Pecharsky V.K., Zavalij P.Y. Fundamentals of Powder Diffraction and Structural Characterization of Materials
Подождите немного. Документ загружается.

Chapter
I
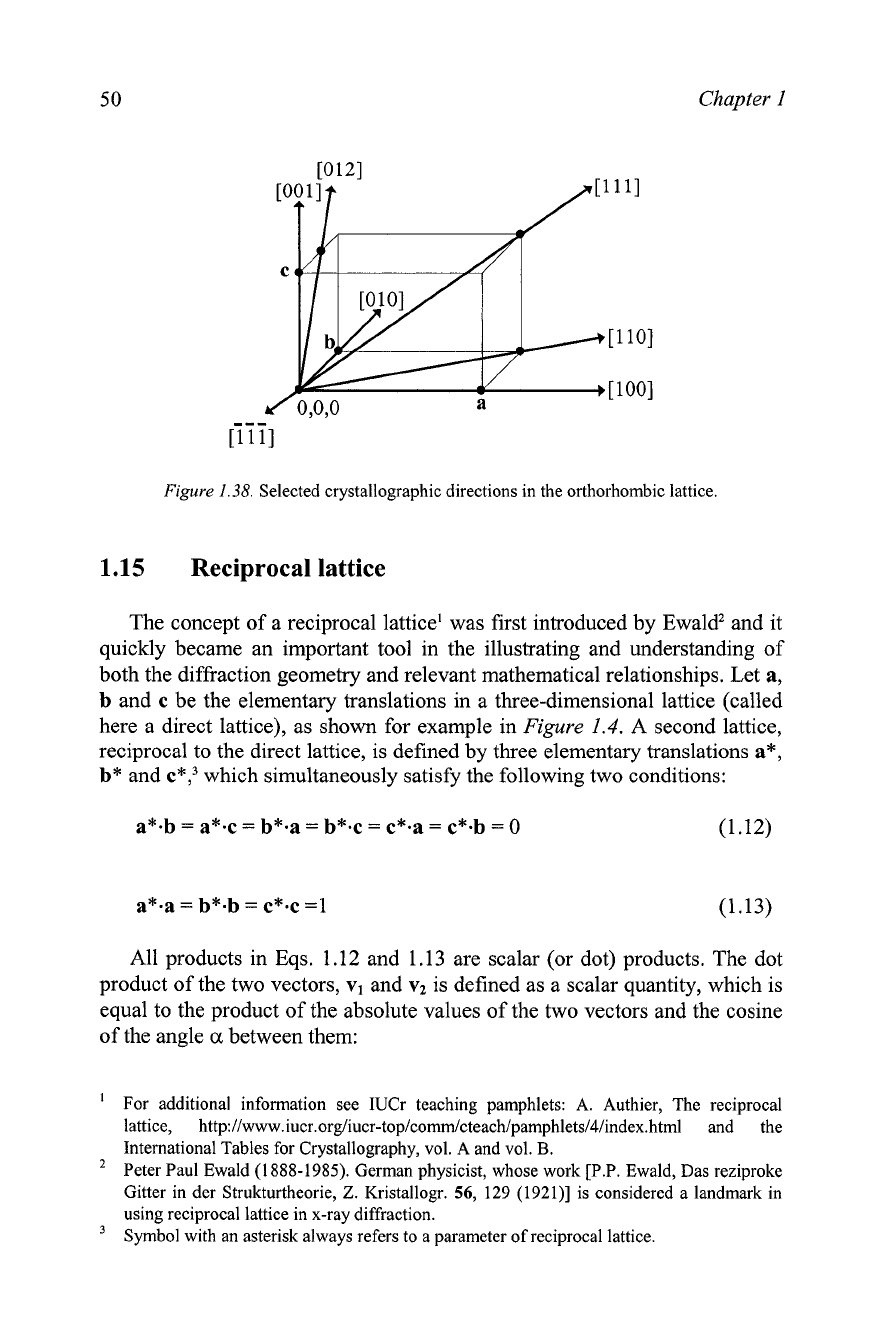
Figure
1.38.
Selected crystallographic directions in the orthorhombic lattice.
1.15
Reciprocal lattice
The concept of a reciprocal lattice' was first introduced by Ewald2 and it
quickly became an important tool in the illustrating and understanding of
both the diffraction geometry and relevant mathematical relationships. Let a,
b
and c be the elementary translations in a three-dimensional lattice (called
here a direct lattice), as shown for example in
Figure
1.4.
A second lattice,
reciprocal to the direct lattice, is defined by three elementary translations a*,
b*
and c*,~ which simultaneously satisfy the following two conditions:
All products in Eqs. 1.12 and 1.13 are scalar (or dot) products. The dot
product of the two vectors,
vl
and
vz
is defined as a scalar quantity, which is
equal to the product of the absolute values of the two vectors and the cosine
of the angle
a
between them:
'
For additional information see IUCr teaching pamphlets: A. Authier, The reciprocal
lattice,
http://www.iucr.org/iucr-top/comm~cteach/pamphlets/4/index.html
and the
International Tables for Crystallography, vol. A and vol.
B.
Peter Paul Ewald (1888-1985). German physicist, whose work [P.P. Ewald, Das reziproke
Gitter in der Strukturtheorie,
Z.
Kristallogr.
56,
129 (1921)l is considered a landmark in
using reciprocal lattice in x-ray diffraction.
Symbol with an asterisk always refers to a parameter of reciprocal lattice.
Fundamentals of crystalline state
5
1
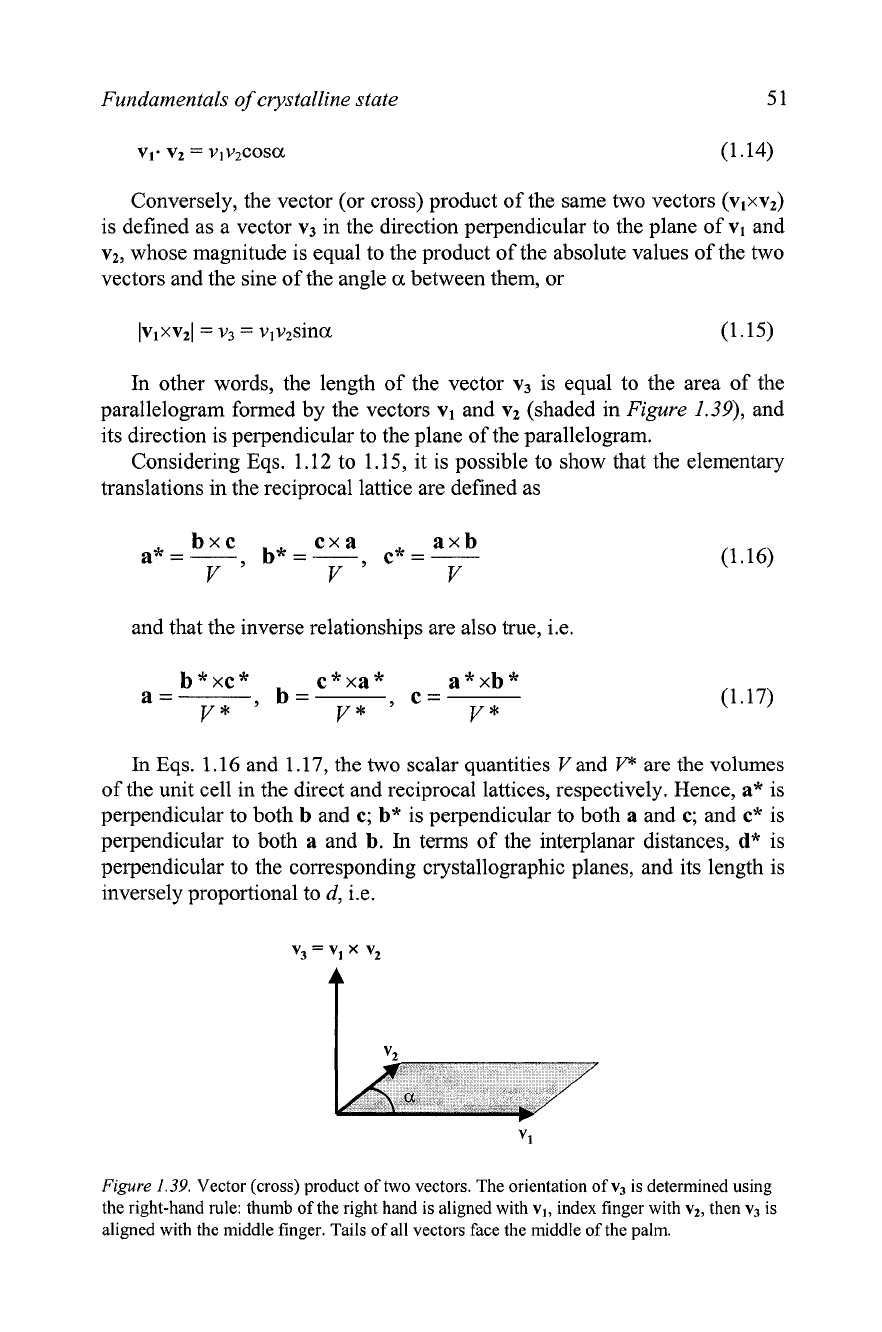
Conversely, the vector (or cross) product of the same two vectors (vlxv2)
is defined as a vector v3 in the direction perpendicular to the plane of vl and
vz, whose magnitude is equal to the product of the absolute values of the two
vectors and the sine of the angle
a
between them, or
In
other words, the length of the vector v3 is equal to the area of the
parallelogram formed by the vectors vl and vz (shaded in
Figure
1.39),
and
its direction is perpendicular to the plane of the parallelogram.
Considering Eqs. 1.12 to 1.15, it is possible to show that the elementary
translations in the reciprocal lattice are defined as
bxc cxa axb
a*=__ b*=-,
c*=-
v
v v
and that the inverse relationships are also true, i.e.
In
Eqs.
1.16
and
1.17,
the two scalar quantities
V
and
P
are the volumes
of the unit cell in the direct and reciprocal lattices, respectively. Hence,
a*
is
perpendicular to both
b
and
c; b*
is perpendicular to both
a
and
c;
and
c*
is
perpendicular to both
a
and
b.
In
terms of the interplanar distances,
d*
is
perpendicular to the corresponding crystallographic planes, and its length is
inversely proportional to
d,
i.e.
Figure
1.39.
Vector (cross) product of two vectors. The orientation
of
v3
is determined using
the right-hand rule: thumb of the right hand is aligned with
vl,
index finger with
vt,
then
v3
is
aligned with the middle finger. Tails
of
all vectors face the middle
of
the palm.
5
2
Chapter
I
An important consequence of Eq. 1.18 is that a set, which consists of an
infinite number of crystallographic planes in the direct lattice, is replaced by
a single vector or by a point at the end of the vector in the reciprocal lattice.'
Furthermore, Eqs. 1.16 and 1.17 can be simplified in the orthogonal crystal
systems to
a*
=
lla, b*
=
llb, c*=llc (1.19)
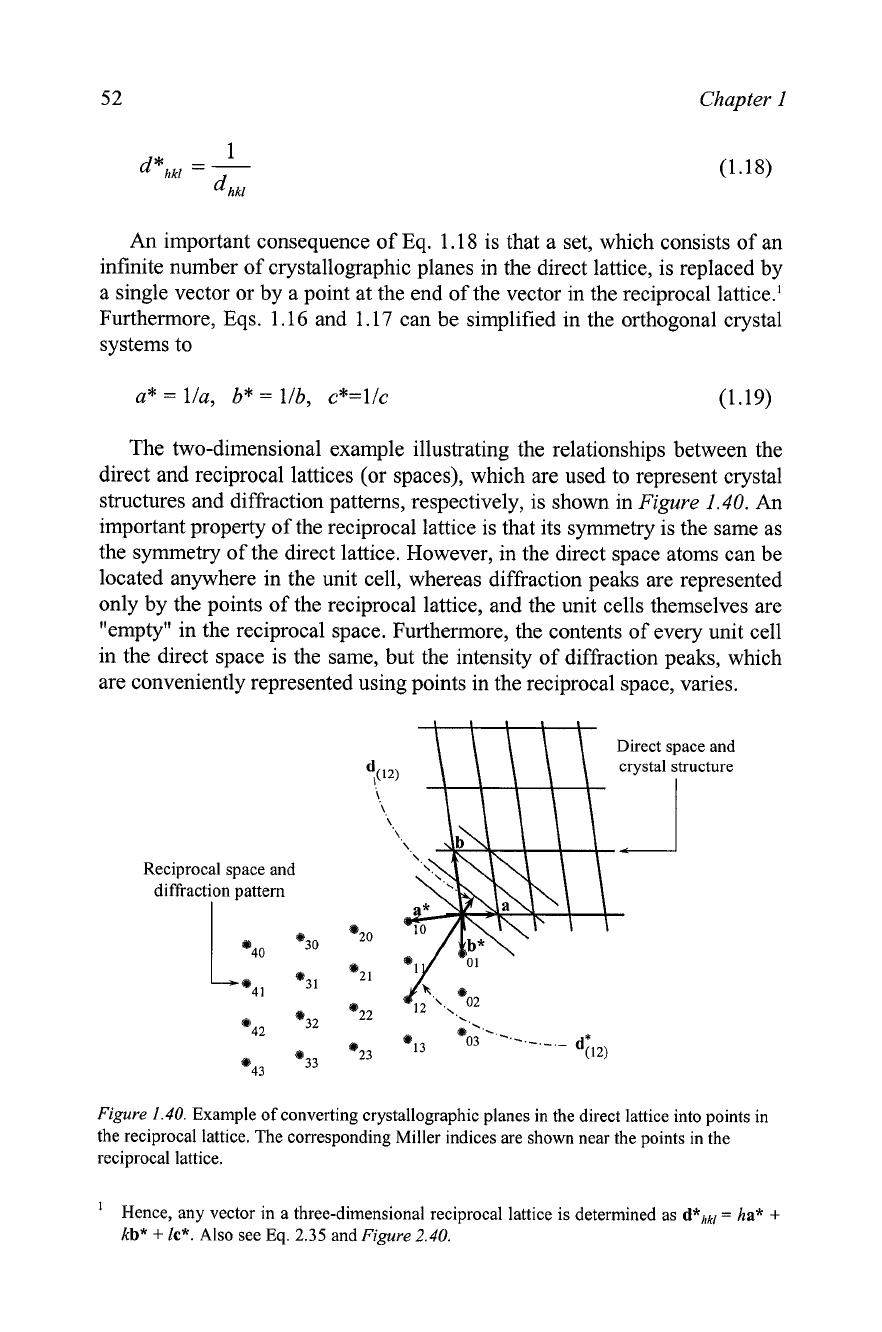
The two-dimensional example illustrating the relationships between the
direct and reciprocal lattices (or spaces), which are used to represent crystal
structures and diffraction patterns, respectively, is shown in
Figure
1.40.
An
important property of the reciprocal lattice is that its symmetry is the same as
the symmetry of the direct lattice. However, in the direct space atoms can be
located anywhere in the unit cell, whereas diffraction peaks are represented
only by the points of the reciprocal lattice, and the unit cells themselves are
"empty" in the reciprocal space. Furthermore, the contents of every unit cell
in the direct space is the same, but the intensity of diffraction peaks, which
are conveniently represented using points in the reciprocal space, varies.
Reciprocal space and
diffraction pattern
L
a,,
'30
CP41
'31
@42
*32
0S43
@33
Xrect space and
$12)
\ \ \ \ \
crystal structure
Figure
1.40.
Example of converting crystallographic planes in the direct lattice into points in
the reciprocal lattice. The corresponding Miller indices are shown near the points in the
reciprocal lattice.
'
Hence, any vector in a three-dimensional reciprocal lattice is determined as
d*hkl
=
ha*
+
kb*
+
lc*.
Also see
Eq.
2.35
and
Figure
2.40.

Fundamentals
of
crystalline state
53
1.16
Crystallographic space groups
Similar to finite symmetry elements, which can be combined in point
groups (see section 1.10), various combinations of the same symmetry
elements plus allowed translations, while obeying the rules described in
section 1.7, result in the so-called crystallographic space groups. As follows
from the differences in their names, symmetry elements in a space group are
spread over the space of an infinite (continuous) object, contrary to point
groups, where all symmetry elements have at least one common point.
Therefore, each point of a continuous object can be moved in a periodic
fashion through space by the action of symmetry elements that
form a space
group symmetry, whereas at least one point of the object remains unmoved
by the action of symmetry elements that belong to a point group symmetry.
Given the limitations on the allowed rotations and translations (see
sections 1.4 and 1.13), there is a total of 230 three-dimensional
crystallographic space groups,' which were derived and systematized
independently by Fedorov2 and S~honflies.~ A complete list of space groups
is found in
Table
1.1
7,
where they are arranged according to seven crystal
systems (see section 1.8) and
32
crystallographic point groups (see section
1.10).
1.16.1
Relationships between space and point groups
The symbols of 230 crystallographic space groups, which are found in
Table
1.1
7,
are known as short international or short Hermann-Mauguin
symbols. They are based on the symbols of the corresponding point groups.
The orientation of symmetry elements with respect to the three major
crystallographic axes in a space group is the same as in the parent point
group (see
Table
1.8) and it depends on the position of the element in the
symbol. The rules that govern space group symbolic are quite simple:
When vector directions (e.g. magnetic moments) are included into consideration, the
number of space groups increases dramatically. Thus, a total of 1651 dichromatic (or
Shubnikov) symmetry groups are used to treat symmetry of magnetically ordered
structures. See A.V. Shubnikov and V.A. Koptsik, Symmetry in science and art, Plenum
Press (1974) for a brief description of color symmetry groups. A complete treatment of
dichromatic groups is found in
V.A.
Koptsik, Shubnikov groups, Moscow University
Press (1966).
Evgraf Stepanovich Fedorov (1 853-1919). Russian mineralogist and crystallographer who
by applying the theory of finite groups to crystallography derived 230 space groups in
1891. Fedorov's last name is spelled as Fyodorov or
Fedoroff in some references.
Arthur Moritz Schonflies (1853-1928). German mathematician who derived
230
space
groups independently of E.S. Fedorov in 1891. See
http:Nwww-gap.dcs.st-and.ac.uk/
-history/Mathematicians/Schonflies.html
for a brief biography.
54
Chapter
I
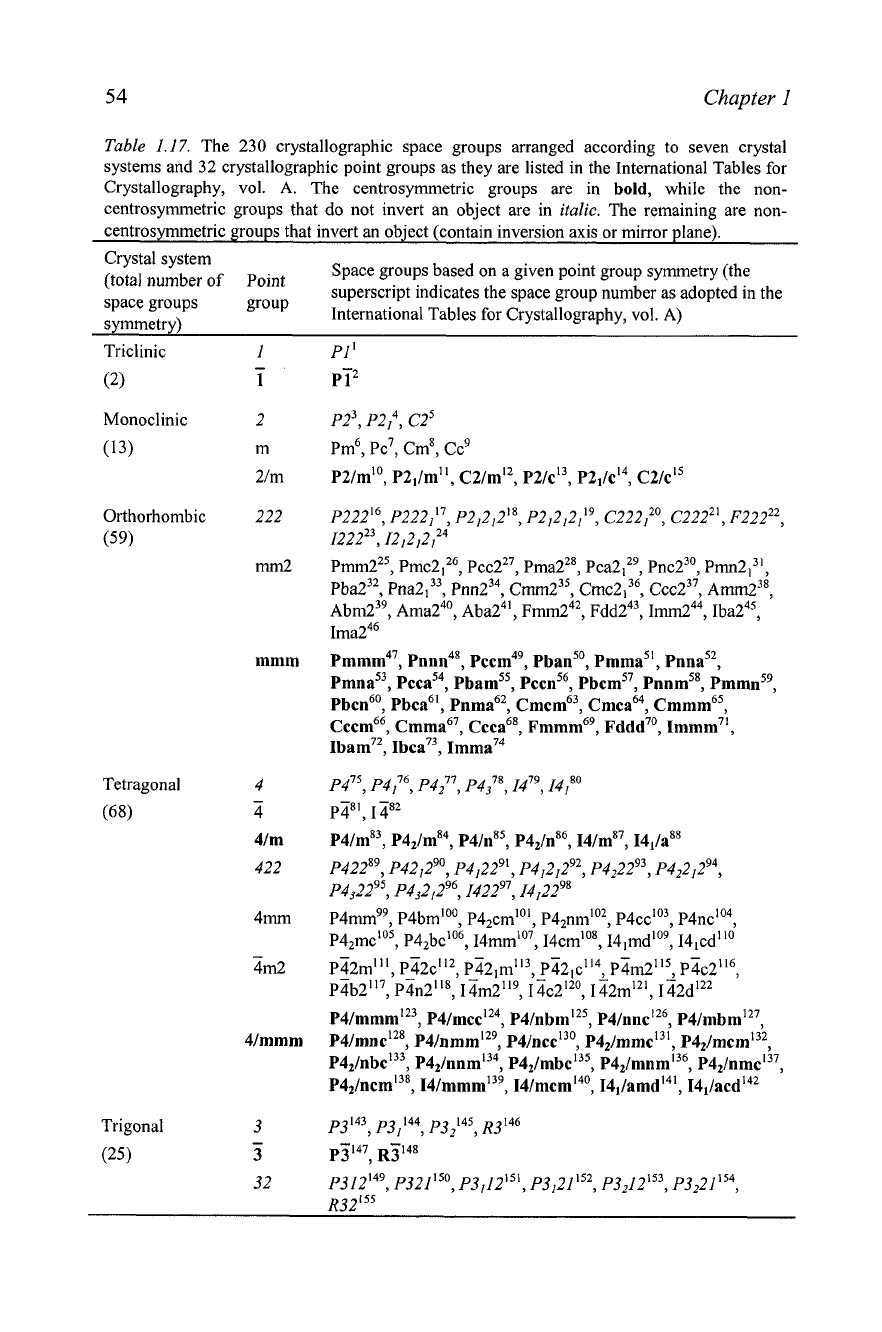
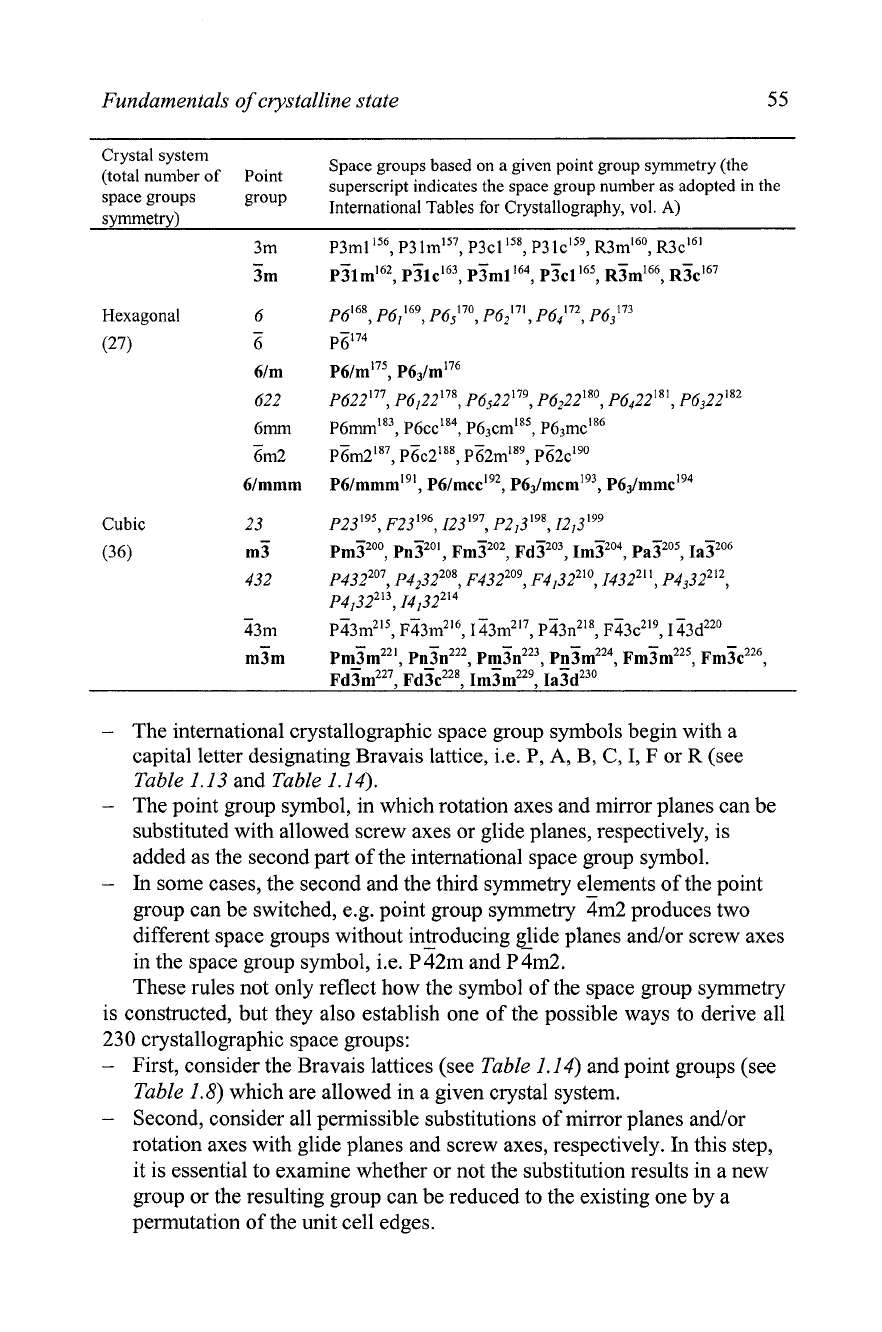
Table
1.17.
The
230
crystallographic space groups arranged according to seven crystal
systems and
32
crystallographic point groups as they are listed in the International Tables for
Crystallography, vol. A. The centrosyrnmetric groups are in
bold,
while the non-
centrosymmetric groups that do not invert an object are in
italic.
The remaining are non-
centrosymmetric groups that invert an object (contain inversion axis or mirror plane).
Crystal system
(total number of Point
Space groups based on a given point group symmetry (the
superscript indicates the space group number as adopted in the
space groups
symmetry)
International Tables for Crystallography, vol. A)
Triclinic
(2)
Monoclinic
(13)
Orthorhombic
(59)
Tetragonal
(68)
Trigonal
(25)
I
-
1
2
m
2/m
222
mrn2
mmm
4
-
4
4/m
422
4mm
-
4m2
Wmmm
3
-
3
32
Fundamentals
of
crystalline state
55
Crystal system
Space groups based on a given point group symmetry (the
(total number of Point
superscript indicates the space group number as adopted in the
space groups
symmetry)
International Tables for Crystallography,
vol.
A)
3m
-
P3m1 '56, P3 ~m'~~, P3c1
15',
~31c'~~, R3mI6O, R3cI6'
3
m
~31m'~', P~ICI~~, ~?ml'~~, ~?cl'~~, ~3m~~~,
~5'~~
Cubic
(36)
-
The international crystallographic space group symbols begin with a
capital letter designating Bravais lattice, i.e.
P,
A,
B,
C,
I,
F
or
R
(see
Table
1.13
and
Table
1.14).
-
The point group symbol, in which rotation axes and mirror planes can be
substituted with allowed screw axes or glide planes, respectively, is
added as the second part of the international space group symbol.
-
In
some cases, the second and the third symmetry elements of the point
group can be switched, e.g. point group symmetry am2 produces two
different space groups without introducing glide planes
andlor screw axes
in the space group symbol, i.e. ~42m and ~-4rn2.
These rules not only reflect how the symbol of the space group symmetry
is constructed, but they also establish one of the possible ways to derive all
230 crystallographic space groups:
-
First, consider the Bravais lattices (see
Table
1.14)
and point groups (see
Table
1.8)
which are allowed in a given crystal system.
-
Second, consider all permissible substitutions of mirror planes and/or
rotation axes with glide planes and screw axes, respectively.
In
this step,
it is essential to examine whether or not the substitution results in a new
group or the resulting group can be reduced to the existing one by
a
permutation of the unit cell edges.

Chapter
I
For example, think about the monoclinic point group m in the standard
setting, where m is perpendicular to
b
(Table
1.8). According to
Table
1.14,
the following Bravais lattices are allowed in the monoclinic crystal system: P
and C. There is only one finite symmetry element (mirror plane m) to be
considered for replacement with glide planes (a, b, c, n and d):
-
The first and obvious choice of the crystallographic space group is Pm.
-
By replacing m with a, we obtain new space group, Pa.
-
Replacing m with b is prohibited since the plane is perpendicular to b.
-
By replacing m with c, we obtain space group PC. This space group
symmetry is identical to Pa, which is achieved by switching
a
and c
because glide plane, a, translates a point by 112 of full translation along a,
and glide plane, c, translates a point by
112 of full translation along c. PC
is a standard choice (see
Table
1.17).
-
By replacing m with n, we obtain space group Pn. This space group can
be converted into PC when the following transformation is applied to the
unit cell vectors: anew
=
-aold, bnew
=
bold, cnew
=
aold
+
cold.
-
Glide plane, d, is incompatible with the primitive Bravais lattice.
-
By repeating the same process in combination with the base-centered
lattice, C, two new space groups symmetry, Cm and Cc can be obtained.
Therefore, the following four monoclinic crystallographic space groups
(Pm,
PC, Cm and Cc) result from a single monoclinic point group (m) after
considering all possible translations in three dimensions.
1.16.2
Full international symbols of crystallographic space groups
The 230 crystallographic groups listed in
Table
1.1
7
are given in the so-
called standard orientation (or setting), which includes proper selection of
both the coordinate system and origin. However, there exist a number of
publications in the scientific literature, where space group symbols are
different from those provided in
Table
1.17. Despite being different, these
symbols refer to one of the same 230 crystallographic space groups but using
a non-standard setting or even using a non-standard choice of the coordinate
system. These ambiguities primarily occur because of the following:
1. The crystal structure was solved using a non-standard setting, since most
of the modern crystallographic software enables minor deviations from
the standard, and the results were published as they were obtained,
without converting to a conventional orientation. It is worth noting that
many, but not all technical journals allow certain deviations from
crystallographic standards.
2.
The crystal structure contains some specific molecules, blocks, layers, or
chains of atoms or molecules, which may be easily visualized or
represented using space group symmetry in a non-standard setting.

Fundamentals
of
crystalline state
5
7
Deviations from the standard are most often observed in the monoclinic
crystal system, because there are many different ways that result in a non-
standard setting in this crystal system. This uncertainty is even reflected in
the International Tables for Crystallography, where there are two different
settings in the monoclinic crystal system. When the unique two-fold axis is
parallel to
b
(i.e. to Y-axis), this setting is considered a standard choice, but
when it is parallel to
c
(or to Z-axis), this is an allowed alternative setting. In
addition, the unique two-fold axis can be chosen to be parallel to
a
(i.e. to
X-
axis), which is considered a non-standard setting.
To reflect or to emphasize a non-standard choice of space group
symmetry, the so-called full international or full Herrnann-Mauguin symbols
can be used. In the full symbol, both the rotation axes parallel to the specific
direction (see
Table
1.8)
and planes perpendicular to them (if any) are
specified for each of the three positions. If the space group has no symmetry
element parallel or perpendicular to a given direction, the symbol of the one-
fold rotation axis is placed in the corresponding position. For example,
P121/al and P1121/a, both refer to the same space group symmetry where the
orientation of the crystallographic axes,
Y
and
2,
is switched. Full
international symbols may also be used to designate space group symmetry
in a standard setting, such as when the short notation Pmmm is replaced by
the full Hermann-Mauguin notation P2/rn2/m2/m. The full symbol in this
case emphasizes the presence of the three mutually perpendicular two-fold
axes and mirror planes that are parallel and perpendicular, respectively, to
the three major crystallographic axes.
Thus, one of the most commonly observed in both natural and synthetic
crystals, space group symmetry
P2Jc (standard notation with the unique
two-fold screw axis parallel to Y, and
a
=
y
=
90" and
P
#
90") can be listed
as follows:
-
P21hll and P21/cll, when the unique two-fold screw axis is chosen to
be parallel to
X.
Both symbols represent non-standard settings of this
group, in which
a
#
90".
-
P121/cl (or sh0rtP2~/c, whichis standard) andP121/al (or sh0rtP2~/a,
which is also standard except for the choice of the glide plane), when the
unique two-fold screw axis is chosen to be parallel to Y.
-
P 1 12Ja and P 1 12,h, when the unique two-fold screw axis is chosen to
be parallel to Z (both represent alternative setting, where the unique two-
fold screw axis is parallel to Y, and therefore,
y
z
90").
-
P2,/nll, P121/nl (or short P2Jn) or P1 121/n, when the unique two-fold
screw axis is chosen to be parallel to
X,
Y
or Z, respectively, but the
selection of the diagonal glide plane, n, represents a deviation from the
standard.

5
8
Chapter
1
Since the selection of the crystallographic coordinate system is not
unique, conventionally, a right-handed set' of basis vectors a,
b,
c
is chosen
in compliance with Table
1.12
and in a way that the combination of
symmetry elements in the space group is best visualized. The selection of the
conventional origin is usually more complicated, but in general the origin of
the coordinate system is selected at the center of inversion, if it is present, or
at the point with the highest site symmetry, if there is no center of inversion
in the group.
Additional information about each of the 230 three-dimensional
crystallographic space groups can be found in the International Tables for
Crystallography, vol. A. It includes their symbols, diagrams of all symmetry
elements present in the group together with their orientation with respect to
crystallographic axes, the origin of the coordinate system and more, for both
the conventional and alternative (if any) settings. The format of the
International Tables for Crystallography and some relevant issues are briefly
discussed in the following section 1.17.
1.16.3 Visualization
of
space group symmetry in three dimensions
An
excellent way to achieve a better understanding of both the infinite
symmetry elements and how they are combined in crystallographic space
groups is to use three-dimensional illustrations coupled with computer
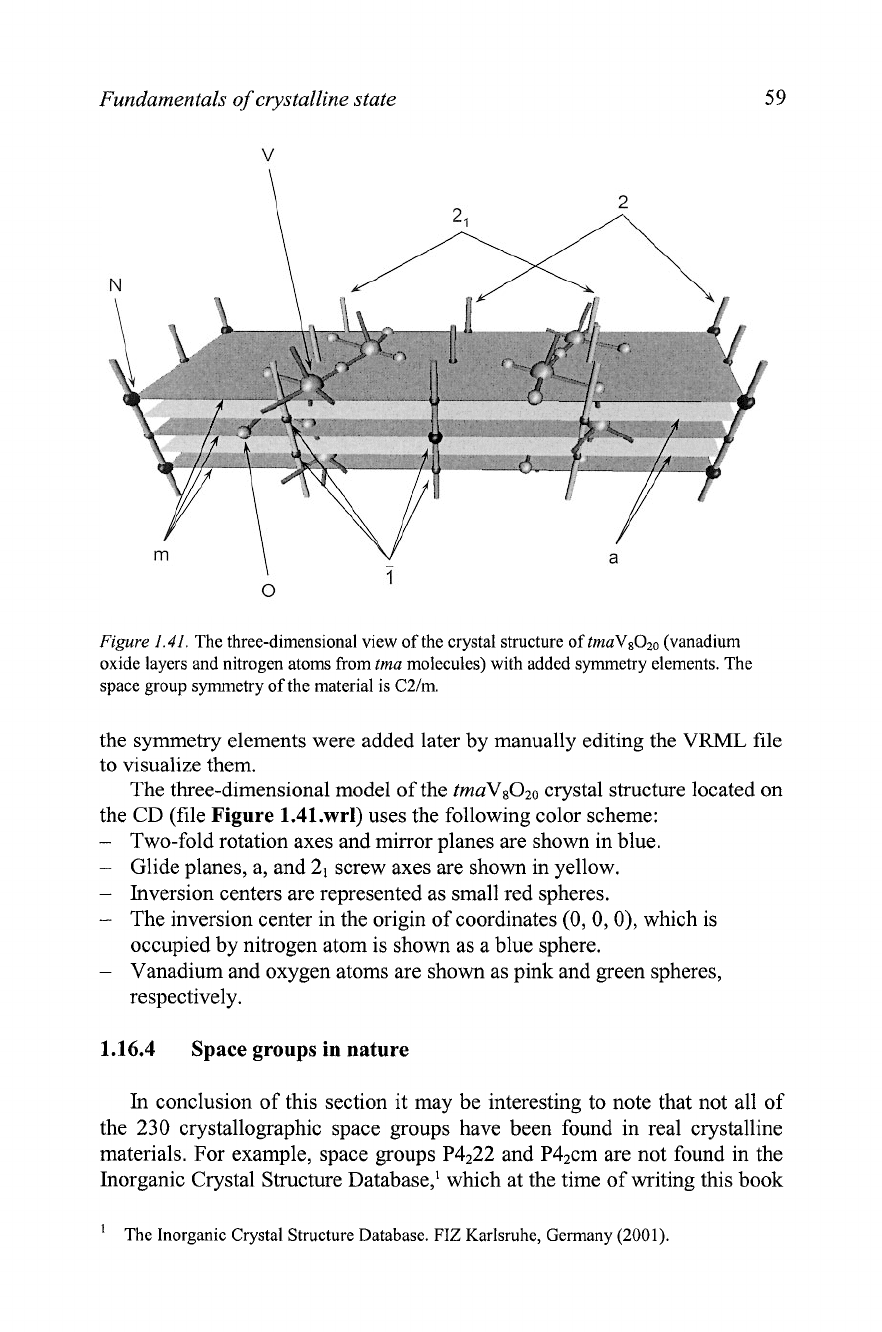
animation capabilities. For example, the crystal structure of the vanadium
oxide layer in
tmaVsOzo (tma is short for tetramethylammonium,
[N(CH&]+)~
is shown as a still image in Figure
1.41,
and it is also available
as an electronic figure on the CD, which accompanies this book. The crystal
structure is shown with the unit cell and its symmetry elements in the VRML
(Virtual Reality Modeling Language) file format (Figure 1.41.wrl). The file
may be displayed using a Web browser, which includes a VRML viewer as a
plug-in. Standalone VRML viewers can be downloaded from the Web,
e.g.
from the Web3D repository
(http://www.web3d.org/vrml/browpi.htm).
In addition to visualizing the crystal structure in three dimensions, using
VRML enables one to move and rotate it. It is also possible to virtually "step
inside" the lattice or the structure and to examine them from there. The
pseudo three-dimensional drawing of the trnaVs020 crystal structure on the
CD
was created using the General Structure Analysis System (GSAS),3 and
'
In a right-handed set the positive directions of basis vectors
a,
b,
c
are chosen from the
middle of the palm of the right hand towards the ends of thumb, index and middle fingers,
respectively.
T.
Chirayil,
P.Y.
Zavalij, M.S. Whittingham, Synthesis and characterization of a new
vanadium oxide,
tmaVsOzo,
J.
Mater. Chem.
7,
2193 (1997).
'
A.C. Larson and R.B. Von Dreele, General Structure Analysis System (GSAS), Los
Alamos National Laboratory Report, LAUR 86-748 (2000).
Fundamentals
of
crystalline state
5
9
Figure
1.41.
The three-dimensional view of the crystal structure of
tmaVsOzo
(vanadium
oxide layers and nitrogen atoms from
tma
molecules) with added symmetry elements. The
space group symmetry of the material is C2lm.
the symmetry elements were added later by manually editing the VRML file
to visualize them.
The three-dimensional model of the trnaVs02,, crystal structure located on
the CD (file Figure 1.41.wrl) uses the following color scheme:
-
Two-fold rotation axes and mirror planes are shown in blue.
-
Glide planes, a, and 2, screw axes are shown in yellow.
-
Inversion centers are represented as small red spheres.
-
The inversion center in the origin of coordinates (0, 0, 0), which is
occupied by nitrogen atom is shown as a blue sphere.
-
Vanadium and oxygen atoms are shown as pink and green spheres,
respectively.
1.16.4 Space groups in nature
In
conclusion of this section it may be interesting to note that not all of
the 230 crystallographic space groups have been found in real crystalline
materials. For example, space groups P4222 and P42cm are not found in the
Inorganic Crystal Structure Database,' which at the time of writing this book
The Inorganic Crystal Structure Database.
HZ
Karlsruhe, Germany (2001).
