Polaina J., MacCabe A.P. (ed.). Industrial Enzymes. Structure, Function and Applications
Подождите немного. Документ загружается.

LACCASES 463
the reasons for the sensitivity towards chelating agents. The selective removal of
Cu by chelating agents (EDTA, dimethyl glyoxime, N N
-diethyldithiocarbamate,
NTA) leads to a loss of catalytic activity. Laccases generally are more stable
at alkaline pH than at acidic pH, probably due to the OH
−
inhibition of
auto-oxidation.
3. STRUCTURAL AND FUNCTIONAL FEATURES
Phylogenetic analyses based on sequence alignments have shown that laccase
copper-binding domains are highly conserved even when the rest of the molecule
shows wide variability. The topology of the phylogenetic trees obtained suggests
that a single monophyletic branch might exist for the fungal laccases (Valderrama
et al., 2003). It is assumed that laccases are evolutionarily very ancient enzymes,
and that the enzyme activity linked to three different copper sites must have arisen
early in the course of evolution. In a recent study, sequence alignments of more than
100 plant and fungal laccases resulted in the identification of a sequence signature
that uniquely characterizes the laccases as a distinctive subgroup of enzymes of
the multi-copper family. This signature, comprising 4 ungapped sequence segments
L1-L4 ranging from 8 to 24 residues in length and scattered across almost the
entire length of the protein, contains the twelve amino acid residues that serve
as the copper ligands (Kumar et al., 2003). This structural conservation reflects
a common reaction mechanism for copper oxidation and O
2
reduction in these
enzymes.
3.1. Metal Content
Laccases contain four copper atoms per monomer and no other co-factor (Davies,
2002). The four copper atoms are bound to 3 redox sites designated T1, T2 and
T3. The four complexed copper atoms are designated Type I, Type II and Type III,
according to their spectroscopic and paramagnetic properties.
Paramagnetic “blue” Cu1 (copper Type I, T1) is bound as a mononuclear cluster
and confers the beautiful greenish-blue colour to laccases (in the oxidized resting
state,
610nm
∼ is in the range 4900–5700 M
−1
cm
−1
). This intense absorption band
results from the covalent copper-cysteine bond. In fact the Cys → Cu
2+
charge
transfer yields an electron paramagnetic resonance (EPR) spectrum with an excep-
tionally small hyperfine splitting in the low-field region. Cu T1 is available to
solvents, including water. It can be removed from the enzyme molecule by various
copper complexones, or substituted by cobalt. It can also be displaced by mercury
but this affects severely enzyme activity.
Paramagnetic “normal or non-blue” Cu2 (copper Type II, T2) behaves as a
mononuclear site with normal EPR spectrum parameters attesting the presence
of tetragonal surroundings. It has only weak and insignificant absorption in the
UV-VIS region but is EPR-active.
464 ALCALDE
Diamagnetic spin-coupled Cu3–Cu4 pair (coppers Type III, T3) form a binuclear
site in which the two copper atoms are anti-ferromagnetically coupled (the so
called Cu3–Cu4 dyad) through a bridging ligand – an hydroxyl bridge-. The main
consequence is the total absence of an EPR signal. The T3 pair are most likely
responsible for a shoulder in the UV spectrum at about 330nm (oxidized form)
which disappears upon reduction of the active site. Together the T2 and T3 coppers
form a trinuclear cluster where reduction of molecular oxygen and the release of
water takes place. The T1 copper is involved in the oxidation of the reducing
substrate, capturing electrons that are then transferred to the T2 and T3 copper
centres.
3.2. Redox potential, E
Redox potential E
i.e. the energy required to capture one electron from a reducing
substrate with the corresponding formation of a cation radical, is one of the most
significant features of laccases. The E
of laccases is typically determined using
an appropriate couple such as K
3
FeCN
6
−K
4
FeCN
6
+433mV. Despite the
strong similarities in their EPR parameters, the redox potentials of T1 centres
can vary widely between laccases from different sources. For example, the E
of fungal laccases is far higher than that of plant or bacterial laccases. Thus, the
Rhus vernicifera laccase has a Cu T1 E
of about +400 mV, whereas the E
of
fungal laccases range from +400 to +800 mV. Ligninolytic peroxidases are able
to oxidize substrates of extremely high electropotential, up to 1.49V (e.g. lignin
peroxidase can convert veratryl alcohol whose E
= 122 V). Laccases, however,
cannot oxidize non-phenolic substrates with electropotentials greater than 1.06 V
(Table 1).
The reactivity of laccases has been correlated with their redox potential which is
thought to play a major role in the overall performance of these enzymes (Xu et al.,
1998). It has been demonstrated that the oxidation rate depends on the E
difference
between the reducing substrate and the Cu T1. A lower E
of substrate or a higher
E
of laccase (Cu T1) often results in a higher rate of substrate oxidation. The broad
difference observed between the E
values of different laccases (from +465mV
for Myceliophthora thermophyla laccase to +780 mV of Trametes – Polyporus or
Coriolus – versicolor) has been the subject of studies aimed to determine which
parameters modulate E
. It is well known that a hydrophobic residue (either Phe
or Leu) at the axial position of the T1 Cu site is implicated in the elevated redox
potential of fungal laccases (see Table 1). However, there are broad differences in
redox potentials even amongst the fungal laccases; thus it is clear that a Leu or Phe
residue at the T1 site cannot be the sole contributor to these effects. In this sense,
the significance of a highly conserved pentapeptide segment located near the Cu
T1 has been described (Xu et al., 1998). Recently, analysis of sequence alignments,
site-directed mutagenesis experiments and crystallographic studies have allowed to
propose mechanisms that explain how laccases can tune their redox potential by as
much as 200 mV (Piontek et al., 2002).

LACCASES 465
Table 1.E
of several blue multicopper enzymes.
Species Organism Enzyme CuT1E
(V) Potential
axial
ligand
Ref
Trametes
versicolor
Basidiomycete Laccase +0.79 Phe (Alcalde et al.,
2002)
Trametes
villosa
Basidiomycete Laccase +0.79 Phe (Kumar et al.,
2003)
Neurospora
crassa
Ascomycete Laccase +0.78 Leu (Piontek et al.,
2002)
Rhizoctonia
solani
Deuteromycete Laccase +0.71 Leu (Kumar et al.,
2003)
Coprinus
cinereus
Basidiomycete Laccase +0.55 Leu (Kumar et al.,
2003)
Scytalidium
thermophilum
Basidiomycete Laccase +0.51 Leu (Kumar et al.,
2003)
Homo sapiens Mammalian Ceruloplasmin +0.49 Met (Kumar et al.,
2003)
Myrothecium
verrucaria
Fungi mitosporic Bilirubin
oxidase
+0.48 Met (Kumar et al.,
2003)
Myceliophthora
thermophila
Ascomycete Laccase +0.47 Leu (Alcalde et al.,
2002)
Rhus
vernicifera
Plant Laccase +0.43 Met (Yaropolov
et al., 1994)
Zucchini
(Cuburbita
pepo)
Plant Ascorbate
oxidase
+0.34 Met (Kumar et al.,
2003)
3.3. Protein Structure
The crystal structures of one bacterial laccase (the spore coat protein from Bacillus
subtilis, BsL) (Enguita et al., 2003) and three fungal laccases (Coprinus cinereus
(CcL) Ducros et al., 1998; Trametes versicolor (TvL) Piontek et al., 2002;
Melanocarpus albomyces (MaL) Hakulinen et al., 2002) have been reported. All
were resolved at high resolutions: 1.7; 2.2, 1.90 and 2.4 Å, respectively. In the cases
of MaL and TvL, the structures of the active enzymes containing a full complement
of copper atoms were determined. Deglycosylation carried out to obtain diffracting
crystals of CcL resulted in the loss of copper (CuT2), therefore the available
structure corresponds to a catalytically incompetent state.
Fig. 1 shows the overall structure of laccase from M. albomyces (MaL).
The structures of MaL and TvL are similar. Both are monomers consisting of
three cupredoxin-like domains. One of these domains (domain 3) contains the
mononuclear site T1. The trinuclear cluster (T2 and T3) is embedded between
domains 1 and 3 both of which provide residues for the coordination of the coppers.
Domain 2 contains residues that participate in substrate binding. MaL and TvL are
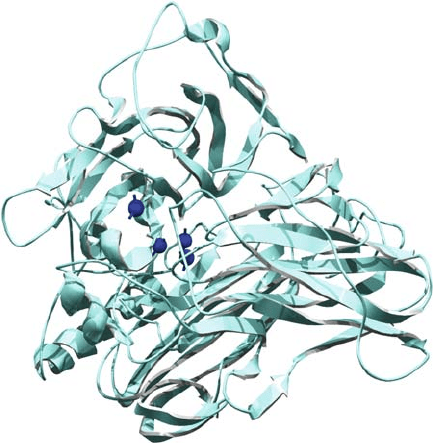
466 ALCALDE
Figure 1. Overall structure of Melanocarpus albomyces laccase (MaL) according to Hakulinen et al.,
(2002). Copper atoms are drawn as dark spheres. Disulfide bonds are included as stick models
both heavily glycosylated (between 5–9 glycosylation sites) and their structures are
stabilized by two (TvL) or three (MaL) disulfide bridges.
Fig. 2 shows the disposition of the copper atoms in MaL. The mononuclear site
contains one Cu T1, which is trigonally coordinated to two ND atoms from two
His residues and a SG atom from a Cys. Additionally, the T1 copper is connected
to the trinuclear cluster by a His-Cys-His tripeptide which is highly conserved
among the blue multicopper oxidases. The closest distance between the T1 and
T2/T3 coppers is about 12 Å. The type-1 centres of the blue multicopper family
usually have an additional axial ligand provided by a methionine sulphur atom
(i.e. ascorbate oxidase has a SD atom from a Met residue 2.9 Å away from Cu T1,
see Table 1). Fungal laccases, however, have a Phe or Leu residue at the equivalent
position (Phe for TvL and Leu for MaL). In the TvL and MaL structures, Phe and
Leu are 3.6 and 3.7 Å from the Cu T1, respectively, and do not participate in the
coordination. Furthermore, no residue occupies the axial position on the other side,
so this position is free for the substrate. Thus, the coordination – trigonal coplanar
- of the T1 copper in TvL or MaL (and in general in all fungal laccases) is different
from that of the rest of the blue multicopper family which consists of two histidines,
one cysteine and one axial methionine and is therefore 4-fold.
Axial coordination has been considered to be one factor affecting the redox
potential of copper enzymes. The geometry of the T2/T3 cluster is very similar
to that found in the crystal structure of ascorbate oxidase. The three coppers are
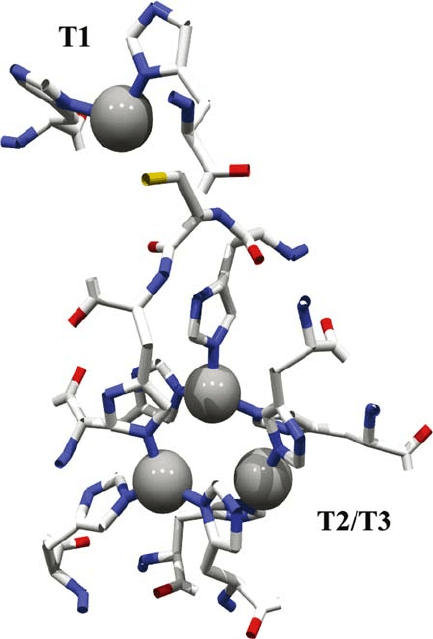
LACCASES 467
Figure 2. Geometry of the four copper atoms at the T1 and T2/T3 sites in the Melanocarpus albomyces
laccase structure (Hakulinen et al., 2002)
arranged in an almost perfect regular triangle. In this site two T3 coppers (Cu3 and
Cu4) are coordinated symmetrically to six His N atoms. The ligation of each of
these coppers is therefore 4-fold, and their coordination sphere can be best described
as being a distorted tetrahedron. Cu T2 is coordinated to two His N atoms and to
one atom that is probably a chloride ion. Cu T2 is more exposed and more labile
compared with the other two coppers at the T3 site. In this regard it is the T2 copper
site that is deficient in copper in the copper-depleted forms of both laccase and
ascorbate oxidase. Two channels, one broad and one narrow, facilitate access of
solvent molecules to the trinuclear site in ascorbate oxidase and also in MaL, CcL
and TvL. The narrow channel leads to the T2 copper. The broad channel, which is
approximately 10 Å long and leads to one of the type-3 coppers (Cu3), is located
between the trinuclear centre and the protein surface. It is worth noting that the
crystal structures reveal one exclusive feature in MaL that TvL does not have: a
468 ALCALDE
plug formed by the last four C-terminal residues of the polypeptide. This plug is
a consequence of the C-terminal processing common to other ascomycete laccases
(Kiiskinen and Saloheimo, 2004; Bulter et al., 2003). From crystallographic and
phylogenetic studies one can conclude that the active site and its environment
are structurally highly conserved. This is true for the copper geometry, for the
two channels which provide access for molecular oxygen and for the conserved
His-Cys-His tripeptide implicated in the electron transfer pathway between the T1
copper and the trinuclear cluster. This structural conservation reflects a common
mechanism for copper oxidation and O
2
reduction in these enzymes.
3.4. Catalytic Mechanism
Neither the electron transfer mechanism nor the oxygen reduction to water are fully
understood. However several facts are well established:
1) Laccases react oxidizing the reducing substrate by T1 Cu
2+
-mediated abstraction
of one electron. Consequently a free (cationic) radical is formed. This radical
can further undergo laccase-catalysed oxidation (e.g. phenol to quinone) or
non-enzymatic reactions (e.g. hydration or polymerisation).
2) As a one-electron substrate oxidation is coupled to the four-electron reduction
of oxygen the reaction mechanism cannot be entirely straightforward. Laccase
can be thought to operate as a battery, storing electrons from individual
oxidation reactions in order to reduce molecular oxygen. Hence the oxidation
of four reducing substrate molecules is necessary for the complete reduction of
molecular oxygen to water.
3) Each electron extracted from the four monoelectronic oxidations at the T1 site
is transferred to the trinuclear cluster where O
2
is bound. Thus the T2 and T3
sites are the locations where reduction of molecular oxygen and the release of
water occurs.
A “two-site Ping-Pong Bi-Bi” reaction mechanism has been established for laccase,
which means that products are released before the binding of new substrate occurs.
It appears that the solvent channels of the blue copper oxidases are well suited to
allow fast access of dioxygen molecules to the trinuclear cluster and subsequent
easy release of water. Although many catalytic schemes have been proposed for
this (Torres et al., 2003; Davies, 2002) the major unknown is the reductive part of
the cycle: the mechanism by which the trinuclear cluster is reduced.
4. BIOLOGICAL FUNCTIONS
Different physiological roles have been suggested for laccases, including plant
wounding response, the development of fruiting bodies, cell-wall reconstitution and
metabolic turnover of soil humic matter. Laccases are also believed to play roles in
pathogenesis (fungal virulence factors), sporulation, fungal spore pigmentation and
LACCASES 469
fungal morphogenesis in general. One of the most controversial and more studied
biological functions of laccases is related to the process of lignification of plant cell
walls and lignin polymerisation during white rot of wood.
Lignin, which is a structural component of the plant cell wall, is a heteroge-
neous and complex polyphenolic biopolymer that consists of phenyl propanoid
units linked by various non-hydrolysable C-C and C-O bonds. The considerable
abundance of wood-rotting Basidiomycete fungi as laccase producers seems to
indicate that the main role of fungal laccase is to depolymerise lignin. However, this
function contrasts with that of laccases in plants which are components of the lignin-
synthesizing system. Whereas enzymes in general are highly substrate specific, a
remarkable property of lignolytic enzymes is the breadth of their substrate range. The
most important lignin degrading enzymes are lignin peroxidases, manganases perox-
idases and laccases but some other enzymes such as celobiose:quinone oxidore-
ductase, cellobiose dehydrogenase, glyoxalate oxidase, glucose oxidases (glucose
1-oxidase and pyranose 2-oxidase), veratryl alcohol oxidase and some esterases
may also play roles in the complex process of natural wood decay. Consequently,
the lignin biodegradation process involves the synergistic effects of many enzymes
and non-enzymatic components (mediators) that interact to reach an equilibrium
between enzymatic polymerisation and depolymerisation. Indeed, some experi-
mental evidence suggests that laccase acting on lignin may display both activ-
ities. What is clear is that the enzymes assumedly involved in lignin cleavage
produce highly reactive (and hence highly toxic) species from which the fungal
mycelium must be protected. It might be that one of the functions of laccase is to
scavenge these compounds by promoting polymerisation before they can enter the
hypha. At the present however, the information in hand is insufficient for definite
conclusions to be reached on the role of laccases in the lignification/delignification
process.
5. INDUSTRIAL APPLICATIONS
Laccases are increasingly being used in a wide variety of industrial oxidative
processes such as delignification, dye or stain bleaching, bioremediation, plant-
fibre modification, ethanol production, biosensors, biofuel cells etc. Industrial
uses require overproduction of the enzyme, generally in a heterologous host, as
an indispensable prerequisite. Indeed, most commercial laccases are produced in
Aspergillus hosts. The functional expression of the Myceliophthora thermophila
laccase in S. cerevisiae by directed molecular evolution has been reported, which
enables this system to be tuned up for new and challenging applications (Bulter
et al., 2003). In recent work an efficient transformation and expression system
was developed for the basidiomycete Pycnoporus cinnabarinus and this was used
to transform a laccase-deficient monokaryotic strain with the homologous lac1
laccase gene. The yield obtained was as high as 1.2 g of laccase per litre and
represents the highest laccase production reported for recombinant fungal strains
(Alves et al., 2004).
470 ALCALDE
5.1. The Laccase-mediator System (LMS)
Over the last 10 years the versatility of laccase has been broadened by the invention
of the laccase-mediator system (LMS). The combination of the enzyme with
low molecular weight molecules such as 2 2
-azinobis (3-ethylbenzthiazoline-6-
sulfonate) (ABTS) or 1-hydroxybenzotriazole (HBT) not only leads to higher rates
in the conversion of known substrates but also adds new reactions for which laccase
alone had no or only marginal activity. The study of the LMS in the pulp-kraft
bleaching industry or in the removal of harmful xenobiotics (e.g. polycyclic aromatic
hydrocarbons, PAHs) is well documented (Call and Mucke, 1997). Generally, all
mediators are substrates of laccases (e.g. syringaldazine). They are easily oxidized
at the T1 site, in some cases producing very unstable and reactive cationic radicals
which can in turn oxidize more complex substrates. In this mechanism, the mediator
acts as a diffusible electron carrier (Majcherczyk and Johannes, 2000) permitting
higher molecular weight substrates such as lignin can be oxidised. The electrons
acquired by the laccase molecule are finally transferred to oxygen to form water
(at the T2/T3 trinuclear cluster). The action of a mediator is advantageous as
it enables laccases to achieve two goals: i) the oxidation of polymers by side-
stepping the inherent steric hindrance problems (enzyme and polymer do not have to
interact in a direct manner) and ii) increased substrate range, being able to oxidize
compounds having redox potentials exceeding their own. An effective mediator
does not necessarily have to have a redox potential higher than that of the laccase.
A potential higher than that of the substrate and free diffusiblity are generally more
important. It is also worth pointing out that the synergistic effects of mixtures of
mediators can improve the oxidation by laccases (Pickard et al., 1999). However,
chemical mediators are mostly toxic, unstable or expensive. Moreover, they lead to
by-products and inactivate the enzyme: by oxidizing the mediator, laccase is gener-
ating a strongly oxidizing intermediate the “co-mediator” which apart from acting
as a diffusible electron carrier also interacts with the laccase inactivating the biocat-
alyst. Novel approaches to overcome this shortcoming are currently being developed
(from searching for natural mediators e.g. tyrosine (Johannes and Majcherczyk, 2000;
Camarero et al., 2005) to the directed evolution of laccases (Bulter et al., 2003).
5.2. LMS in the Pulp and Paper Industry
The removal of lignin from woody tissues is a process that has attracted a very
great deal of research, especially due to its importance in the pulp and paper
industry. Bleaching of pulp for paper manufacture is carried out in some instance
using harmful and polluting chemicals. Traditional techniques utilize chlorine-based
agents – including Cl
2
- that can lead to the release of toxic contaminants to the
environment. Bourbonnais and Paice (1990) demonstrated for the first time that
a laccase from T. versicolor efficiently demethylated and delignified kraft pulp.
Since then a number of studies have been carried out analysing most of the factors
LACCASES 471
involved in the process (Call and Call, 2005; Sigoillot et al., 2005; Bajpai, 2004;
Call and Mucke, 1997). The application of laccases in pulp-kraft bleaching may
result in higher pulp yields and energy savings.
5.3. LMS and Polycyclic Aromatic Hydrocarbons
Polycyclic aromatic hydrocarbons (PAHs) are a class of highly dangerous xenobi-
otics (mutagenic, carcinogenic) widely distributed in terrestrial and aquatic environ-
ments. Laccase is able to oxidize both low molecular weight PAHs and the more
recalcitrant ones (i.e. benzo[a]pyrene) to more benign, less toxic compounds.
To exert this remarkable biotransformation, laccase needs the presence of redox
mediators (basically HBT and ABTS) (Alcalde et al., 2002; Majcherczyk and
Johannes, 2000; Majcherczyk et al., 1998; Collins et al., 1996; Johannes
et al., 1996). Natural mediators (tyrosine, 4-hydroxybenzoic acid, hydroxybenzilic
alcohol) secreted by white-rot fungi have also been demonstrated to be useful for
PAH oxidation to quinones (Johannes and Majcherczyk, 2000). It has been reported
that the biodegradation of PAHs and their metabolites increases with their oxidation
state. This suggests that enzymatic oxidation followed by the action of micro-
organisms could be an effective PAH remediation strategy. Additionally, quinones
are significantly less mutagenic and carcinogenic than their corresponding PAHs
(Torres et al., 2003).
5.4. Use of Laccases in Organic Solvents
Due to the hydrophobicity and low aqueous solubility of many laccase substrates
(e.g. lignin, PAHs, steroids), reactions are usually performed in the presence of
organic solvents (Torres et al., 2003; Gianfreda et al., 1999; Yaropolov et al.,
1994). However, under these conditions laccases are fairly unstable being either
denaturated or inhibited (Rodakiewicz-Nowak et al., 2000; Luterek et al., 1998). In
this regard strategies have been reported for improving the performance of laccases
in organic solvents e.g. enzyme immobilization (Duran et al., 2002) and directed
molecular evolution of laccases (Alcalde et al., 2005). In this latter approach the
Myceliophthora thermophila laccase expressed in S. cerevisiae was engineered by
in vitro evolution in the presence of increasing concentrations of acetonitrile and
ethanol. Screening was focused towards higher activity and stability. The turnover
rates of mutant enzymes at high concentrations of organic solvents were several
fold improved.
5.5. Enzymatic Bioremediation with Laccases
In addition to their effects on PAHs, laccases can diminish the toxicity of phenols,
trichlorophenols, organophosphorus pesticides and azo dyes, among others. Phenols
and their derivatives (chlorophenols, dimethoxyphenols and nitrophenols) are among
the most common organic chemicals found in industrial wastewaters and in sanitary
472 ALCALDE
waste sites. They can be oxidized by laccase (Ullah et al., 2000) resulting in the
generation of o-benzoquinones which are less toxic than phenols. Organophos-
phorus insecticides and nerve agents that contain the P-S bond are highly resistant
to enzymatic hydrolysis (Jauregui et al., 2003). Laccases from different sources
can perform their oxidation in the presence of chemical mediators (ABTS) (Amitai
et al., 1998). An ample range of azo dyes are oxidized by laccases in the
presence of mediators. The effect of HBT and ABTS has been extensively studied
(Almansa et al., 2004). The recent finding of natural mediators for dye bleaching open
new perspectives in the application of the LMS (Camarero et al., 2005).
5.6. Mediator-less Electroreduction of Oxygen to Water
Laccase is one of the few enzymes able to catalyse enzymatic and electrochemical
reactions even in the absence of a low molecular weight electron carrier (Call
and Mucke, 1997; Yaropolov et al., 1994). A cathode can be used to substitute
one of the substrates of the enzymatic reaction as an electron donor. Electrons
are therefore taken by the Cu T1 of the enzyme directly from the electrode. The
reaction is called mediator-less electroreduction which runs without the formation of
hydrogen peroxide as an intermediate. The electrode potential established as a result
of the reaction is close to the equilibrium oxygen potential. The overall reaction
is the electroreduction of molecular oxygen to water. The most effective inorganic
catalyst for this reaction is a specially treated form of platinum (equilibrium oxygen
potential: 1.23 V). In contrast, laccase immobilized on the surface of electrodes
of various materials is able to change the potentials in the range of 1.2–0.6V
depending on the amount of enzyme fixed. It should be noted that the Trametes
versicolor laccase does not catalyse water decomposition at potentials greater than
1.2 V. This is due to the irreversible inactivation of the laccase at higher poten-
tials by overoxidation of catalytically important groups of the enzyme. A number
of potentiometric biosensors based on this principle have been developed (Freire
et al., 2003; Freire et al., 2002; Milligan and Ghindilis, 2002). Another signif-
icant application is in the engineering of biofuel cells (Shleev et al., 2005).
One of the most brilliant works done in this field reported a miniature biofuel
cell made from two 7m-diameter, 2cm long carbon fibre electrodes, glucose
oxidase being coupled to the anode and laccase immobilized on the cathode
(Chen et al., 2001).
5.7. Medium Density Fibreboards (MDF)
Laccases are being used in the enzymatic cross-linking of lignin-based materials to
produce medium density fibreboards (MDF) which are nowadays employed in the
construction of furniture. Formerly the manufacture of MDF was based on non-
enzymatic cross-linking by harmful and polluting compounds such as formaldehyde
(Widsten et al., 2004; Felby et al., 2002).
