Pecharsky V.K., Zavalij P.Y. Fundamentals of Powder Diffraction and Structural Characterization of Materials
Подождите немного. Документ загружается.


502
Chapter
6
Nonetheless, even when the gravimetric density cannot be measured,
Eq.
6.4
still can be used, especially when the chemical composition of the material is
known precisely or when working with molecular compounds.
In
these
cases,
Z
can be estimated from restrictions imposed by symmetry and from
approximately known range of densities for a specific class of compounds.
For example,
Eq.
6.4 is easily rearranged as
where n is the minimum number of molecules per unit cell, which is usually
the multiplicity of a general site position' and
z
is a variable integer, which
corresponds to the number of molecules with mass
M
in the asymmetric
(lln-th) part of the unit cell. For instance, in the space group P2Jm, n
=
4,
and by varying z, the acceptable value of density
p
can be achieved.
Consider tetraphenylphosphonium (TPP) tetravanadate, which
crystallizes in the monoclinic crystal system in the space group
P2Jc. Its unit
cell volume is
V
=
6806.6(6) A3. The molecular weight of [(C6H5)4P]2V~01~
is 1058.55 a.m.u. The density range for this class of compounds (not the
easiest case but certainly a good example) may be estimated between 1.2 and
1.6
glcm3 given the presence of both the metal oxide core and large organic
molecule. The multiplicity of the general site position in this space group is
4. The V4Ol1 unit, however, can be centrosymmetric because it has four V
and ten
0
atoms plus an additional
0
atom that can be located in the center
of inversion, and there are two
TPP
molecules in the formula unit. Therefore,
a minimum multiplier for the number of molecules per unit cell (n) should be
set to 412
=
2.
We now consider densities while varying z. When
z
=
1 and z
=
2 the densities are 0.5 16 and 1 .O33 g/cm3, respectively, which are too low.
When z
=
4, the density is 2.065 &m3, which is too high. Therefore,
z
=
3
is
the only possibility left and it results in the density of 1.549 g/cm3. The latter
value falls into the expected range, so the resulting total number of the
formula units in the unit cell
Z
=
nz
=
6. Considering that the multiplicity of
the general site is 4, there are 1.5 formula units in the asymmetric part of the
sinks.
A
high-density fluid is then slowly added until neutral buoyancy of the particle is
reached. The gravimetric density of the particle is then determined from the density of the
mixture of two fluids, provided their amounts are known. Obviously, the two fluids should
form an ideal solution, i.e. volume effects of mixing should be negligible.
'
In general, n in
Eq.
6.5
can be the multiplicity of a general or any special position or their
sum. The latter makes it complicated, but still keeps the solution integer with respect to
z.
Note that symmetry of any special position and the molecule should agree; furthermore,
some special positions, i.e, those at the center of inversion where all three coordinates are
fixed, can be occupied by only one atom.

Crystal structure solution 503
unit cell. Thus, there should be
3
TPP ions in the general position, while the
vanadate molecules
(V40,,)
may occupy 1 general and 1 special, or
3
special
positions, where special positions are such that one of the
0
atoms are
located in the centers of inversion.
6.4
Pearson's classification
As noted in section 6.2, when the material of interest is an intermetallic
alloy, the solution of its crystal structure may be simplified because
intermetallics often form series of isostructural compounds.
In
contrast to
conventional inorganic and molecular compounds, stoichiometries of the
majority of intermetallic phases are not restricted by "normal" valence and
oxidation states of atoms and ions; therefore, crystal structures of metallic
alloy phases are conveniently coded using the classification suggested by
W.B. Pearson.' According to
Pearson, each type of the crystal structure is
assigned a specific code (symbol), which is constructed from three
components as follows:
-
The first position in a structure type symbol is occupied by a small letter
designating the crystal system of the material:
c
for cubic,
t
for
ietragonal,
h
for hexagonal, trigonal and rhombohedral, o for
orthorhombic,
m
for monoclinic, and a for triclinic (anorthic).
-
-
The second position in the symbol is occupied by a standard notation of
Bravais lattice. Thus, the first two elements in the Pearson's symbol are
letters and they classify all available alloy structures according to 14
Bravais lattices, as shown in Table
6.1.
-
The third (and last) position in Pearson's symbols is occupied by the total
number of atoms located in one unit cell of the compound.
For example, considering the crystal structure of copper, which has cubic
face-centered lattice (Figure
6.2)
and a total of 4 atoms in the unit cell, its
Pearson's symbol is cF4. On the other hand, if the material has Pearson's
symbol 0132, this means that its crystal structure is orthorhombic, and one
body-centered unit cell contains a total of 32 atoms.
Pearson's classification is insensitive to both chemical compositions and
stoichiometries of metallic alloys. It is quite useful because all known
intermetallic crystal structures are grouped according to their structural
symbols, which are quite simple. Thus, once the symmetry and the content
of the unit cell of a new alloy phase have been established, it only makes
sense to search for potentially isostructural compounds among those that
have identical Pearson's symbols.
'
W.B. Pearson, Handbook of lattice spacings and structures of metals, vol. 2, Pergamon
Press, New York (1967);
W.B.
Pearson, The crystal chemistry and physics of metals and
alloys, Wiley-Interscience, New York (1972).
5
04
Chapter
6
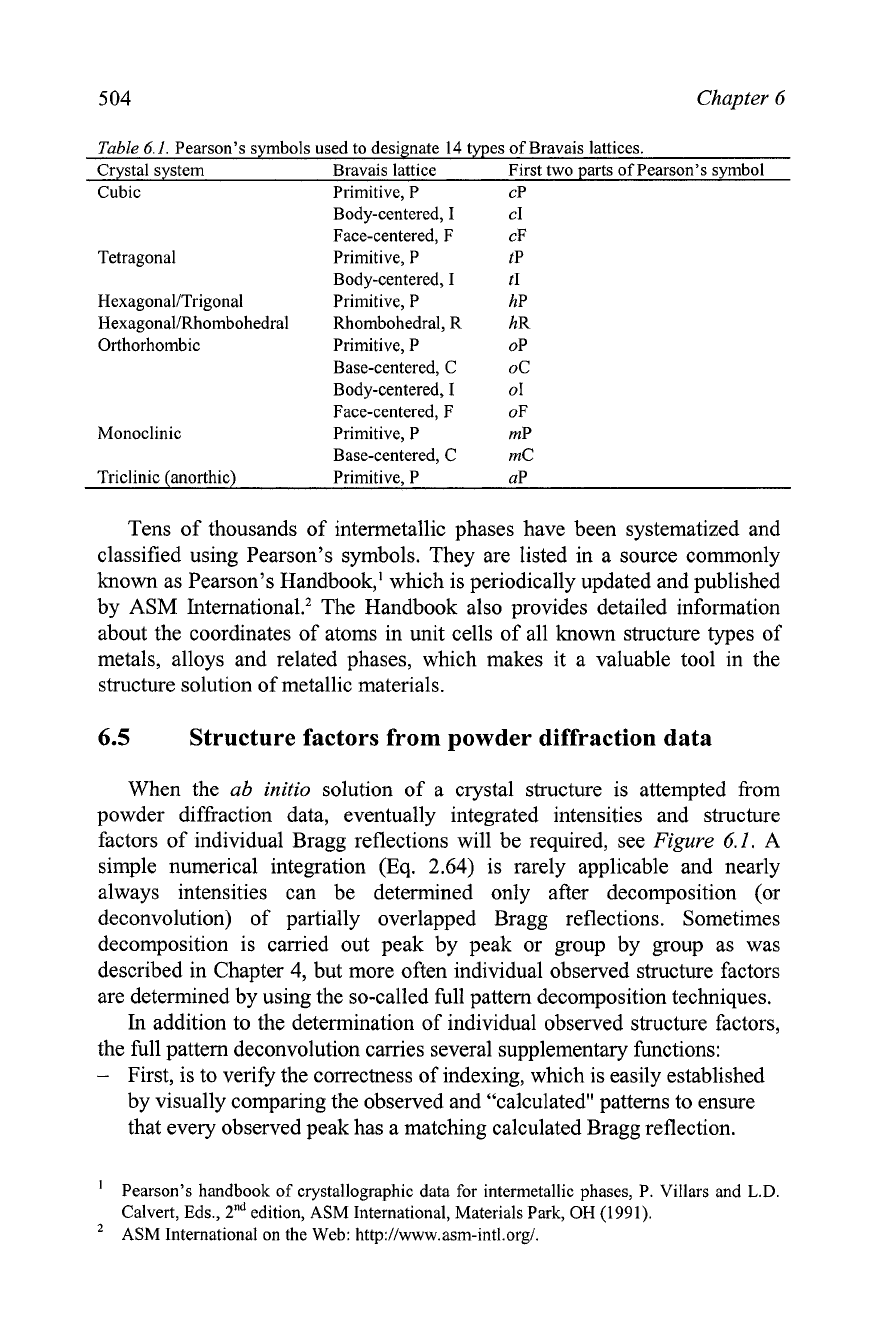
Table
6.1.
Pearson's symbols used to designate
14
types of Bravais lattices.
Crystal system Bravais lattice First two parts of Pearson's symbol
Cubic Primitive, P
CP
Body-centered, I CI
Face-centered, F cF
Tetragonal Primitive, P tP
Body-centered, I tI
Hexagonal/Trigonal Primitive, P hP
Hexagonal/Rhombohedral Rhombohedral, R hR
Orthorhombic Primitive, P OP
Base-centered, C OC
Body-centered, I 01
Face-centered, F OF
Monoclinic Primitive, P mP
Base-centered, C mC
Triclinic (anorthic) Primitive, P UP
Tens of thousands of intermetallic phases have been systematized and
classified using Pearson's symbols. They are listed in a source commonly
known as Pearson's Handbook,' which is periodically updated and published
by ASM Internati~nal.~ The Handbook also provides detailed information
about the coordinates of atoms in unit cells of all known structure types of
metals, alloys and related phases, which makes it a valuable tool in the
structure solution of metallic materials.
6.5
Structure factors from powder diffraction data
When the
ab
initio
solution of a crystal structure is attempted from
powder diffraction data, eventually integrated intensities and structure
factors of individual Bragg reflections will be required, see
Figure
6.1.
A
simple numerical integration (Eq. 2.64) is rarely applicable and nearly
always intensities can be determined only after decomposition (or
deconvolution) of partially overlapped Bragg reflections. Sometimes
decomposition is carried out peak by peak or group by group as was
described in Chapter 4, but more often individual observed structure factors
are determined by using the so-called full pattern decomposition techniques.
In addition to the determination of individual observed structure factors,
the full pattern deconvolution carries several supplementary functions:
-
First, is to verify the correctness of indexing, which is easily established
by visually comparing the observed and "calculated" patterns to ensure
that every observed peak has a matching calculated Bragg reflection.
'
Pearson's handbook of crystallographic data for intermetallic phases, P. Villars and L.D.
Calvert, Eds.,
2nd
edition, ASM International, Materials Park, OH
(1991).
ASM International on the Web:
http:Nwww.asm-intl.org/.

Crystal structure solution
505
-
Second, is to precisely determine the unit cell dimensions without
performing a semi-manual profile fitting (see Chapter 4).
-
Third, is to estimate the best figures of merit (see section 6.7), achievable
in a Rietveld refinement using the existing set of diffraction data.
The two related full pattern decomposition methods in common use today
were suggested by Pawley' and by Le Bail
et
aL2
Pawley's approach is based
on Eq. 2.48 and full pattern decomposition in the case of dual wavelength
data, when
Kal/Ka2
doublets are present, is performed by solving the
following system of equations using a least squares minimization:
The notations used in Eq.
6.6
are identical to Eq. 2.48. Individual
integrated intensities (Ik) are treated as free least squares parameters. Peak
shape function parameters are represented as described in section 2.9, and
Bragg peak positions, which affect the values of xk, are established by the
unit cell dimensions, see section 2.8. The background,
bi,
where
1
I
i
I
n
and
n
is the total number of measured data points, is modeled by one of the
several functions described in Chapter 4 (see Eqs.
4.1
to 4.6).
When peak shape functions and their parameters, including Bragg
reflection positions, are
known precisely and the background is modeled by
a polynomial function with
j
coefficients, the solution of Eq.
6.6
is trivial
because all equations are linear with respect to the unknowns (Bj, see Eq.
4.1, and Ik). It facilitates the use of a linear least squares algorithm described
in section 5.13.1. In practice, it is nearly always necessary to refine both
peak shape and lattice parameters in addition to
Bj and
Ik
to achieve a better
precision of the resultant integrated intensities. Thus, a non-linear least
squares minimization technique (see next section) is usually employed
during full pattern decomposition using Eq. 6.6.
'
G.S. Pawley, Unit-cell refinement from powder diffraction scans,
J.
Appl.
Cryst.
14
,
357
(1981).
A.
Le Bail, H. Duroy, and J.L. Fourquet,
Ab
initio
structure determination of LiSbW06 by
x-ray powder diffraction, Mat. Res. Bull.
23,
447
(1988). The method is also commonly
known as Le Bail extraction.

506
Chapter
6
Pawley's method works best when the complexity of a powder
diffraction pattern is relatively low (few hundreds or so Bragg peaks total).
When the number of independent Bragg peaks
(m)
exceeds several thousand,
the size of the least squares normal equation matrix increases proportionally
to
m2.
As a result, Pawley's algorithm may become unstable, especially if
there are multiple Bragg reflections with low intensity and/or when there is a
severe overlap. The latter is quite normal when the total number of
measurable Bragg peaks exceeds -1000 in the scanned range. In the case of
complex patterns, this algorithm has problems in keeping values of all
integrated intensities
Ik
non-negative, which is obvious from Eq. 6.6.
Le Bail's approach is also based on Eq. 6.6 and it differs from the
Pawley's method in that the individual intensities remain unaltered during
each least squares cycle. They are extracted from a total observed intensity
of the pattern between the least squares cycles after subtraction of the
background. The extraction is performed using a decomposition approach
that has been employed in the Rietveld method since its development,' in
which intensity observed in every point of the powder pattern is divided
among different reflections proportionally to their calculated intensities:
cnlc
Yk,i
yi;!
=
pk$i
(Y?~'
-
bi
),
where
pk%i
=
In
Eq.
6.7,
yk,Pb5s the pseudo-observed intensity of the
kth
reflection in the
ith
point,
pk,i
is the fractional contribution of the
k'
reflection to the
ith
point,
yrb9s the observed total intensity in the
ith
point,
yk,ic"'c
is the calculated
intensity of the
kt"
reflection in the
ith
point. The main difference between
Rietveld and Le Bail decompositions is in the calculated intensities. The
former technique uses intensities computed from the model of the crystal
structure, while the latter approach uses intensity obtained from the previous
cycle during the decomposition. Initially, all "calculated" intensity values in
the Le Bail's method are set to arbitrary identical quantities, typically unity.
It is worth noting that in the Le Bail's decomposition, the number of free
least squares variables becomes independent of the number of Bragg
reflections and only background, peak shape and lattice parameters are
refined during each least squares cycle. A small inconvenience of Le Bail's
approach is that the unit cell dimensions should be known with a greater
precision than in Pawley's method. It also takes more least squares
'
H.M. Rietveld, Line profiles of neutron powder-diffraction peaks for structure refinement,
Acta Cryst.
22,
151 (1967);
H.M. Rietveld, A profile refinement method for nuclear and
magnetic structures,
J.
Appl. Cryst.
2,
65 (1969).

Crystal structure solution
507
refinement cycles to complete but the fit converges in much more complex
cases. The disadvantage of the approach suggested by Le Bail is the separate
handling of intensities for
Kal and Ka2 peaks during the decomposition
according to Eq. 6.7 and correction for the proper intensity ratio after the
decomposition, which increases the time required for the completion of the
fitting process. As far as suitable software is of concern, it is essential to
check the manual and find out how the presence of the
KallKa2 doublet is
handled because some computer codes do not address this issue and the
resulting intensities of Kal and Ka2 components may become unreasonable.
There is a variety of freely available software, which enables one to
deconvolute a powder diffraction pattern and determine either or all:
individual intensities, lattice and peak shape function parameters, and
observed structure factors of all possible Bragg reflections.
Freeware codes
include EXPO, Fullprof, GSAS, LHPM-Rietica, and others.'
In
addition to
free programs, nearly all manufacturers of commercial powder
diffractometers offer software for sale either as a package with the sale of the
equipment or as stand-alone
product^.^
6.6
Non-linear least squares
Both the full pattern decomposition and Rietveld refinement are based on
the non-linear least squares minimization of the differences between the
observed and calculated profiles. Therefore, the non-linear least squares
method is briefly considered here. Assume that we are looking for the best
solution of a system of
n
simultaneous equations with m unknown
parameters
(n
>>
m), where each equation is a non-linear function with
respect to the unknowns, xl, x2,
.
..,
x,.
In
a general form, this system of
equations can be represented as
'
The most extensive sources of various software links are found at the International Union
of Crystallography (www.iucr.org) and/or Collaborative Computational Project No.
14
(http://www.ccpl4.ac.uk)
Web sites.
Commercial manufacturers, which offer a variety of software products for processing
powder diffraction data are: Bruker
(http://www.bruker-axs.com/production/indexnn.htm),
Philips
(http://www-us.analytical.philips.com/products/xrd),
Rigaku/Molecular Structure
Corporation
(http://www.rigaku.com/xrd/index.jsp),
STOE
&
Cie, Gmbh
(http://www.stoe.com/products/index.htm)
and many others.
508
Chapter
6
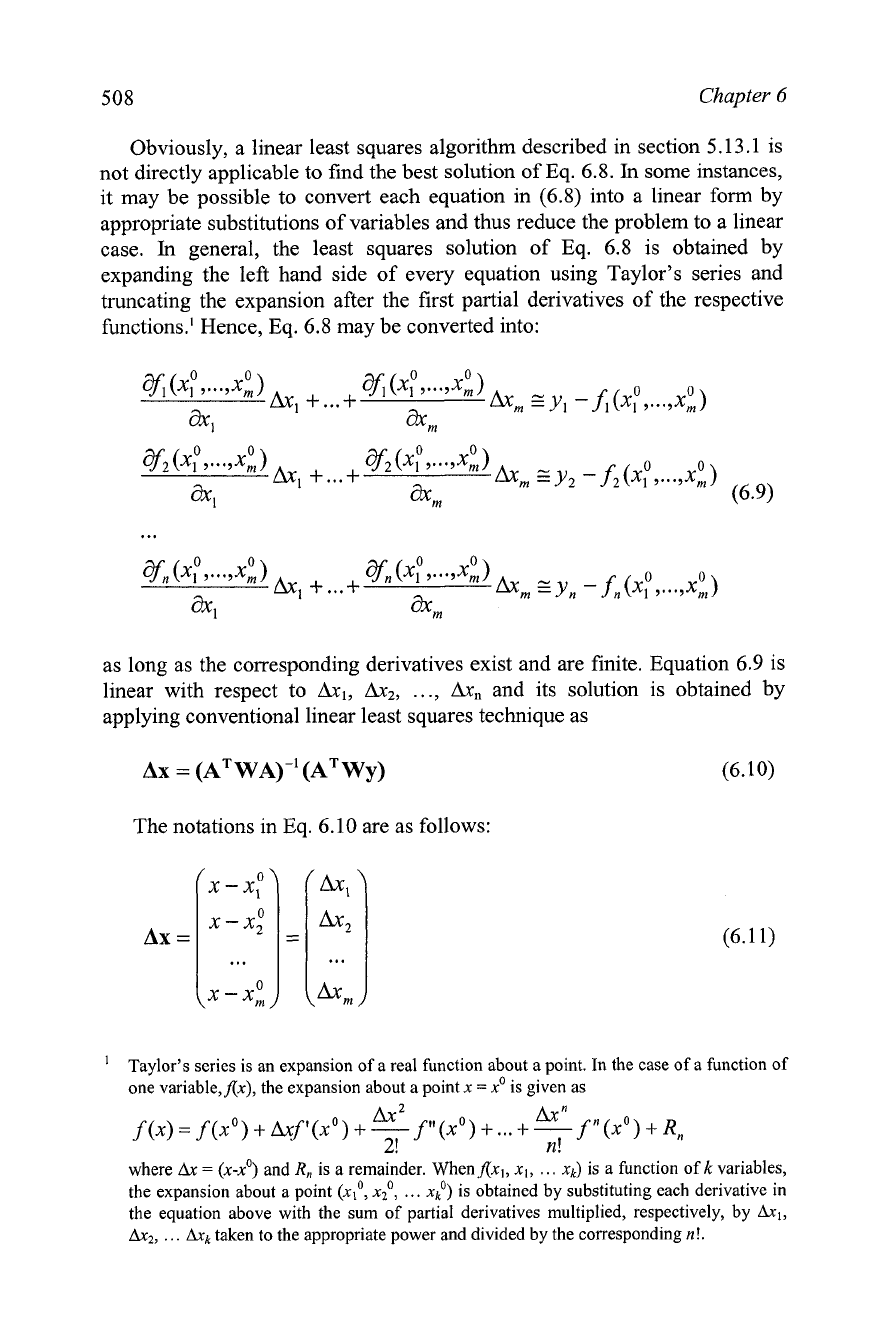
Obviously, a linear least squares algorithm described in section 5.13.1 is
not directly applicable to find the best solution of Eq.
6.8.
In
some instances,
it may be possible to convert each equation in (6.8) into a linear form by
appropriate substitutions of variables and thus reduce the problem to a linear
case.
In
general, the least squares solution of Eq. 6.8 is obtained by
expanding the left hand side of every equation using Taylor's series and
truncating the expansion after the first partial derivatives of the respective
functions.' Hence, Eq. 6.8 may be converted into:
0
%cxP
,-.,
x,)
Ax,
+...+
%
(XP,...,~
0
hm
EY, -fi(xP,...,x,)
a1
&m
0
iY;(xP ,...,x
Ax,
+...+
v2(x:7'*',xi)
0
Ax,
Y2
-f2
7...7xm)
(6.9)
0
vn
<xP,...,xm)
Ax,
+
...+
vn<.P7...,xi)
0
Axm EYn
-fn&
,...,x:
)
a1
am
as long as the corresponding derivatives exist and are finite. Equation 6.9 is
linear with respect to Ax1, AxZ,
...,
Ax,
and its solution is obtained by
applying conventional linear least squares technique as
The notations in Eq. 6.10 are as follows:
'
Taylor's series is an expansion of a real function about a point. In the case of a function of
one variable,f(x), the expansion about a point x
=
x0 is given as
where
Ax
=
(x-xO) and
R,
is a remainder. Whenfi,, xi,
. . .
xk) is a function of
k
variables,
the expansion about a point (xI0, xzo,
.
.
.
xko) is obtained by substituting each derivative in
the equation above with the sum of partial derivatives multiplied, respectively, by
Ax1,
Axz,
. . .
Axk
taken to the appropriate power and divided by the corresponding
n!.
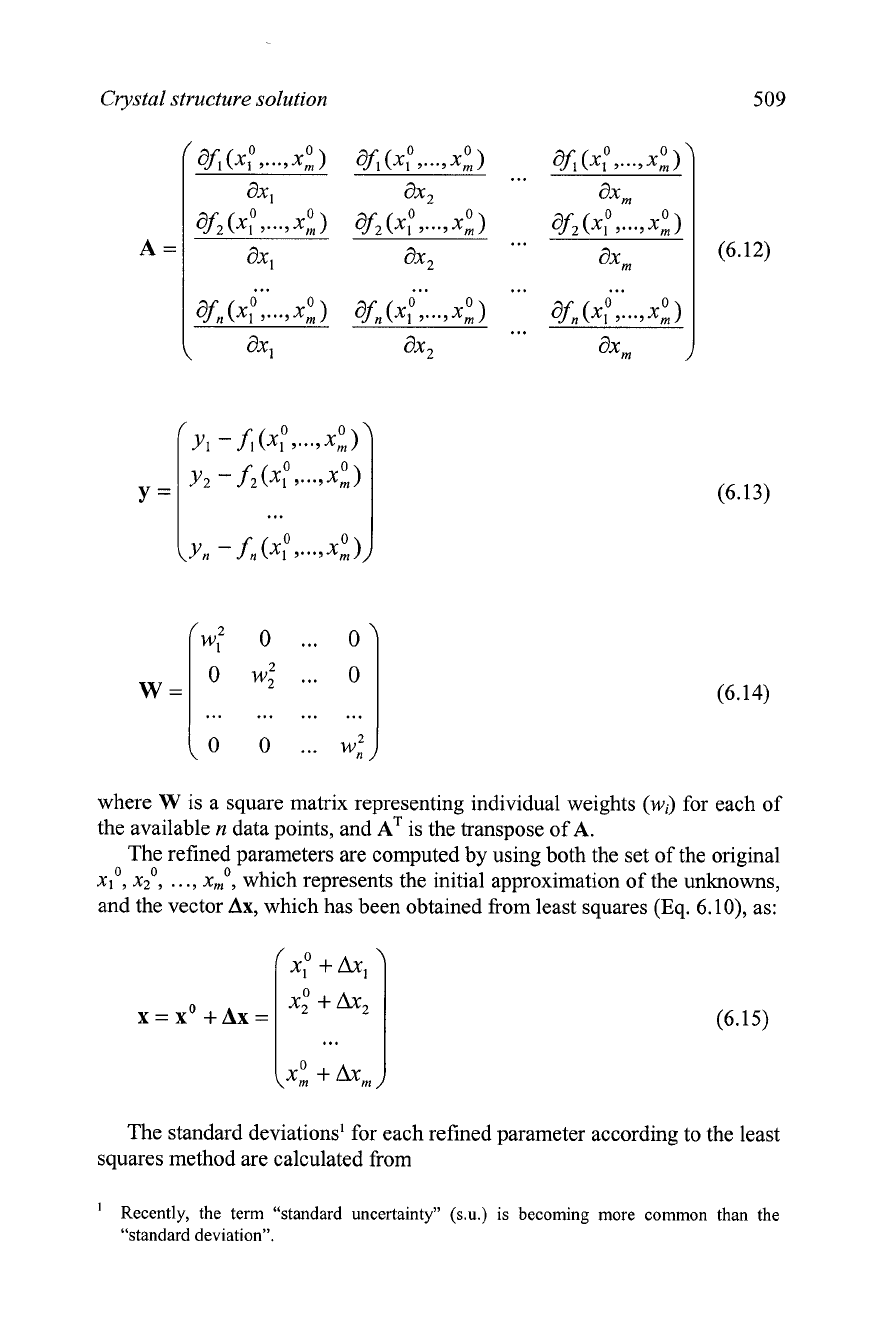
Crystal structure solution 509
where
W
is a square matrix representing individual weights
(wi)
for each of
the available
n
data points, and
is the transpose of
A.
The refined parameters are computed by using both the set of the original
0 0 0
,...,
,
xl
,
x2
X,
which represents the initial approximation of the unknowns,
and the vector
Ax,
which has been obtained from least squares (Eq.
6.
lo), as:
xp
+
Ax,
x;
+
Ax,
...
x:
+
Ax,
The standard deviations' for each refined parameter according to the least
squares method are calculated from
'
Recently, the term "standard uncertainty" (s.u.) is becoming more common than the
"standard deviation".

(ATWA);
f:Wi(yi)2
i=l
(3(xj)
=
,
j
=
1,
...,
m
n-m
Chapter
6
(6.16)
where:
-
n
is the number of equations in Eq. 6.9,
-
m
is the number of unknown parameters in Eq. 6.9,
-
(A~wA),~' is the corresponding diagonal element of the inverse
normal equation matrix,
-
wi
is the corresponding weight,
-
yi
is the corresponding element of the vector
y.
The major differences between the non-linear least squares technique and
the linear least squares method, described in section 5.13.1, are as follows:
-
The substitution of the original Eq. 6.8 with Eq. 6.9 requires the
knowledge of initial (i.e, approximate) values of parameters to be refined,
0 0 0
which are represented by the set
xl
,
x2
,
.
.
.,
x,
.
-
The least squares solution (Eq. 6.10) results in the shifts (vector
Ax,
Eq.
6.1 I), which shall be added to the corresponding initial parameters, as
shown in Eq. 6.15.
-
Because Eq. 6.9 is not exact, usually more than one cycle of a least
squares refinement is necessary to achieve a full convergence: during the
second and following least squares cycles, the new set of parameters as
obtained in the previous step from Eq. 6.15 is used as the initial
approximation. Thus, non-linear least squares refinement is an iterative
process, where the result of the next iteration depends on the result
obtained during the prior iteration.
-
Because of the iterative nature of non-linear least squares, convergence
may be difficult to achieve, especially when the initial approximation is
far from correct (Figure 6.3, left) or when the minimized function (see
Eqs. 5.33, 5.34 and 6.9) is poorly defined. The latter often occurs when
certain least squares parameters correlate.' Instead of converging, non-
linear least squares may diverge and become unstable, as illustrated in
Figure 6.3, right. Therefore, various numerical conditioning techniques
should be employed to improve both the convergence and stability of the
method. Their detailed consideration exceeds the scope of this book and
the reader is referred to a large amount of special literature covering this
subject.
'
Considering, for example, Eqs.
2.65
and
2.89,
when the phase scale
(K)
is refined together
with the population parameters of all atoms
(g'),
a complete correlation results: an increase
of the phase scale by a factor
k
will be completely offset by the reduction of all population
parameters by the factor
dk.
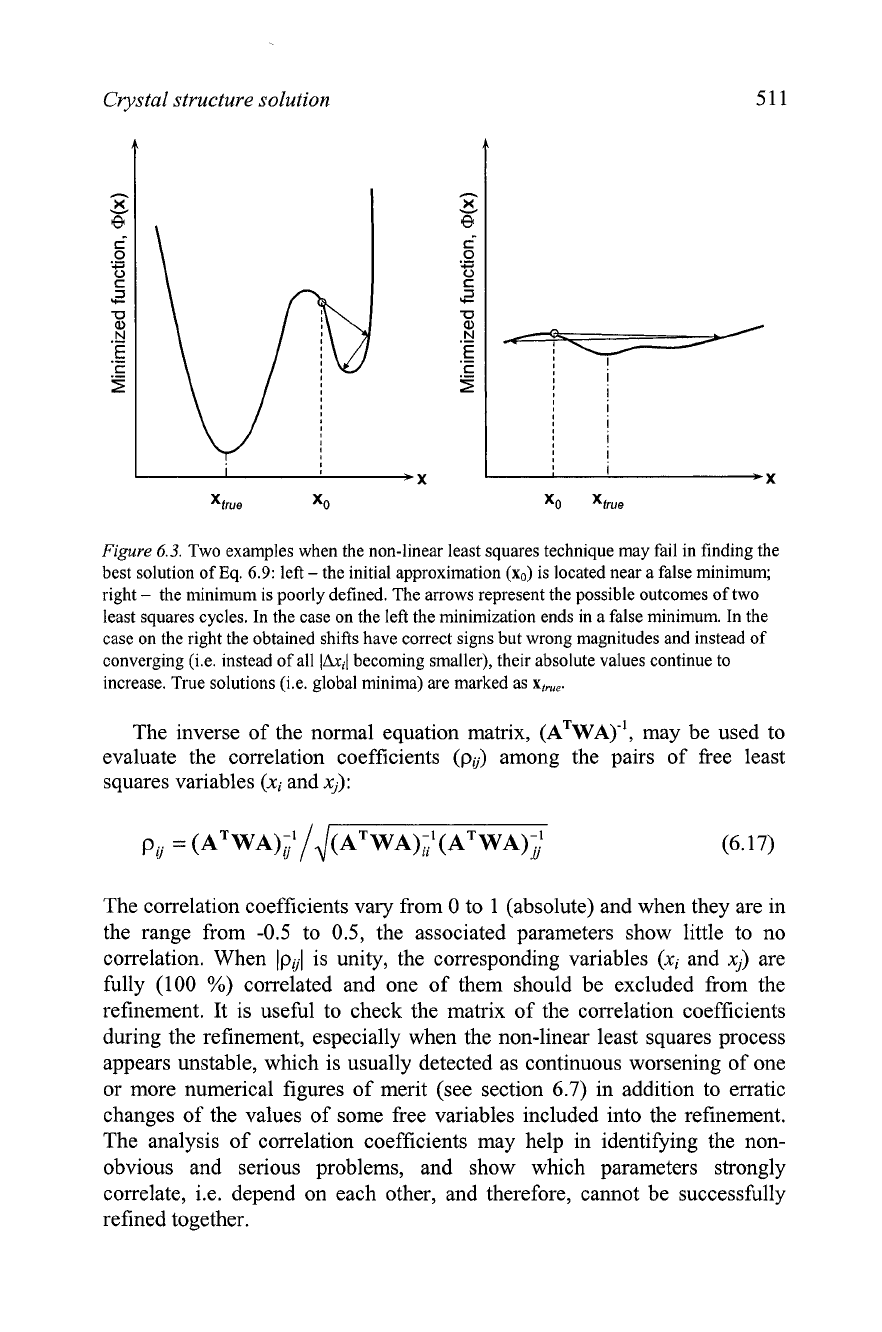
Crystal structure solution
Figure
6.3.
Two examples when the non-linear least squares technique may fail in finding the
best solution of Eq.
6.9:
left
-
the initial approximation
(xo)
is located near a false minimum;
right
-
the minimum is poorly defined. The arrows represent the possible outcomes of two
least squares cycles. In the case on the left the minimization ends in a false minimum. In the
case on the right the obtained shifts have correct signs but wrong magnitudes and instead of
converging (i.e. instead of all
becoming smaller), their absolute values continue to
increase. True solutions (i.e. global minima) are marked as
x,,,.
The inverse of the normal equation matrix, (ATwA)-', may be used to
evaluate the correlation coefficients
(pu)
among the pairs of free least
squares variables (xi and
xj):
The correlation coefficients vary from 0 to 1 (absolute) and when they are in
the range from -0.5 to 0.5, the associated parameters show little to no
correlation. When
lpUl
is unity, the corresponding variables (xi and
xj)
are
fully (100
%)
correlated and one of them should be excluded from the
refinement. It is useful to check the matrix of the correlation coefficients
during the refinement, especially when the non-linear least squares process
appears unstable, which is usually detected as continuous worsening of one
or more numerical figures of merit (see section
6.7)
in addition to erratic
changes of the values of some free variables included into the refinement.
The analysis of correlation coefficients may help in identifying the non-
obvious and serious problems, and show which parameters strongly
correlate,
i.e. depend on each other, and therefore, cannot be successfully
refined together.
