Murray J.D. Mathematical Biology: I. An Introduction
Подождите немного. Документ загружается.


256 7. Biological Oscillators and Switches
and sketch the domain. Indicate how the domain for periodic solutions changes as
the inhibition parameter K varies.
7 The two-variable FitzHugh–Nagumo model for space-clamped nerve axon firing
with an external applied current I
a
is
dv
dt
= v(a −v)(v − 1) −w + I
a
,
dw
dt
= bv −γw,
where 0 < a < 1andb, γ and I
a
are positive constants. Here v is directly related
to the transmembrane potential and w is the variable which represents the effects of
the various chemical ion-generated potentials.
Determine the local maximum and minimum for the v null cline in terms
of a and I
a
and hence give the corresponding piecewise linear approximate form.
Show that there is a confined set for the model system. Using the piece-
wise linear model, determine the conditions on the parameters such that the positive
steady state is stable but excitable. Find the conditions on the parameters, and the rel-
evant window (I
1
, I
2
) of applied currents, for the positive steady state to be linearly
unstable and hence for a limit cycle solution to exist. For a fixed set of parameters
a, b and γ , find the period of the small amplitude limit cycle when I
a
is just greater
than the bifurcation value I
1
. [Use the Hopf bifurcation result near bifurcation.]
8 Consider a simplified model for the control of testosterone secretion given by
dR
dt
= f (T ) − b
1
R,
dT
dt
= b
2
R(t −τ) − b
3
T,
where R denotes the luteinising hormone releasing hormone (LHRH), T denotes
the hormone testosterone and f (T ) is a positive monotonic decreasing function of
T . The delay τ is associated with the blood circulation time in the body and b
1
, b
2
and b
3
are positive constants. When τ = 0, show that the steady state is stable. Using
the method in Section 7.6 investigate the possibility of periodic solution behaviour
when τ>0.
8. BZ Oscillating Reactions
8.1 Belousov Reaction and the Field–K
¨
or
¨
os–Noyes (FKN) Model
The reaction known as the Belousov–Zhabotinskii reaction is an important oscillating
reaction discovered by the Russian Boris Belousov (1951), a biochemist, and is de-
scribed in an unpublished paper, which was contemptuously rejected by a journal editor;
at the time the accepted dogma was that oscillating reactions were simply not possible.
A translation of the original article is given in the book edited by Field and Burger
(1985). Eventually Belousov (1959) published a brief note in the obscure proceedings
of a Russian medical meeting. Basically he found oscillations in the ratio of concen-
trations of the catalyst; in Belousov’s reaction it was cerium in the oxidation of citric
acid by bromate. The oscillation manifested itself via a colour change as the cerium
changed from Ce
3+
to Ce
4+
although it is more dramatic with an iron ion, ferroin
where the colour is brick red when in the Fe
2e
state and bright blue in the Fe
3e
state.
The study of this reaction was continued by Zhabotinskii (1964) and is now known
as the Belousov–Zhabotinskii reaction or simply the BZ reaction. When the details of
this important reaction and some of its dramatic oscillatory and wavelike properties
finally reached the West in the 1970’s it provoked widespread interest and research.
Belousov’s seminal work was finally, but posthumously, recognised in 1980 by his be-
ing awarded the Lenin Prize. Winfree (1984) gives a brief interesting description of
the history of the Belousov–Zhabotinskii reaction. When the reactants can also diffuse
a diverse menagerie of complex patterns can be formed and it is the latter which has
sustained the continuing widespread interest among both biological and physical scien-
tists. It provided an enormous incentive to those who were interested in the fundamental
question of spatiotemporal self-organisation with all the implications for the generation
of biological pattern and form. We discuss such aspects in considerable detail in later
chapters.
Before discussing the reaction in detail it is highly pertinent to mention its analogy
with real biological oscillators, not at the molecular level, of course, but in the similarity
it has with an increasing number of real biological cyclical behaviour. Approximate
equations for the BZ reaction are identical to those that have arisen in a realistic model
for the periodic signalling exhibited by cells during the self-organisation of the slime
mould Dictyostelium discoideum (see, for example, the book by Goldbeter 1996). There
is, of course, no analogy at the molecular level but nevertheless a knowledge of the
behaviour from similar equations can still be very useful. Winfree (1987) also used it in a
258 8. BZ Oscillating Reactions
highly original and elegant way in modelling three-dimensional activity in the ventricle
of the heart. The analogy has also been applied by Tyson (1991) in his investigations
into the molecular biology of the cell cycle. This reaction is certainly not just some
academic curiosity.
There are now many such chemical reactions which can exhibit periodic behaviour
and the term BZ reaction now refers to a general class of such reactions, essentially
where an organic substance is oxidised by bromate ions facilitated by a metal ion in an
acid medium. Typical metal ions are cerium and ferroin. Although it is a chemical rather
than a biological oscillator the BZ reaction is now considered the prototype oscillator.
The detailed reactions involved are more or less understood, as are many, but certainly
not all, of the complex spatial phenomena it can exhibit: we shall describe some of the
wavelike properties in Chapter 1, Volume II. The book of articles edited by Field and
Burger (1985) is a good and varied introduction to the BZ reaction. It also has several
articles on chemical oscillators and wave phenomena. The book by Winfree (2000),
among other things, also discusses some of the reaction’s properties, both temporal
and spatial. The article by Tyson (1994) is a succinct and more recent summary of the
phenomena exhibited by the BZ kinetics, both temporal and spatial. In this chapter we
consider the reaction in some detail not only because of its seminal importance in the
field, but also because it illustrates techniques of analysis which have wide applicability.
References to the more detailed kinetics are given at the appropriate places. Almost all
the phenomena theoretically exhibited by reaction and reaction diffusion mechanisms
have been found in this real and practical reaction—but many of these only after the
mathematics predicted them.
The BZ reaction (the general class of them) is probably the most widely studied
oscillating reaction both theoretically and experimentally. Here we briefly describe the
key steps in the reaction and develop the Field–K
¨
or
¨
os–Noyes (Field and Noyes 1974)
model system which quantitatively mimics the actual chemical reactions (Field et al.
1972). The models for the BZ reaction are prototypes to study since the theoretical
developments can be tested against experiments. The experience gained from this is
directly transferable to biochemical oscillators as mentioned above. The literature on
the subject is now large. A succinct review of the detailed reaction and its properties is
given by Tyson (1994).
In the original Belousov (1951) reaction, the basic mechanism consists of the ox-
idation of malonic acid, in an acid medium, by bromate ions, BrO
3
−
, and catalyzed
by cerium, which has two states Ce
3+
and Ce
4+
. Sustained periodic oscillations are
observed in the cerium ions. With other metal ion catalysts and appropriate dyes, for
example, iron Fe
2+
and Fe
3+
and phenanthroline, the regular periodic colour change
is visually dramatic, oscillating as mentioned between red and blue. It is not only the
catalyst ion concentrations which vary with time, of course, other reactants also vary.
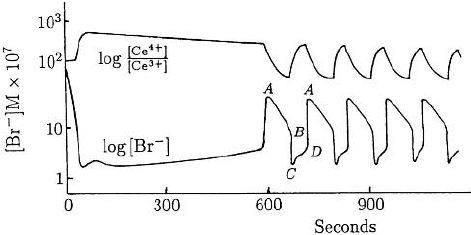
Figure 8.1 illustrates the temporal variations in the bromide ion concentration [Br
−
]and
the cerium ion concentration ratio [Ce
4+
]/[Ce
3+
] measured by Field et al. (1972), who
studied the mechanism in depth; see Tyson (1994) for more references and technical
details.
Basically the reaction can be separated into two parts, say I and II, and the concen-
tration [Br
−
] determines which is dominant at any time. When [Br
−
] is high, near A in
Figure 8.1, I is dominant and during this stage Br
−
is consumed; that is, we move along
8.1 Belousov Reaction and Field–K
¨
or
¨
os–Noyes Model 259
Figure 8.1. Experimentally measured periodic limit cycle type of temporal variation in the concentrations in
the ratio of the cerium metal ion concentration [Ce
4+
]/[Ce
3+
] and the bromide ion concentration [Br
−
]in
the Belousov–Zhabotinskii reaction. (Redrawn from Field et al. 1972)
AB, and the cerium ion is mainly in the Ce
3+
state. As [Br
−
] decreases further it passes
through a critical value, B, and then drops quickly to a low level, that is, C in Figure 8.1.
At this stage process II takes over from I. During II the Ce
3+
changes to Ce
4+
.However,
in the II-process Ce
4+
reacts to produce Br
−
again while it reverts to the Ce
3+
state.
Now [Br
−
] increases, that is, along CDA, and, when its value is sufficiently high, pro-
cess I again becomes dominant. The whole sequence is continually repeated and hence
produces the observed oscillations. This qualitative description is not, of course, suffi-
cient to show that the reaction will actually oscillate. The two pathways, I and II, could
simply reach some steady state of coexistence. In fact for certain parameter ranges this
is exactly what can happen. The parameter range required for oscillatory behaviour is
derived in detail below and depends on a careful analysis of the kinetics equations.
The rapid variation along BC and DAis typical of a relaxation oscillator, which is
just an oscillator in which parts of the limit cycle are traversed quickly. This behaviour
suggests a particular asymptotic technique which often allows us to get analytical results
for the period in terms of the parameters; we discuss this later in Section 8.4.
Although there are many reactions involved they can be rationally reduced to 5 key
reactions, with known values for the rate constants, which capture the basic elements of
the mechanism. These five reactions can then be represented by a 3-chemical system in
which the overall rate constants can be assigned with reasonable confidence. This model
is known as the Field–K
¨
or
¨
os–Noyes or FKN model and is the specific model proposed
by Field and Noyes (1974) based on the Field–K
¨
or
¨
os–Noyes (1972) mechanism. We
give this simpler model system and derive the 3-species model. A complete derivation
from the chemistry together with estimates for the various rate constants are given by
Tyson (1994).
The key chemical elements in the 5-reaction FKN model are
X = HBrO
2
, Y = Br
−
, Z = Ce
4+
,
A = BrO
−
3
, P = HOBr,
(8.1)
and the model reactions can be approximated by the sequence

260 8. BZ Oscillating Reactions
A +Y
k
1
→ X + P, X + Y
k
2
→ 2P
A + X
k
3
→ 2X + 2Z, 2X
k
4
→ A + PZ
k
5
→ fY,
(8.2)
where the rate constants k
1
,... ,k
5
are known and f is a stoichiometric factor, usually
taken to be 0.5. The first two reactions are roughly equivalent to the process I, described
above, while the last three relate approximately to process II. The form used by Field
and Noyes (1974) is actually slightly different in that the last reaction is B + Z →
fY + Q where B represents organic acids and Q is CO
2
. [B] is a constant and can
be incorporated into the rate constant in the model equation system. It is reasonable to
take the concentration [A] of the bromate ion to be constant; the concentration [P] is
not of interest here. So, using the Law of Mass Action on (8.2), we get the following
third-order system of kinetics equations for the concentrations, denoted by lowercase
letters:
dx
dt
= k
1
ay − k
2
xy +k
3
ax − k
4
x
2
,
dy
dt
=−k
1
ay − k
2
xy + fk
5
z,
dz
dt
= 2k
3
ax − k
5
z.
(8.3)
This oscillator system is sometimes referred to as the ‘Oregonator’ since it exhibits limit
cycle oscillations and the research by Field et al. (1972) was done at the University of
Oregon.
Oscillatory behaviour of (8.3) depends critically on the parameters involved. For
example, if k
5
= 0, the bromide ion (Br
−
) concentration y decays to zero according to
the second equation, so no oscillations can occur. On the other hand if f = 0.5andk
5
is
very large, the last reaction in (8.2) is very fast and the third and fifth reactions in (8.2)
effectively collapse into the single reaction
A + X
k
3
→ 2X +Y.
The system then reduces to a two-species mechanism, which is bimolecular, and so
cannot oscillate (Hanusse 1972). There is clearly a domain in the ( f, k
5
) plane where
periodic behaviour is not possible.
The only sensible way to analyse the system (8.3) is in a dimensionless form. There
are usually several ways to nondimensionalise the equations; see, for example, Murray
(1977) and Tyson (1982, 1985), both of whom give a fuller description of the chemistry
and justification for the model. Here we give only the more recent one suggested by
Tyson (1985) which incorporates the estimated values of the rate constants in the above
model (8.3). Often different nondimensionalisations highlight different features of the
oscillator. Following Tyson (1985), introduce
x
∗
=
x
x
0
, y
∗
=
y
y
0
, z
∗
=
z
z
0
, t
∗
=
t
t
0

8.2 FKN Model and Limit Cycle Solutions 261
x
0
=
k
3
a
k
4
≈ 1.2 ×10
−7
M, y
0
=
k
3
a
k
2
≈ 6 ×10
−7
M,
z
0
=
2(k
3
a)
2
k
4
k
5
≈ 5 ×10
−3
M, t
0
=
1
k
5
≈ 50s,
ε =
k
5
k
3
a
≈ 5 ×10
−5
,δ=
k
4
k
5
k
2
k
3
a
≈ 2 ×10
−4
,
q =
k
1
k
4
k
2
k
3
≈ 8 ×10
−4
,(f ≈ 0.5)
(8.4)
which we substitute into (8.3). Field and Noyes (1974) suggested the value for f , based
on experiment. As said before, since the model telescopes a number of reactions the
parameters cannot be given unequivocally; the values are ‘best’ estimates. For our pur-
poses here we need only the fact that ε, δ and q are small. With (8.4) we get the fol-
lowing dimensionless system, where for algebraic convenience we have omitted the
asterisks,
ε
dx
dt
= qy − xy + x(1 − x),
δ
dy
dt
=−qy − xy + 2 fz,
dz
dt
= x − z.
(8.5)
In vector form with r = (x, y, z)
T
we can write this as
dr
dt
= F(r;ε, δ, q, f ) =
ε
−1
(qy − xy + x − x
2
)
δ
−1
(−qy − xy +2 fz)
x − z
. (8.6)
8.2 Linear Stability Analysis of the FKN Model and Existence of
Limit Cycle Solutions
Even though the system (8.5) is third-order, the linear stability analysis procedure is
standard and described in detail in Chapter 3; namely, first find the positive steady state
or states, determine the eigenvalues of the linear stability matrix and look for a confined
set, which is a finite closed surface S enclosing the steady state such that any solution at
time t
0
which lies inside S always remains there for all t > t
0
. This was done by Murray
(1974) for the original, but only slightly different, FKN equation system; it is his type
of analysis we apply here to (8.6).
The nonnegative steady states (x
s
, y
s
, z
s
) of (8.5) are given by setting the left-hand
sides to zero and solving the resulting system of algebraic equations, to get
262 8. BZ Oscillating Reactions
(0, 0, 0) or z
s
= x
s
, y
s
=
2 fx
s
q + x
s
,
x
s
=
1
2
(1 − 2 f − q) +[(1 −2 f −q)
2
+4q(1 + 2 f )]
1/2
.
(8.7)
The other nonzero steady state is negative.
Linearising about (0, 0, 0) we obtain the stability matrix A with eigenvalues λ given
by
| A −λI |=
ε
−1
−λ qε
−1
0
0 −qδ
−1
−λ 2 f δ
−1
10−1 −λ
= 0
⇒ λ
3
+λ
2
(1 +qδ
−1
−ε
−1
) − λ[ε
−1
(1 + qδ
−1
) − qδ
−1
]−
q(1 + 2 f )
εδ
= 0.
If we simply sketch the left-hand side of this cubic as a function of λ for λ ≥ 0wesee
that there is at least one positive root. Alternatively note that the product of the three
roots is q(1+2 f )/εδ > 0, which implies the same thing. Thus the steady state (0, 0, 0)
is always linearly unstable.
If we now linearise (8.5) about the positive steady state (x
s
, y
s
, z
s
) in (8.7) the
eigenvalues λ of its stability matrix are given, after a little algebra, by
| A −λI |=
1 −2x
s
− y
s
ε
−λ
q − x
s
ε
0
−y
s
δ
−
x
s
+q
δ
−λ
2 f
δ
10−1 −λ
= 0
⇒ λ
3
+ Aλ
2
+ Bλ +C = 0,
(8.8)
where, on using the quadratic for x
s
, the simultaneous equations for x
s
, y
s
from (8.6)
and some tedious but elementary algebra, we get
A = 1 +
q + x
s
δ
+
E
ε
,
E = 2x
s
+ y
s
−1 =
x
2
s
+q(x
s
+2 f )
q + x
s
> 0,
B =
q + x
s
δ
+
E
ε
+
(q + x
s
)E + y
s
(q − x
s
)
εδ
,
C =
(q + x
s
)E − 2 f (q − x
s
) + y
s
(q − x
s
)
εδ
=
x
2
s
+q(2 f + 1)
εδ
> 0.
(8.9)
Note that A > 0, since E > 0, and that C > 0, on using the expression for x
s
from (8.7);
8.2 FKN Model and Limit Cycle Solutions 263
B can be positive or negative. It follows from Descartes’ rule of signs (see Appendix B)
that at least one eigenvalue λ in (8.8) is real and negative. The remaining necessary and
sufficient condition for all of the solutions λ to have negative real parts is, from the
Routh–Hurwitz conditions (see Appendix B), AB − C > 0. Substituting for A, B and
C from (8.9) gives a quadratic in 1/δ for the left-hand side and hence the condition for
stability of the positive steady state in (8.7). This is given by
AB −C = φ(δ, f,ε) =
Nδ
2
+ Mδ + L
δ
2
> 0,
L = (q + x
s
)
(q + x
s
) +
x
x
(1 −q − 4 f ) + 2q(1 +3 f )
ε
,
N =[x
2
s
+q(x
s
+2 f )]
1 + E/ε
ε(q + x
s
)
> 0
(8.10)
with M also determined as a function of x
s
, f , q and ε; we do not require it in the
subsequent analysis and so do not give it here. With x
s
from (8.7), L, M and N are
functions of f , q and ε. Thus for the steady state to be linearly unstable, δ, f and ε must
lie in a domain in (δ, f,ε) space where φ(δ, f,ε) < 0. The boundary or bifurcation
surface in (δ, f,ε)space is given by φ(δ, f,ε) = 0.
We can get an indication of the eigenvalue behaviour asymptotically for large pos-
itive and negative B.IfB 1 the asymptotic solutions of (8.8) are given by
λ ∼−
C
B
, −
A
2
±i
√
B, (8.11)
while if B < 0and| B |1,
λ ∼
C
| B |
, ±
| B |. (8.12)
So, for large positive B condition (8.10) is satisfied and from (8.11), Re λ<0andthe
steady state is linearly stable, while if B is large and negative, it is unstable.
When the parameters are such that B = C/A, the bifurcation situation, we can
solve for the roots λ in (8.8), namely,
λ =−A, ±i
√
B, when B =
C
A
. (8.13)
If B = (C/A) − ω,0<ω 1, it can be seen by looking for asymptotic solutions to
(8.8) in the form λ =±i(C/A)
1/2
+ O(ω) that the O(ω) term has a positive real part.
Thus, near the bifurcation surface in the unstable region, the steady state is unstable by
growing oscillations. The conditions of the Hopf bifurcation theorem (see, for example,
Strogatz 1994) are satisfied and so in the vicinity of the surface φ(δ, f,ε) = 0the
system exhibits a small amplitude limit cycle solution with period
T =
2π
C
A
1/2
. (8.14)

264 8. BZ Oscillating Reactions
With the parameter values obtained from experiment, and given in (8.4), the amplitudes
of the oscillations in fact are not small, so the last expression is of pedagogical rather
than practical use. However, the bifurcation surface φ(δ, f,ε) = 0 given by (8.10) is of
practical use and this we must discuss further.
The surface φ(δ, f,ε) = 0, namely, Nδ
2
+ Mδ + L = 0 in (8.10), where N > 0,
is quadratic in δ. So, for the steady state to be unstable, that is, δ, f and ε make φ<0,
δ must be such that
0 <δ<
1
2N
−M +[M
2
−4LN]
1/2
. (8.15)
But there is a nonzero range of positive δ only if the right-hand side of this inequality is
positive, which requires, with L from (8.10),
L = (q + x
s
)
(q + x
s
) +
x
s
(1 −q − 4 f ) + 2q(1 +3 f )
ε
< 0.
With x
s
from (8.7) this gives an algebraic equation relating f , q and ε. From (8.4) ε is
small, so to first-order the last inequality gives the numerator in the ε
−1
term equal to
zero, which reduces to
(1 −4 f − q){(1 −2 f −q) +[(1 −2 f − q)
2
+4q(1 +2 f )]
1/2
}
+4q(1 + 3 f )<0,
(8.16)
which (with an equals sign in place of <) defines the critical f , f
c
,foragivenq. In fact
there are two critical f ’s, namely,
1
f
c
and
2
f
c
and f must lie between them:
1
f
c
< f <
2
f
c
.
With q = 8 ×10
−4
we can determine accurate values for
1
f
c
and
2
f
c
by exploiting the
fact that 0 < q 1. The critical f
c
are given by (8.16) on replacing the inequality sign
with an equals sign. Suppose first that (1 −2 f −q)>0; that is, 2 f < 1toO(1).Then
on letting q → 0,
(1 −4 f )(1 −2 f ) ≈ 0 ⇒
1
f
c
≈
1
4
.
With (1 −2 f −q)<0, that is, 2 f > 1toO(1), the limiting situation q → 0hastobe
done carefully; this gives
2
f
c
.ToO(q), (8.16), again with an equals sign, now becomes
(1 −4 f )
−(2 f + q −1) +(2 f + q −1)
1 +
2q(1 + 2 f )
(2 f + q −1)
2
+4q(1 + 3 f ) ≈ 0,
which reduces to
8.3 Nonlocal Stability of the FKN Model 265
4 f
2
−4 f − 1 ≈ 0 ⇒
2
f
c
≈
1 +
√
2
2
.
So, for small q, the range of f for which the positive steady state in (8.7) is linearly
unstable is
1
4
≈
1
f
c
< f <
2
f
c
≈
1 +
√
2
2
. (8.17)
Finally the stability bifurcation curve of δ against f for each ε is given by (8.15),
namely,
δ =
1
2N
−M +[M
2
−4LN]
1/2
. (8.18)
with
1
f
c
< f <
2
f
c
, where the critical f
c
are obtained from (8.16), and L, M and N
are defined by (8.10), with (8.9), in terms of f , q and ε.
8.3 Nonlocal Stability of the FKN Model
We showed in the last section that for each ε,ifδ and f lie in the appropriate domain, the
positive steady state is linearly unstable, and indeed by growing oscillations if (δ, f ) are
close to the bifurcation curve. Wherever (δ, f ) lies in the unstable domain, we must now
consider global stability. Even though we do not have the equivalent of the Poincar
´
e–
Bendixson theorem here since we are dealing with a third-order system, the existence
of a periodic solution with finite amplitudes requires the system to have a confined set,
S say. That is, with n the unit outward normal to S,wemusthave
n ·
dr
dt
< 0, r on S, (8.19)
where dr/dt is given by (8.6).
Although the existence of a confined set and a single unstable steady state (by grow-
ing oscillations) is not sufficient to prove the existence of a periodic limit cycle, they
give sufficient encouragement to pursue the analysis further. With three equations it is
possible to have chaotic solutions, such as we found with discrete models in Chapter 2.
Chaotic behaviour in this sense has been found in the BZ reaction (see, for example,
Scott 1991). (The now classical Lorenz (1963) model is a 3-equation system and it ex-
hibits chaos; it models a fluid dynamics situation.) Hastings and Murray (1975) gave a
rigourous proof, together with a procedure which determines the general trajectory path
over a cycle, showing that the FKN model system (8.5) possesses at least one limit cycle
periodic solution. The procedure they developed has wider applications to a fairly broad
class of feedback control systems as has been demonstrated by Hastings et al. (1977).
Let us look for the simplest surface S, namely, a rectangular box defined by the
faces
