amagasakiense with non-
covalently bound FAD represented by MOLSCRIPT (Kraulis, 1991)
2.2. Galactose Oxidases
Galactose oxidase (D-galactose:oxygen 6-oxidoreductase, GAO, EC 1.1.3.9.) is a
Type II (non-blue) copper protein (Giordarno et al., 1974) with a molecular mass of
69 kDa. It catalyses the oxidation of a wide range of primary alcohols and polysac-
charides (e.g. D-galactose) to the corresponding aldehydes, coupling this reaction to
the reduction of O
2
to H
2
O
2
RCH
2
OH+O
2
→RCHO+H
2
O
2
(Bretting and Jacobs,
1987). The substrate specificity of GAO is very broad, ranging from small alcohols
to polysaccharides but the enzyme is highly stereospecific and does not oxidise
either D-glucose or L-galactose (Klibanov et al., 1982; Goudsmit et al., 1984). The
physiological function of GAO is unclear.
Different fungal species: Fusarium dendroides (Dactylium dendroides),
Gibberella fujikuroi and G. zeae as well as the basidiomycete Polyporus circinatus,
can be used for industrial-scale GAO production. GAO genes can also be expressed
efficiently in either Pichia pastoris (Whittaker and Whittaker, 2000) or Aspergillus
oryzae (Xu et al., 2000). GAO contains a single copper ion in addition to a protein
base redox site (Whittaker and Whittaker, 1988, 1990) (Fig. 3), which comprises a
444 ADÁNYI ET AL.
N
Cu
2+
N
H
2
O
O
O
Tyr495
Tyr272
S
Cys228
His581
His496
RCH
2
OH
N
Cu
2+
N
O
O
O
Tyr495
Tyr272
S
Cys228
H
H
R
H
S
R
–H
2
O
H
+
abstraction
N
Cu
2+
N
O
O
HO
Tyr495
Tyr272
S
Cys228
C
H
R
H
S
R
H atom abstraction
N
Cu
2+
N
O
O
HO
Tyr495
Tyr272
S
Cys228
C
H
R
R
e- transfer
N
Cu
+
N
O
O
HO
Tyr495
Tyr272
S
Cys228
C
H
R
R
H
H
–H2O2
O
2
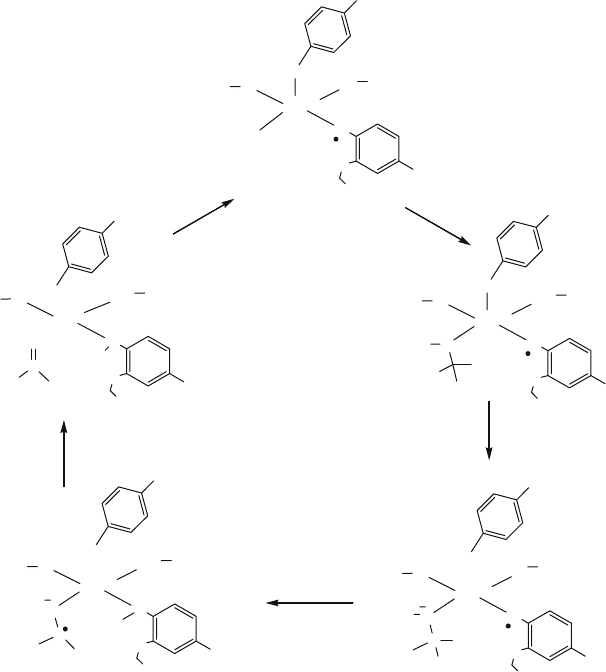
Figure 3. Proposed mechanism for D-galactose oxidation by galactose oxidase according to Whittaker
(1988). R represents the pyranose ring associated with D-galactose
tyrosine residue (Tyr272) covalently linked to a cysteine (Cys228) through thioether
bond, forming a cysteinyl-tyrosine (C-Y) “built-in” cofactor (Ito et al., 1991). The
enzyme undergoes self-processing in the presence of oxygen and copper to generate
this covalent cross-link, which is required for enzyme activity (Xie and van der
Donk, 2001). The cross-linked tyrosine serves as an axial ligand to the copper and
is oxidized to the radical form in the active state of the protein. The Cu(II)/Tyr
cofactor on GAO carries out the two-electron oxidation of primary alcohols to the
corresponding aldehydes via a radical mechanism.
The enzyme uses a Ping-Pong mechanism with respect to the substrate and
O
2
. Tyr495 functions as a base for abstracting a proton from the bound substrate
followed by hydrogen atom abstraction by the Cys-Tyr radical generating an
H
2
O
2
PRODUCING AND DECOMPOSING ENZYMES 445
alkoxide radical and diamagnetic Cys-Tyr (Whittaker and Whittaker, 1990). From
the reactive alkoxide radical one electron transfers to Cu
2+
reducing it to Cu
+
and
forming the aldehyde product. O
2
converts Cu
+
back to Cu
2+
(Fig. 3).
2.3. Cholesterol Oxidases
Cholesterol oxidases (cholesterol:oxygen oxidoreductase, ChOx, EC 1.1.3.6.) are
bifunctional flavoenzymes that catalyse two reactions at a single active site. The
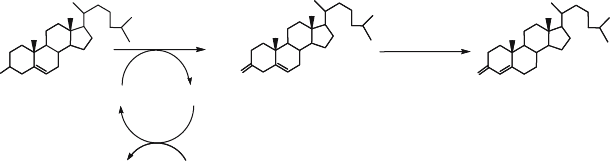
first is the oxidation of cholesterol to cholest-5-ene-3-one and the isomerisation of
the labile cholest-5-ene-3-one intermediate to cholest-4-ene-3-one product (Fig. 4).
Although the enzymes exhibit a wide-range of steroid specificities, the 3-hydroxyl
group is essential for their activity (Inouye et al., 1982).
ChOxs are produced by bacteria including species from the Rhodococcus,
Streptomyces and Nocardia genera. However, several Brevibacterium, Proactino-
myces, Pseudomonas and Cellulomonas species also possess ChOx activity suitable
for industrial production (Goodhue and Risley, 1978; Watanabe et al., 1986;
MacLachlan et al., 2000). Recombinant Escherichia coli strains expressing bacterial
ChOx genes have been reported (Sakka et al., 1994). Several fungi from the Basid-
iomycete taxon (e.g. Lentinus edodes, Oudemansiella radicate, Coprinus comatus
and Auricularia polytricha) are also promising candidates for future industrial ChOx
production (Matsui et al., 1982). In non-pathogenic bacteria, e.g. Streptomyces,
ChOx is secreted and is a component of the metabolic pathway for utilizing choles-
terol as a carbon source (Fukuda et al., 1973; Cheetman et al., 1982). Pathogenic
bacteria, e.g. Rhodococcus equi and slow-growing Mycobacterium spp., require
ChOx for invasion of the host macrophage and cholesterol regulates the expression
of this enzyme (Fernanandez-Garayzabal et al., 1996; Navas et al., 2001). ChOx is
a water soluble, interfacial enzyme that binds transiently to the membrane surface
during catalysis (Sampson et al., 1998).
ChOxs utilise a Ping-Pong mechanism with respect to the steroid substrate and
O
2
. In the reductive half-reaction the cholesterol reduces FAD by hydride transfer
from the 3C of cholesterol to N5 of the flavin moiety yielding cholest-5-ene-3-one
HO
3
5
O
O
O
H
cholesterol
Isomerization
Oxidation
FAD
FADH + H
+
O
2
H
2
O
2
cholest-5-ene-3-one cholest-4-ene-3-one
Figure 4. Cholesterol oxidation and isomerization catalysed by cholesterol oxidase
446 ADÁNYI ET AL.
and 1,5-dihydroflavin (FADH
2
(Medina et al., 1997). In the oxidative half-reaction
the reduced flavin (FADH
2
is converted back to the active, oxidized form (FAD)
by O
2
with the generation of H
2
O
2
.
2.4. Catalases
Catalases (hydrogen-peroxide:hydrogen-peroxide oxidoreductase, CAT, EC
1.11.1.6.), more correctly hydroperoxidases, catalyse the degradation of H
2
O
2
to
H
2
O and O
2
2H
2
O
2
→2H
2
O+O
2
.
Considering the possibility of industrial enzyme production, a variety of different
micro-organisms including bacteria (e.g. Micrococcus, Bacillus, Microscilla, Alcali-
genes spp.), moulds (e.g. Aspergillus, Penicillium, Thermomyces, Thermoascus,
Acremonium spp.) and yeasts (e.g. Saccharomyces, Candida, Mycotorula spp.)
are known as good CAT producers. CATs from animal sources (e.g. bovine
liver) are generally cheap, therefore the production of microbial CATs will only
be economical when better producer (preferably recombinant) strains and cheap
technology can be used or CATs with special properties (e.g working at high or low
temperatures or at alkaline or acidic pH) are produced (Fusho and Yajima, 1995;
Kou et al., 1998; Takeuchi and Isobe, 1999).
In general, three distinct types of proteins, which do not share either sequence or
structural homologies, exhibit significant CAT activity. The class I CATs, which
are the most widely spread in nature, consists of monofunctional haem-containing
enzymes which can be divided into two subclasses having large > 75KDa or small
< 60 kDa subunits (Klotz et al., 1997). The second, less widespread class includes
bifunctional haem-containing CAT-peroxidases, which are closely related to plant
peroxidases. The third class comprises members of the non-haem or Mn-containing
CATs (Wu et al., 2004).
Monofunctional haem-containing CATs all possess a two-stage mechanism for
the degradation of H
2
O
2
. The first, oxidative, step in the catalyses is the mono-
oxygen transfer from H
2
O
2
to the iron centre with the release of water (Fig. 5).
Under physiological conditions the resting state of haem CATs contains iron (III).
In the oxidation, one molecule of H
2
O
2
oxidizes the haem to an oxy-ferryl species,
in which one electron is removed from the iron and one electron is removed from
the coordinated porphyrin ring to generate a porphyrin radical cation (Fig. 5). In
the second stage, another H
2
O
2
molecule is utilised to regenerate the enzyme by
reducing the porphyrin radical coordinated oxy-ferryl species to the resting haem
Enz(Por
+.
–Fe
IV
= O) + H
2
O
2
Enz(Por–Fe
III
) + H
2
O + O
2
Enz(Por–Fe
III
) + H
2
O
2
Enz(Por
+.
–Fe
IV
= O) + H
2
O
(1)
(2)
Figure 5. The two-stage process of H
2
O
2
disproportionation catalysed by catalases
H
2
O
2
PRODUCING AND DECOMPOSING ENZYMES 447
releasing H
2
O and O
2
(Matsunaga and Shiro, 2004). The haem reactivity is enhanced
by the axial phenolate ligand of Tyr.
CATs do not follow classical Michaelis-Menten kinetics except across a certain
range of substrate concentrations (Switala and Loewen, 2002). Most small subunit
enzymes are inactivated by H
2
O
2
at concentrations above 300–500 mM and never
reach the maximum rate extrapolated from the Michaelis–Menten curve determined
at lower substrate concentrations. Large subunit enzymes show a greater resistance
to H
2
O
2
damage even at concentrations above 3 M and the observed rates exceed
the theoretical Michaelis-Menten maximum rate (Switala and Loewen, 2002).
Most of the monofunctional haem-containing CATs are uniquely stable and resist
proteolysis due to their very rigid structure that resists unfolding (Fig. 6). Enzymes
with larger subunit such as HPII CAT from E. coli, show enhanced thermal stability,
the activity of the enzyme starting to drop at temperatures above 80
C (Switala
et al., 1999).
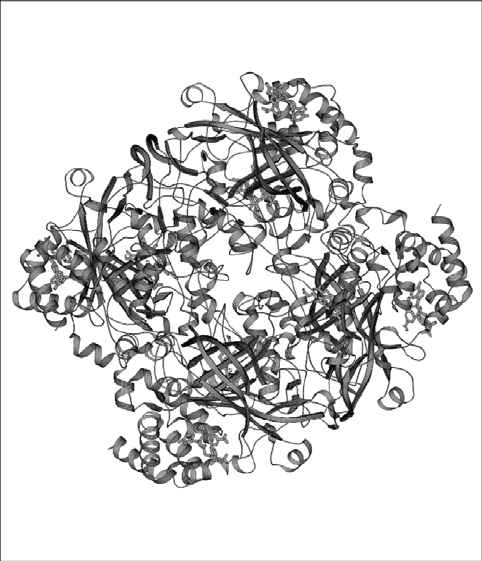
Figure 6. The tetrameric structure of catalase from beef liver. Each domain contains haem and NADP
cofactor represented with ribbon diagram (Kraulis, 1991)
448 ADÁNYI ET AL.
3. BIOSENSORS BASED ON OXIDASES AND CATALASE
3.1. Biosensors Based on Glucose Oxidase
Currently, GOx is the most important model enzyme in both basic and applied
biosensor research because its enzymological properties are well understood and
it is very cheap in comparison to other oxidases. Different design approaches
have been considered and employed in the construction of GOx-based biosensors,
including needle type sensors, sensors for in vivo monitoring, flow injection analysis
(FIA) applications, combinations of glucose biosensors with microdialysis sampling
techniques and disposable sensors (Wilson and Gifford, 2005).
Electrochemical glucose biosensors prepared by immobilising GOx on the
surface of carbon film electrodes using glutaraldehyde and bovine serum albumin
(BSA) with or without Nafion (a sulfonated tetrafluorethylene copolymer)
have been reported by Florescu and Brett (2005). An ISFET (Ion Sensitive
Field Effect Transistor) biosensor containing GOx and horseradish peroxidase
(HRP) co-immobilized with BSA using glutaraldehyde and covered by poly(4-
vinylpyridine-co-styrene) polymeric film could also be used for glucose deter-
mination (Volotovsky and Kim, 1998). Monolayer enzyme electrodes have been
made by covalently or electrostatically binding the recognition molecule onto
electrodes modified with conducting polymer films (e.g. self-assembled monolayers,
Langmuir–Blodgett films; Malhotra and Singhal, 2003). A flow-through electro-
chemical micro-cell can be used as an on-line detector in microdialysis-based assays.
The micro-detector modified by enzyme-based chemistry can be directly connected
to the outlet of the microdialysis probe (Gáspár et al., 2004).
Redox couples, or mediators, are able to shuttle electrons between the redox
centre of the enzyme and the electrode. The electron transfer mediator must be
chemically stable in both the reduced and oxidised forms, have a relatively low and
stable redox potential, and should be easy to immobilize at the electrode surface
(Harwood and Pouton, 1996). Carbon paste electrodes or graphite–Teflon rigid
composite biosensors offer the possibility of co-immobilization of several enzymes
via simple physical inclusion in the bulk of the electrode matrix by non-covalent
linkages in the presence of a mediator like ferrocene (Guzman-Vázquez de Prada
et al., 2004). Yu et al. (2003) constructed a biosensor by immobilizing GOx with
titanium isopropoxide forming GOx-titania sol-gel film, which retains the native
structure and activity of the entrapped enzymes. Carbon film resistor electrodes
can be modified with Prussian Blue (PB, ferric hexacyanoferrate) and then covered
with a layer of covalently immobilized enzyme. These enzyme electrodes can be
used to detect glucose via the oxidation of H
2
O
2
at +50mV vs. Ag/AgCl in the
low micromolar range, while also avoiding or reducing electrochemical interference
(Ricci and Palleschi, 2005). Electrodes produced with the ‘screen printing’ thick-
film technique can be chemically modified with PB prior to enzyme immobilisation
(Newman and Turner, 2005).
The use of metallized carbon electrodes resulted in remarkably selective amper-
ometric biosensors because these transducers eliminated major electroactive
H
2
O
2
PRODUCING AND DECOMPOSING ENZYMES 449
interference. Platinization has been used to increase electrode surface area
and thus electrode sensitivity, immobilizing GOx by the electropolymer-
ization of o-phenylenediamine (Reyes De Corcuera et al., 2005). Glucose
nanosensors based on the co-electrodeposition of an osmium redox polymer/enzyme
composite on low-noise carbon fibre nanoelectrodes have been constructed
(Fei et al., 2005). Chromium and manganese half-sandwich complexes
were developed as mediators for GOx since their sizes are similar to
that of ferrocene derivatives and they contain a -ligand for interaction
with the enzyme co-factor (Forrow and Walters, 2004). Screen-printed
electrodes modified with ruthenium dioxide were investigated as amperometric
sensors to immobilize GOx onto the electrode surface using Nafion films
(Kotzian et al., 2005).
Recently there has been considerable interest in the development of single-
use sensor strips especially in the fields of decentralized medical diagnostics
or on-site environmental monitoring (Wang et al., 1995). These strips can be
regarded as disposable electrochemical cells onto which a sample droplet is
placed. A miniaturized total analytical system (TAS) for on-line monitoring
of glucose in biological systems based on integrated microdialysis sampling
and photolithographically-prepared glucose electrodes has been developed
(Wang, 1999).
Interest in optical fibre biosensors is growing fast since they confer several
advantages compared to biochemical and clinical analyses due to their simple
and flexible construction. Malhotra et al. (2005) presented a glucose biosensor in
which GOx was immobilized onto poly-3-hexyl thiophene mixed with stearic acid
(P3HT/SA) LB films. The activity of GOx-containing LB films was measured by
a colorimetric method using o-dianisidine as a chromogenic dye with absorbance
at 540 nm. Capillary electrophoresis integrated with an optical biosensor was
built by modifying the detector part of the electrophoresis equipment with the
redox-sensitive polymer film polyaniline (PANI) containing immobilized GOx
(Bossi et al., 2003). Measuring molecular fluorescence is a very promising
technique in biosensing because of its high sensitivity. Fibre-optic glucose sensors
based on this phenomenon have been developed by immobilizing GOx onto an
oxygen optrode composed of either decacyclene in silicone (ex 385 nm, em 450–
600 nm) or the ruthenium complex tris(1,10-phenanthrolene)ruthenium chloride
(ex 447 nm, em 604 nm). The formation of hydrogen peroxide can be followed
by measuring chemiluminescence (luminol) or via the formation of a fluorescent
oxidation product from non-fluorescent p-hydroxyphenyl acetic acid (HPA) or
homovanillic acid (HVA) in the presence of HRP. A decrease in the pH in
the enzyme layer can be determined taking advantage of the light absorption
(e.g. bromocresol green) or fluorescence (e.g. hydroxypyrenetrisulphonate) of
acid/base indicators. In a different type of glucose biosensor GOx itself (the
prosthetic group, FAD), or GOx chemically modified with a fluorescein derivative
(GOx-FS), served as an indicator for recording changes in fluorescence (Pickup
et al., 2005).
450 ADÁNYI ET AL.
3.2. Multienzyme Biosensors Based on Glucose Oxidase
Multienzyme systems involving GOx have been developed for the rapid, repro-
ducible and cheap determination of different disaccharides and polysaccharides.
For example, the concentration of maltose was determined using a bienzyme cell
in which amyloglucosidase and GOx were co-immobilized on a protein membrane
using glutaraldehyde followed by amperometric detection. For lactose sensing,
-galactosidase, GAO and GOx enzymes were co-immobilized either on the surface
of a measuring electrode using a triacetate cellulose membrane, or on the surface
of different resins, or on the controlled-pore glass used in analytical reactors, or on
protein membranes by covalent coupling (Adányi et al., 1999). Sensors for total
D-glucose + and sucrose have been developed using pectin as a novel matrix
to enhance enzyme entrapment and stabilisation on rhodinised carbon electrodes.
Total D-glucose can be measured by enzymatically transforming all -glucose
anomers to the form using mutarotase. For sucrose detection, a multienzyme
system involving invertase, mutarotase and GOx were used (Jawaheer et al., 2003).
With the incorporation of lysozyme during the immobilization step, considerable
enhancement of the operational stability of a biosensor has been demonstrated in the
case of sucrose determination (Gouda et al., 2002). Marconi et al. (2004) developed
a method for the determination of gelatinised starch in processed cereal foods by
co-immobilization of amyloglucosidase and GOx on a Pt electrode surface while
the third enzyme, -amylase, was added to the solution under analysis. Wu et al.
(2005) proposed a biosensor for the determination of glucosinolates employing a
bienzyme system involving myrosinase and GOx co-immobilized onto an eggshell
membrane on the surface of a dissolved oxygen electrode.
3.3. Biosensors Based on Galactose Oxidase
GAO is used in biosensors developed for the determination of galactose or dihydrox-
yacetone in different biofermentation processes, or for measuring lactose primarily
in food samples. Schumacher et al. (1994) constructed an electrode for the deter-
mination of galactose and galactose-containing disaccharides in which GAO was
immobilized in gelatine between two dialysis membranes and stuck to an electrode.
Malhotra et al. (2005) prepared an amperometric biosensor to measure galactose
in milk and blood serum by immobilizing the enzymes in Langmuir–Blodgett
(LB) films of poly-3-hexyl thiophene (P3HT) mixed with stearic acid (SA), and
depositing the LB film onto indium tin-oxide (ITO) coated glass plates. Hasebe
and Uchiyama (2000) demonstrated that the substrate specificity of GAO from
F. dendroides changed dramatically in the presence of L-histidine resulting in
catalysis of the air oxidation of L-ascorbate. Using this observation a flow-
type system was developed for the indirect measurement of histidine. Since the
catalytic activity of GAO is inhibited by free radicals a biosensor based on enzyme
inhibition was developed to detect superoxide and nitrogen oxide radicals using
GAO entrapped in a gel-like -carrageenan membrane coupled to an amperometric
oxygen electrode (Campanella et al., 2000).
H
2
O
2
PRODUCING AND DECOMPOSING ENZYMES 451
3.4. Biosensors Based on Cholesterol Oxidase
To measure free and total cholesterol contents in biological samples, ChOx-
based biosensors and bienzyme cells containing immobilized cholesterol esterase
(ChEt) and ChOx were developed, respectively. Ram et al. (2001) reported a
biosensor in which ChOx and ChEt were bound to either a collagen membrane
or to conducting polymer matrices. The electrochemical redox processes taking
place in enzyme-layered films deposited either on platinum or ITO-coated glass
plates have been investigated. Gobi and Mizutani (2001) constructed a direct
amperometric biosensor by layer-by-layer nanothin film formation using ChOx
and poly(styrenesulfonate) on a monolayer of HRP covalently immobilized on
Au-alkanethiolate electrodes. ChOx and ChEt were entrapped within polypyrrole
(PPy) films on a platinum disc electrode during electrochemical polymerisation
(Singh et al., 2004). Situmorang et al. (1999) studied the conversion of cholesterol
esters by flow injection potentiometry using a tungsten electrode and monitoring
ferricyanide/ferrocyanide conversion. Hall and Turner (1991) described an amper-
ometric organic-phase enzyme electrode (OPEE) using ChOx to determine the
concentration of cholesterol in a chloroform/hexane mixture. Pena et al. (2001)
reported on a bienzyme amperometric composite biosensor for the determination of
free and total cholesterol in food samples. ChOx and HRP together with potassium
ferrocyanide as a mediator were incorporated into a graphite-Teflon (30%–70%)
matrix. The enzyme membrane was prepared from ChEt and ChOx using a photo-
sensitive polymer and an ultra-thin dialysis membrane, and this was applied in a flow
system with a Clark-type oxygen electrode (Endo et al., 2003). An amperometric
cholesterol biosensor using carbon nanotubes has been produced by layer-by-layer
deposition of a cationic polyelectrolyte (poly(diallyldimethylammonium) chloride;
PDDA) and ChOx on a multi-walled carbon nanotube-modified gold electrode (Guo
et al., 2004). The hybrid composite film was used to immobilize ChOx on the
surface of PB-modified glass carbon electrode (Tan et al., 2005).
The FIA method was investigated by covalent co-immobilization of enzymes
to the silica in packed-bed reactors (IMMER) and using an amperometric
HRP electrode or photometric determination of H
2
O
2
(Baticz and Tömösközi,
2002). Optical cholesterol biosensors have also been constructed by immobilizing
ChOx and octadecyl silica (ODS) particles in either hydrogel network matrices
of copolymers of poly(vinyl alcohol)/hydroxyethyl carboxymethyl cellulose
(PVA/HECMC) or sol-gel (Wu and Choi, 2003).
3.5. Biosensors Based on Catalase
CAT decomposes H
2
O
2
and remains active when kept in appropriate organic
solvent environments. Wang et al. (1995) demonstrated the applicability of CAT
for organic-phase biosensing by immobilising the enzyme onto a glassy carbon
amperometric transducer by casting a mixed enzyme/Eastman-AQ polymer solution.
Horozova et al. (2002) prepared an OPEE by immobilizing the enzyme within a
