North, Gerald R., Erukhimova Tatiana L. Atmospheric Thermodynamics: Elementary Physics and Chemistry
Подождите немного. Документ загружается.


8.5 Equilibrium constant 207
As the reaction proceeds the products of this reaction (C and D) become sufficiently
dense in number that they will begin to react and form A and B through the reverse
reaction:
C + D → A + B. (8.51)
When eventually the rate of the forward reaction (8.50) is the same as the rate of
the reverse reaction (8.51), the system is in chemical equilibrium. We can combine
(8.50) and (8.51) and write
A + B
C + D. (8.52)
If we were trying to find the rate for which the reaction (8.52) proceeds from the
point of view of the individual gas molecules, we would say the rate of increase of
the concentration of C is given by
d
dt
[C] = k
ab
[A][B] − k
cd
[C][D]
=
d
dt
[D]
=−
d
dt
[A]
=−
d
dt
[B] (8.53)
where k
ab
and k
cd
are the reaction rate coefficients for forward and reverse reactions
respectively. The equation simply states that the rate of buildup of C is the sum of
the rates of reactive collisions of A and B minus the reverse process in which
C and D react. The first term must be proportional to the respective number
densities and similarly for the second (loss) term. Since for every creation of a
C molecule there must be a B molecule, these rates of formation must be equal
to each other and equal to the negative of the rates of formation of the A and B
molecules.
In equilibrium the rates of change of the species are zero. This means
d
dt
[C] = 0 equilibrium (8.54)
or
K =
[C][D]
[A][B]
=
k
ab
k
cd
. (8.55)
The constant K is called the equilibrium constant for the reaction. If K is known,
we can determine the ratios of concentrations of the product and reactant gases in
equilibrium.
208 Thermochemistry
For the case of a general reaction
a A + b B → c C + d D, (8.56)
the equilibrium constant is (as derived in physical chemistry texts):
K =
[C]
c
[D]
d
[A]
a
[B]
b
(8.57)
The rule can be generalized to cases where more than two species are on each side
of the equation.
It is seen from (8.57) that when the inverse reaction rate is very small, which
means that products dominate over reactants, K is large. Small K means there will
be a relatively large concentration of the reactant species.
The equilibrium constant depends on collision dynamics and in principle
should have a strong temperature dependence, since the intermolecular relative
velocity will be an important factor in the rearrangements. To find the temperature
dependence of the equilibrium constant, let us write the reaction rate coefficients
for the forward and reverse reactions (8.52). Using the Arrhenius equation (8.59)
the reaction rate coefficient for the forward reaction, k
1
,is
k
1
(T ) = A
1
exp
−E
act
R
∗
T
. (8.58)
The reaction rate coefficient for the reverse reaction, k
2
,is
k
2
(T ) = A
2
exp
−E
act
+ H
◦
R
∗
T
. (8.59)
The equilibrium constant
K(T ) =
k
2
k
1
∝ exp
−
H
◦
R
∗
T
(8.60)
Hence, knowing the standard enthalpy associated with a reaction provides
information about the equilibrium concentrations of the species.
Example 8.9 Consider the recombination of OH and O:
OH + O → O
2
+ H. (8.61)
Write the expression for the equilibrium constant. By using formula (8.60) find out
whether the products will dominate over reactants at high or low temperatures.
8.5 Equilibrium constant 209
Answer: In order for the reaction to proceed, an OH molecule has to bump into an
O atom in the same tiny volume. The rate of growth term must be proportional to
[OH][O]. The equilibrium constant is
K =
[O
2
][H]
[OH][O]
. (8.62)
To find the temperature at which the forward reaction will be favored we need to
find the standard enthalpy:
H
◦
= H
◦
(O
2
) + H
◦
(H) − H
◦
(OH) − H
◦
(O)
= (0 + 217.97 − 38.96 − 249.17) kJ mol
−1
=−70.2 kJ mol
−1
. (8.63)
Since
H
◦
is negative, from (8.60) we have that the equilibrium constant K is
larger with lower temperature. This explains why the products of reaction (8.61)
will dominate over reactants at the lower temperatures.
The next step is to find the connection between the equilibrium constant and
the Gibbs energy. The equilibrium of the chemical reaction implies that at a given
temperature there exist partial pressures of the gases involved in the reaction with
which the rate of the forward reaction is equal to the rate of the reverse reaction. Let
us express the equilibrium constant in terms of partial pressures of each constituent.
Using the Ideal Gas Law for the molar concentrations we obtain:
K =
(p
C
/R
∗
T )
c
(p
D
/R
∗
T )
d
(p
A
/R
∗
T )
a
(p
B
/R
∗
T )
b
=
p
c
C
p
d
D
p
a
A
p
b
B
(R
∗
T )
, (8.64)
where the p
i
are partial pressures and = (a +b) −(c +d ). We can rewrite (8.64)
in the form:
K = K
p
(R
∗
T )
, (8.65)
where
K
p
=
p
c
C
p
d
D
p
a
A
p
b
B
[equilibrium constant for ideal gases]. (8.66)
K
p
is used as an equilibrium constant for chemical reactions involving species in
the gaseous state.
For a reversible transformation at constant temperature the change of Gibbs
energy is (see (4.97))
dG = V dp. (8.67)
210 Thermochemistry
For 1 mol of ideal gas
d
G =
R
∗
T
p
dp. (8.68)
(Note that the molar Gibbs energy
G is also called the chemical potential.) By
integrating this equation from the standard pressure level of 1 atm to some arbitrary
pressure level p holding T constant we obtain
G − G
◦
T
= R
∗
T ln p, (8.69)
where p is in atm. In this formula
G is the Gibbs energy at pressure p and temperature
T and
G
◦
T
is the Gibbs energy at 1 atm and temperature T .
Using (8.69) we can write the change of Gibbs energy for the general chemical
reaction
G =[cG(C) + dG(D)]−[aG(A) + bG(B)] (8.70)
in the form
G =[c
G
◦
T
(C) + dG
◦
T
(D) − aG
◦
T
(A) − bG
◦
T
(B)]
+ cR
∗
T ln p
C
+ dR
∗
T ln p
D
− aR
∗
T ln p
A
− bR
∗
T ln p
B
= G
◦
T
+ R
∗
T ln
(p
C
)
c
(p
D
)
d
(p
A
)
a
(p
B
)
b
. (8.71)
The argument of the logarithmic function in the last formula is the equilibrium
constant K
p
(see (8.66)). Therefore,
G = G
◦
T
+ R
∗
T ln K
p
. (8.72)
At equilibrium
G = 0, and the change of Gibbs energy at a pressure of 1 atm and
arbitrary temperature T ,
G
◦
T
, is related to the equilibrium constant at pressure p
and temperature T , K
p
, by the simple relation:
G
◦
T
=−R
∗
T ln K
p
. (8.73)
The equation (8.73) tells us that if
G
◦
T
is positive, K
p
should be less than unity,
which means that at equilibrium the concentrations of the reactants will exceed
those of the products. If, on the other hand,
G
◦
T
is negative and, moreover, is
large, then K
p
is large and the products will dominatein the equilibrium.

8.6 Solutions 211
For standard conditions at a pressure of 1 atm and a temperature of 25
◦
Cwe
obtain, in joules,
G
◦
=−R
∗
× T
0
× ln K
p
=−2478.9 × ln K
p
(8.74)
The change in Gibbs energy G is especially useful because it simultaneously
takes into account both the First and Second Laws of Thermodynamics. It does so
in such a way that if the temperature and pressure are held constant (and they often
are in atmospheric problems) we have a function which can be applied much more
broadly.
Example 8.10 Consider the reaction of recombination of NO
2
and O
3
:
NO
2
+ O
3
NO
3
+ O
2
. (8.75)
Do the reactants or products dominate for the forward reaction at 1 atm and 25
◦
C?
Answer: We have to find the change of the Gibbs energy for this reaction.
G
◦
= (115.9 + 0 − 51.3 − 163.2) kJ mol
−1
=−98.6 kJ mol
−1
. (8.76)
From (8.74) we obtain K
p
= 1.9 ×10
17
. With such a large value of K
p
the products
will dominate for the forward reaction at equilibrium.
8.6 Solutions
Chemistry refresher A solution is a homogeneous mixture of several components.
Consider a two-component solution. One component has a mole fraction η
w
(the ratio
of the number of moles of a component to the total number of moles), and the other
component has mole fraction η. The component with a greater mole fraction, let it be
η
w
, is called the solvent. The solvent determines the state of matter of the solution
(gas, liquid or solid). The component with the smaller mole fraction, η, is called the
solute. In Chapter 5 we considered a cloud droplet as an example of a solution with
water as a solvent and the salt as a solute. A solution of a salt in a solvent such as
water is saturated when the rates of dissolving and crystallization are equal. In this
case there could be some substance in the crystalline form present in the composite
system. For example, there might be a salt crystal inside a cloud droplet. The amount
of dissolved material (solute) in the saturated solution is called its solubility, which
might depend strongly on temperature and weakly on pressure. We are interested in
the effect of the dissolved solute on the vapor pressure of the solvent.
Raoult’s Law Consider a solution that is in chemical equilibrium. The vapor
pressure of each component of the solution is approximately

212 Thermochemistry
p
i
= p
pure
i
η
i
(8.77)
where p
pure
i
is the vapor pressure of a pure ith component and η
i
is the mole fraction of
an ith component of the solution. The solution is called ideal when both solvent and
solute obey Raoult’s Law. Raoult’s Law applies when the components of the solution
are present in high concentrations. We used Raoult’s Law when we considered the
equilibrium vapor pressure over a droplet containing dissolved electrolytes.
For solutions at low concentrations the vapor pressure of the solute obeys Henry’s
Law. According to Henry’s Law, the vapor pressure of a solute, p, is a product of the
mole fraction of the solute, η, and an empirical tabulated constant, K
H
, expressed in
units of pressure:
p = K
H
η. (8.78)
Generally, the value of K
H
increases with increasing temperature. Thus, at the same
pressure the mole fraction of a solute decreases with increasing temperature.
When the atmospheric pressure decreases, the partial pressure of a gas decreases,
and the molar solubility of a gas decreases. For example, high in the mountains the
atmospheric pressure is low; as a result the solubility of oxygen in human blood
decreases, which can cause respiration problems. At the opposite end, the higher the
pressure, the higher the solubility of gases. You might say the gas is “squeezed” into
the solution.
Example 8.11 Calculate the molar solubility of nitrogen dissolved in1lofwater
at 25
◦
C and atmospheric pressure of 1 atm. Henry’s Law constant for nitrogen at
25
◦
C is 8.68 × 10
9
Pa. The percentage by volume of N
2
in dry air is 78.1.
Answer: The partial pressure of N
2
at 1 atm is p
N
2
= 0.781 × 1 atm = 7.91 ×
10
4
Pa. From Henry’s Law η
N
2
= p
N
2
/K
H
= (7.91 × 10
4
Pa)/(8.68 × 10
9
Pa) =
9.1 × 10
−6
. The mole fraction of nitrogen η
N
2
= ν
N
2
/(ν
N
2
+ ν
H
2
O
) ≈ ν
N
2
/ν
H
2
O
since ν
N
2
ν
H
2
O
. The number of moles of H
2
Oin1lis(1000/18) mol. Then,
ν
N
2
= (9.1 × 10
−6
× 1000/18) mol = 5.05 × 10
−4
mol. The molar solubility of
nitrogen is 5.05 × 10
−4
mol l
−1
.
Example 8.12 Calculate the molar solubility of CO
2
in moles per liter dissolved
in water at 25
◦
C and CO
2
pressure of 2.4 atm (pressure used to carbonate soda).
Henry’s Law constant for CO
2
at 25
◦
C is 1.67 × 10
8
Pa.
Answer: 2.4 atm = 2.43 × 10
5
Pa. The mole fraction of CO
2
according to Henry’s
Law is η
CO
2
= p
CO
2
/K
H
= (2.43 × 10
5
Pa)/(1.67 × 10
8
Pa). Since there is
(1000/18) mol of H
2
O in 1 l, the molar solubility of CO
2
is (2.43 × 10
5
Pa ×
1000/18 mol l
−1
)/(1.67 ×10
8
Pa) = 8.1 ×10
−2
mol l
−1
. When one opens a bottle
of soda, the pressure decreases; as a result the solubility of CO
2
decreases, and the
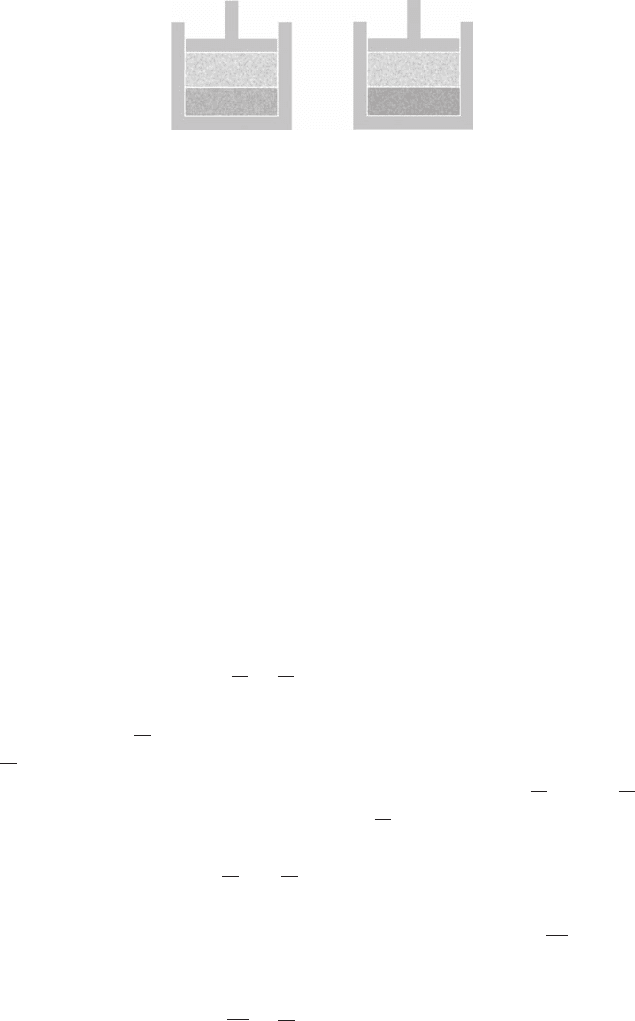
8.6 Solutions 213
(a)
(b)
pure solvent solution
vaporvapor
e
s
e9
Figure 8.7 Notation. (a) Pure solvent in equilibrium with its vapor at pressure e
s
.
(b) Solution in equilibrium with the solvent’s vapor at pressure e
.
bubbles of CO
2
emerge from the solution. At higher temperature the solubility of
CO
2
decreases since Henry’s constant increases (soda from a fridge sparkles less
than soda held at room temperature).
8.6.1 Molar Gibbs energy of an ideal solution
In this section we will find out how the Gibbs energy of a pure solvent changes when
a small amount of solute is added. We will consider liquids that are at equilibrium
with their vapors. This means that the Gibbs energy of the vapor is equal to the
Gibbs energy of the liquid. Let us denote the equilibrium vapor pressure over a
pure solvent as e
s
, and the equilibrium vapor pressure over the solution as e
(see
Figure 8.7). From (8.69) the molar Gibbs energy (the Gibbs energy per mole) of
vapor at pressure e
s
is
G = G
◦
T
+ R
∗
T ln e
s
(8.79)
where e
s
is in atm, G is the molar Gibbs energy
6
at pressure p and temperature T
and
G
◦
T
is the molar Gibbs energy at 1 atm and temperature T . Since at equilibrium
the molar Gibbs energy of a liquid is equal to that of the vapor,
G
vapor
= G
liquid
,
the Gibbs energy of a pure solvent, denoted as
G
w
,is
G
w
= G
◦
T
+ R
∗
T ln e
s
. (8.80)
When a solute is added, the molar Gibbs energy of the solvent,
G
, which is at
equilibrium with its vapor at pressure e
,is
G
= G
◦
T
+ R
∗
T ln e
. (8.81)
6
The molar Gibbs energy is often called the chemical potential.

214 Thermochemistry
Subtracting (8.80) from (8.81), we obtain the difference between the molar Gibbs
energy of the solvent in the solution and the pure solvent:
G
− G
w
= R
∗
T ln
e
e
s
. (8.82)
From Raoult’s Law, e
/e
s
= η
w
, where η
w
is the mole fraction of solvent (e.g.
water) in the solution. Then,
G
− G
w
= R
∗
T ln η
w
. (8.83)
We will consider a dilute solution, when the mole fraction of the solute, η , is much
smaller than that of the solvent, η η
w
. Since η + η
w
= 1, we rewrite (8.83)as
G
− G
w
= R
∗
T ln (1 − η). (8.84)
Taking into account that for η 1, the logarithmic function can be written as
ln(1 −η) ≈−η, we obtain
G
w
− G
= R
∗
T η. (8.85)
We will use (8.85) in the next section to find the temperature at which the solution
boils and freezes.
8.6.2 The elevation of the boiling point and the lowering of the freezing point
of a solution
When some solute is dissolved in a pure solvent, the boiling and freezing points of
the solution are not the same as for the pure solvent. We will show that the change
is proportional to the amount of solute. We will consider nonvolatile solutes (for
example, a salt). In this case the vapor of the solute is a pure gas.
Example 8.13 Show that the addition of a dissolved solute in a solution will elevate
the boiling point compared to the boiling point of pure solvent.
Answer: The boiling point is the temperature at which the saturated vapor pressure
of a liquid is the same as the atmospheric pressure. We consider two cases.
Case 1: equilibrium between a pure solvent and its vapor (see Figure 8.8a). The
pure solvent boils at temperature T
0
. The vapor pressure is 1 atm. At equilibrium,
the molar Gibbs energies of the pure solvent, denoted below as
G
w
, and its vapor,
denoted as
G
v
, are equal to each other:
G
v
(T
0
, p) = G
w
(T
0
, p). (8.86)
8.6 Solutions 215
pure solvent
vapor
vapor
(a)
(b)
G
v
(T, p)
G⬘(T, p)
solution
G
w
(T
0
, p)
G
v
(T
0
, p)
Figure 8.8 Notation. (a) Molar Gibbs energy for the vapor over the pure solvent,
G
v
(T
0
, p), and molar Gibbs energy for the pure solvent, G
w
(T
0
, p). (b) Molar Gibbs
energy of solvent vapor over the solution,
G
v
(T , p), and molar Gibbs energy of
the solvent,
G
(T , p).
Case 2: there is an amount η of nonvolatile solute in the solvent. The solution is
at equilibrium with the solvent’s vapor (see Figure 8.8b). The vapor pressure at the
boiling point is 1 atm, the same as in case 1, but the boiling point differs. Assume
that the solution boils at a temperature T = T
0
+ T where T T
0
. From
(8.85) the molar Gibbs energy of the solvent in the solution is
G
= G
w
− R
∗
T η.
At equilibrium, the molar Gibbs energy of the solvent in the solution,
G
, is equal
to the molar Gibbs energy of the vapor,
G
v
:
G
v
(T
0
+ T , p) = G
(T
0
+ T , p) (8.87)
or
G
v
(T
0
+ T , p) = G
w
(T
0
+ T , p) − R
∗
T η. (8.88)
Since T T
0
we can expand both sides of (8.88) in Taylor’s series retaining
only the linear term:
G
v
(T
0
+ T , p) = G
v
(T
0
, p) +
∂
G
v
∂T
(T
0
,p)
T , (8.89)
G
w
(T
0
+ T , p) = G
w
(T
0
, p) +
∂
G
w
∂T
(T
0
,p)
T . (8.90)
Substituting these expansions in (8.88) and taking into account (8.86), we obtain
R
∗
T
0
η =
∂
G
w
∂T
(T
0
,p)
−
∂
G
v
∂T
(T
0
,p)
T . (8.91)
Here we also replaced T η by T
0
η since η has a small value proportional to T .
216 Thermochemistry
As shown in Section 4.8, the temperature derivative of the Gibbs energy at a
constant pressure is minus the entropy. So,
∂
G
w
∂T
(T
0
,p)
=−s
w
(8.92)
and
∂
G
v
∂T
(T
0
,p)
=−s
v
(8.93)
where
s
w
is the entropy of 1 mol of the solvent and s
v
is the entropy of 1 mol of
the solvent’s vapor. Then, from (8.91) we obtain
R
∗
T
0
η = (s
v
− s
w
)T (8.94)
and
T =
R
∗
T
0
η
s
v
− s
w
. (8.95)
Multiplying and dividing the right-hand side of the last equation by T
0
and taking
into account that (
s
v
− s
w
)T
0
is the amount of heat required to evaporate 1 mol of
the solvent at the boiling point, in other words (
s
v
− s
w
)T
0
=
vap
H
◦
, we obtain
the final formula for the elevation of the boiling point T :
T =
R
∗
T
2
0
η
vap
H
◦
(8.96)
Since T is positive, the boiling point of the solution is higher than that of the pure
solvent. The change in the boiling point is proportional to the amount of solute
η.
Example 8.14 What is the change in the boiling temperature of 1 l of water with
15 g of NaCl dissolved in it?
Answer: The molecular weight of NaCl is 58.44 g mol
−1
. Substituting in (8.96) η =
(15/58.44)/(1000/18), R
∗
= 8.31 J K
−1
mol
−1
, T
0
= 373 K,
vap
H
◦
= 40.656
kJ mol
−1
, we obtain T = 0.13 K.
Example 8.15 Calculate the change in the freezing point of the solution.
Answer: We assume that only pure solvent is frozen, while the solute remains in
the solution. Then the calculation of the freezing point of the solution is similar to
the calculation of the boiling point except we have fusion instead of vaporization.
fus
H
◦
is the heat released when 1 mol of the solvent is frozen at a temperature T
0
;
