Peube J-L. Fundamentals of fluid mechanics and transport phenomena
Подождите немного. Документ загружается.


Thermodynamics of Continuous Media 53
exterior
interior
1
2
n
d
s
dsTfd
21
G
G
o
(a) (b)
(c)
x
1
x
2
x
3
O
V
32
V
31
V
33
7
3
V
11
7
1
V
22
V
21
V
23
7
2
V
V
12
V
V
V
13
V
12
Figure 2.3. Definition and symmetry of the stress tensor
The force density at the point M on the surface
ds
is the
stress
T
:
n
ds
fd
tMT
G
.),(
V
Figure 2.3b shows the stresses on the faces of a parallelepiped whose corners are
parallel to the axes. The reader can easily verify that in the absence of volume sources of
torques, the equality of the moments about the axis
Ox
3
of the stresses exerted on the
four faces parallel to the axis
Ox
3
(see Figure 2.3c) leads to
2112
V
V
. The same goes
for the other components of the stress tensor (
jiij
V
V
).
2.1.3.3.
Volume source equivalent to the fluxes
The balances in a material domain are clearly always effected
on closed surfaces
which comprise the boundary. By applying the Ostrogradski theorem the flux
6
M
G
of
the quantity
G
leaving a closed surface
6
can be written:
¸
¸
¹
·
¨
¨
©
§
w
w
³³³
6
6
D
i
Gi
D
G
GG
dv
x
q
dvqdivdsnq
GG
.
M
[2.6]
This flux can thus be written in the form of a volume integral, which implies
that the total flux of the quantity
G
on a closed surface is equivalent to the
action of the
volume source
G
qdiv
of the quantity G.
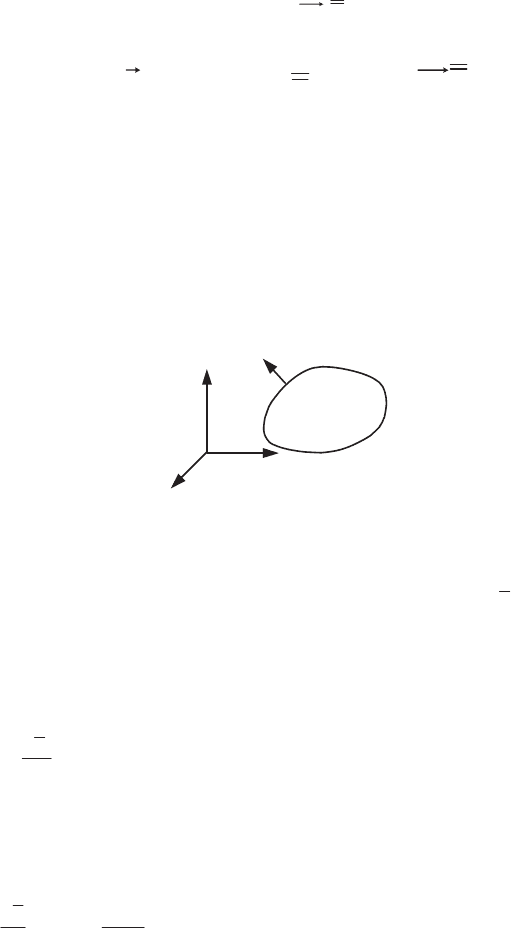
54 Fundamentals of Fluid Mechanics and Transport Phenomena
In the case of stresses in a continuous medium, a force volume source
generated by a stress is equal to the vector
V
div
:
³³³
66 D
dvdivdsndstMT
VV
G
.),(
Reciprocally, all
volume sources
which are mathematically expressed by means of a
divergence operator
(
qdiv
G
for instance) can be interpreted as a transfer by the flux
across a closed surface of vector flux density
q
G
.
2.1.4. Balance equations in continuous media
2.1.4.1
. Balance equation of an extensive quantity
x
1
x
2
x
3
O
n
G
D
6
Figure 2.4. Balance of an extensive quantity
The balance equation of a volumetric extensive quantity
g
consists of
writing that the variation of this quantity in a material domain
D
is due to
contributions from outside, and which have thus crossed the closed surface
6
which constitutes the boundary of
D
(the normal is directed outwards), and to
volume sources of density
V
G
in
D
:
dsnqdvdv
t
g
jGj
D
G
D
³³³
¦
w
w
V
[2.7]
Supposing this relation to be true regardless of the domain
D
, we can deduce the
local equation:
j
Gj
G
x
q
t
g
w
w
w
w
V
[2.8]
Thermodynamics of Continuous Media 55
For a fixed medium, which can thus not be deformed, mass
1
must be strictly
conserved, and we have:
0
w
w
t
U
Balance equation [2.8] for the quantity
G
can also be written, using the mass
quantity
g
:
j
Gj
G
x
q
t
g
w
w
w
w
VU
[2.9]
For a fixed continuous medium, the principal extensive quantities are internal
energy (in its thermal form, and in the absence of possible physicochemical
reactions), volume and the number of moles of a chemical species. Electric
quantities may also be manifested; however, we will not deal with such questions in
this work.
For a material of constant
specific heat C
, in the absence of chemical reactions,
the variation of volumetric internal energy
de
is equal to
U
CdT
, and the balance
equation can be written:
j
Tj
T
x
q
t
T
C
w
w
w
w
VU
[2.10]
Heat-source volume
V
T
is in fact a residuum of another form of energy which is
present in the mass and which is degraded in the form of heat (Joule effect caused by
the passage of an electric current (Ohm’s law), absorption of electromagnetic
radiation, etc.). This result could be obtained in a general way, but such a discussion
is beyond the scope of this work. We will come back to this question in Chapters 3
and 4 when it comes to dealing with mechanical energy.
For a chemical species comprising a number of moles
n
i
per unit volume (molar
concentration), the balance equation can be written:
j
ijn
in
i
x
q
t
n
w
w
w
w
VU
[2.11]
1
Excepting diffusion processes, for which we only give volumic balances in this chapter
(section 2.4.4.2.2).
56 Fundamentals of Fluid Mechanics and Transport Phenomena
The molar flux densities q
ni
and the sources
V
ni
of chemical species are due,
respectively, to diffusion phenomena and chemical reactions.
2.1.4.2. Entropy source
During the natural evolution of a system, entropy is not conserved and it can only
increase. We have already described the entropy-creation mechanism in the case of two
discrete sub-systems that are in contact and have different temperatures (section 1.4.2.3)
and (section 1.4.2.4). The zone where entropy is created was localized in the contact
zone between the two sub-systems. Here the distribution of entropic variables is
continuous, and entropy creation will be diffused and associated with the existence of
gradients with entropic variables.
On account of the local equilibrium hypothesis, relation [1.13] can be written, for the
per mass unit quantities:
t
g
Z
t
e
Tt
s
i
i
w
w
w
w
w
w
U
U
U
[2.12]
By replacing the derivatives
t
g
i
w
w
U
in equation [2.12] by expressions obtained using
balance equations [2.9], the following relation can be obtained:
>@
i
k
Gki
i
Gkik
Gkk
i
Gki
Gkk
x
Z
q
x
qZ
Z
t
e
T
x
q
Z
t
e
Tt
s
w
w
w
w
w
w
¸
¸
¹
·
¨
¨
©
§
w
w
w
w
w
w
V
U
V
U
U
[2.13]
in which the surface flux terms associated with the divergence operator have been
separated from those associated with the volume source as defined in section 2.1.3.3.
The entropy is associated with the extensive quantities and the corresponding entropy
addition are represented by two terms:
–
SGkk
Z
V
V
external entropy source associated with the source
Gk
V
of the
extensive quantity
Gk;
–
SGkik
ZqZ
entropy flux associated with the flux of extensive quantities.

Thermodynamics of Continuous Media 57
The thermodynamic imbalance is characterized by the gradients
i
Zgrad
, which
we designate under the label
thermodynamic forces. The additional term
i
k
Gki
x
Z
q
w
w
of equation [2.13] is the
entropy source associated with the local macroscopic
imbalance
. It is always positive. Its expression is analogous to the expression for the
creation of entropy
21
2,1
ZZ
dt
dX
from formula [1.31].
2.1.5. Phenomenological laws
2.1.5.1. Introduction
An irreversible evolution is characterized by flux density fields of the quantity
Gk
q
G
in a continuous medium. These thermodynamic fluxes are associated with gradients of
intensive entropic quantities
Z
i
. The transfer of a quantity G
i
can occur via relatively
different kinds of processes:
1)
Molecular agitation leads to an exchange of extensive quantities between the
particles involved. These intermolecular actions occur from place to place, because it is
these microscopic particles themselves which transport the extensive quantity considered
(mass, momentum, energy, chemical species, etc.). The interaction zone between two
material domains
D
1
and D
2
separated by the surface S (Figure 2.5) is limited to a
thickness in the order of 2d (d: intermolecular distance in liquids or mean free path in
gases). This extremely thin zone is modeled on the macroscopic scale by the surface
S,
on which we can consider
contact actions.
2
d
S
D
1
D
2
Figure 2.5. Interaction zone between material domains D
1
and D
2
2) On the macroscopic scale, and for turbulent flows, we observe chaotic
velocity fluctuations which lead to fluxes of extensive quantities convected by the
fluid. The effective interaction zone between two material domains is no longer
limited to a surface as before. The flux depends on the structure of the whole
turbulent zone. Considerable difficulties result from this situation in which the cause
of the flux of an extensive quantity can no longer be modeled with a general local
phenomenological law ([COU 89], [MAT 00], [TEN 72]).

58 Fundamentals of Fluid Mechanics and Transport Phenomena
3) In other situations, the flux of an extensive quantity (essentially energy) is due
to the presence of an electromagnetic field. This is the case for energy transfer by
thermal radiation in semi-transparent media, which both emit and absorb at all
points and whose local state results from the emission balance in a macroscopic
volume surrounding the point considered. Here again, we can no longer localize the
cause of the extensive quantity flux on a single surface.
2.1.5.2.
Contact actions and thermodynamic forces
The interaction zone between two material domains D
1
and D
2
is modeled by the
surface
S. The thermodynamic forces, represented by
i
Zgrad
, are the cause of
thermodynamic fluxes.
As with discrete systems (section 1.4.2.6)
all causes of the same tensorial nature
act on
all the corresponding effects and we have a coupling of irreversible
phenomena
: for example, a temperature gradient leads to a material flux (thermal
diffusion). Phenomena of different tensorial orders do not interact.
A rudimentary explanation of these facts can be provided from context of kinetic
gas theory. A gas is a set of molecules which are subjected to a thermal agitation.
Irreversible phenomena are the macroscopic result of this
spontaneous action.
Molecules with different properties (mass, type, kinetic energy, etc.) do not respond
in the same way to non-symmetries in the mean properties of the medium. A
molecular concentration of a given species will be progressively diluted in the rest
of the gas; a temperature gradient (gradient of the molecular kinetic energy) will not
act in the same way on different species of molecules and so may create a
concentration gradient. For example, at equal energy, we notice that smaller, and
therefore faster, molecules can slip in a gas comprising larger molecules, hence the
phenomenon of
thermal diffusion. On the other hand, it is difficult to see how the
static scalar properties of a gas which is macroscopically at rest can spontaneously
generate a vector macroscopic momentum (i.e. a bulk movement) in the absence of
an external influence.
There thus exists a relation between thermodynamic forces and thermodynamic
fluxes of the same tensorial rank. Since in the absence of thermodynamic forces, the
thermodynamic fluxes are zero, the general form of this relation can be written as:
( , 1,..., ) with: 0,0,... 0
ki
lGk
qFgradZ kl K F
GJJG JJJJJG JJGGG G
[2.14]
On account of the principle of action and reaction, the function
k
F
is odd
(
l
k
l
k
ZgradFZgradF ). Relation [2.14] must verify properties of

Thermodynamics of Continuous Media 59
homogeneity and of spatial and material isotropy. In what follows, we will limit our
discussion to cases where the difference from thermodynamic equilibrium is
relatively small, so that we can justify a first order Taylor expansion of the functions
lk
ZgradF
G
:
lkll
k
ZgradLZgradF
For a set of
K extensive quantities,
kl
L
is a square matrix of order K whose
terms are functions of the values of the
K intensive quantities Z
k
at the point Z
ke
,
about which linearization is effected. When the preceding approximation is valid,
we consider that we are dealing with
linear thermodynamics of irreversible
phenomena.
If the medium is considered to be isotropic, the matrix is reduced to a matrix of
dimension
K (see section 2.1.5.3.1).
Thermodynamics does not provide access to any properties associated with the
matrix
kl
L
. However, by means of statistical reasoning the matrix can be shown to
be symmetric (Onsager’s relations). This
symmetry is only verified if the entropic
variables Z
k
are used to define this matrix.
In practice, the local imbalance is characterized using simpler variables than the
intensive entropic variables
Z
k
. For example, we use temperature in place of the
entropic intensive variable
T1
(section 1.3.1.1). Expressions for linearized
thermodynamic force are equivalent to first order, but the matrix coefficients
ij
L
are
modified, such that the symmetric properties generally disappear. An analogous
situation has been observed in the case of discontinuous media (section 1.4.2.6.2).
2.1.5.3.
Some simplifying laws for irreversible transfer
2.1.5.3.1. Fourier’s law and thermal conduction
Consider firstly a case where the medium comprises a pure body, such that
thermal transfer occurs alone, without any coupling with diffusion or electrical
conduction. The relation between the thermal flux density and the thermal gradient
can be written in the context of linear thermodynamics (Fourier’s law) as:
.or
Ti ij
T
j
T
qgradT q
x
s
s
G JJJJJG
ȜȜ
The principal axes of the 3*3 tensor
ij
O
are the symmetric axes of the medium.
If the medium is homogenous (fluids, non-crystalline solids, etc.), the three

60 Fundamentals of Fluid Mechanics and Transport Phenomena
eigenvalues of the tensor
ij
O
are equal and this one is spherical (
ijij
OG
O
); it can
thus be expressed solely as a function of the
thermal conductivity
O
of the medium.
Fourier’s law can be written as:
Tgradq
x
T
q
T
i
Ti
OO
w
w
[2.15]
The thermal conductivity of the medium
O
is expressed in Watts/meter.K.
Heat is the macroscopic form of mechanical energy related to thermal agitation
of a medium. Its transfer in the medium is due to interactions between the
microscopic entities (molecules, atoms, ions, electrons). The values of the
coefficient
O
depends on the nature of the medium.
Metallic media are excellent thermal conductors, thermal conduction being
principally assured by electrons which have a high mobility. The values of the
coefficient
O
lie in a range spanning from a few tens to a few hundred W/m.K.
Solid crystalline media are generally good conductors: as the crystalline structure
presents a reasonably strong coherence on account of its organization, energy can be
transmitted in a vibrational form (phonons). The coefficient values
O
are in the order
of a few W/m.K.
Solid amorphous media or composites have weaker conductivity: well under 1
W/m.K for fibrous materials.
Liquids, comprising a looser structure, are poorer conductors of heat than solids
(of the order of 0.1 to 0.2 W/m.K): intermolecular forces here assure conduction.
Energy exchange in
gases only occurs by means of molecular collisions in the
gaseous medium; the corresponding values of the coefficient
O are of the order of
0.01 to 0.02 W/m.K. Insulating materials are constituted of a matrix (fibers, wools,
foams, etc.) which is as light as possible, thermal insulation being assured by the
gaseous interstices.
The thermal flux across a surface
S can thus be written as:
³³³
w
w
SSS
TT
ds
n
T
dsnTgraddsnq ...
OOM
GGG
We can also define the
thermal diffusivity a:

Thermodynamics of Continuous Media 61
C
a
U
O
The reader can easily verify that this quantity
a can be expressed in m
2
/sec; we
will see the important role that this quantity plays in the heat equation (section
2.3.1).
The thermal diffusivity takes on the following values at 20ºC:
– air: 0.19 × 10
-4
m
2
. s
-1
;
– water: 1.4 × 10
-7
m
2
. s
-1
;
– metals: ~ 10
-4
m
2
. s
-1
.
2.1.5.3.2. Fick’s law and the diffusion of chemical species
As the extensive variable is here the number of molecules of a chemical species,
the corresponding intensive entropic variable is
T
P
(where μ is the chemical
potential (section 1.3.1.1)). In ideal solutions or perfect gases, we replace the
intensive entropic variables with a molar or mass concentration variable. This
simplified approach will suffice here for an exposition and discussion of the
phenomena which we will examine. It will allow us to outline the principal
difficulties associated with fluid movement. The additional complications which
arise when we take account of more complex thermodynamic and chemical
properties are beyond the scope of this work.
The presence of a concentration gradient leads to the existence of a molecular
flux towards regions of weaker concentration. Taking the molecular concentration
n
1
(the number of moles per unit volume) of a component, Fick’s law, which
characterizes the diffusion of the species considered, can be written:
1
1
ngradDq
n
[2.16]
where
D is the diffusion coefficient.
Diffusion is a complex phenomenon, as it implies that the material is not
immobile, and in such cases it is not possible to effect a generalized reasoning
using a single constituent. We will come back to this point at a later stage
(section 2.4), and we will specify the conditions under which law [2.16] is valid.

62 Fundamentals of Fluid Mechanics and Transport Phenomena
2.1.5.3.3. Electrical conduction and Ohm’s law
Electric conduction presents certain analogies with thermal conduction and
diffusion; however, the underlying physics is a little different. Here we are dealing
with an electric field derived from an electric potential
el
V which exerts a force on
mobile electric charge carriers. Ohm’s law has the same form as the preceding laws:
elel
Vgradj
V
where
j
G
is the electric current density and
el
V
is the electrical conductivity.
Thermal molecular agitation, which grows with temperature, slows down the
movement of electric charge-carriers because of collisions; thus, electrical
conductivity is a decreasing function of temperature, whereas for other irreversible
phenomena, thermal agitation is the driving factor of thermal and diffusional fluxes.
2.1.5.3.4. General case: coupled transfer between diffusion and thermal
conduction
In general, the flux of a scalar quantity (energy, chemical species) depends on all
the local thermodynamic forces
,,,etc.,
i
grad T grad n grad p
JJJJJG JJJJJG JJJJJG
associated with these
scalar quantities.
We can schematically write under certain conditions the following relations for
the diffusion of a constituent of binary mixing in the presence of heat transfer:
11
1
1
ngradD
T
Tgrad
Dq
ngradKTgradq
T
n
T
O
The coefficient
T
D
1
characterizes the thermal diffusion (Soret effect), i.e. the
existence of a flux of chemical species which is associated with a temperature
gradient. The symmetric effect of a heat flux caused by a concentration gradient
(Dufour effect) and characterized by the coefficient
K is generally much weaker.
From a physical point of view, the problem is more complex than this, as each
chemical species introduces its own thermal energy (enthalpy at constant pressure,
etc.). Definitions of the preceding coefficients may vary, and we will not provide a
complete discussion of these phenomena, as this would require lengthy discussions
concerning chemical thermodynamics ([BIR 01], [DEG 62], [PRI 68]).
