Magill J., Galy J. Radioactivity Radionuclides Radiation
Подождите немного. Документ загружается.



Joseph Magill
Jean Galy
Radioactivity
·
Radionuclides
·
Radiation

Joseph Magill
Jean Galy
Radioactivity
Radionuclides
Radiation
Including the Universal Nuclide Chart
on CD-ROM
With 130 Figures, 57 in Colour, 43 Tables, a CD-ROM
and with the Fold-out of the Karlsruhe Chart of the Nuclides
Joseph Magill
Jean Galy
European Commission
Joint Research Centre
Institute for Transuranium Elements
P. O. Box 2340
76125 Karlsruhe, Germany
magill@itu.fzk.de
· jean.galy@itu.fzk.de
www.nuclides.net
Library of Congress Control Number: 2004111115
ISBN 3-540-21116-0 Springer Berlin Heidelberg New York
Cover image: The illustration shows a 10 year-old curium oxide (
244
Cm half-life 18.1 y) sample. The
dendritic surface features are the result of transmutation by natural radioactive decay in about 55–60% of
the atoms. More details are given in Chapter 5.
This work is subject to copyright. All rights are reserved, whether the whole or part of the material is
concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting,
reproduction on microfilm or in any other way, and storage in data banks. Duplication of this publication or
parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965,
in its current version, and permission for use must use must always be obtained from Springer-Verlag.
Violations are liable for prosecution under the German Copyright Law.
Springer is a part of Springer Science+Business Media
springeronline.com
© Springer-Verlag Berlin Heidelberg and European Communities 2005
Printed in Germany
The use of registered names, trademarks, etc. in this publication does not imply, even in the absence of
a specific statement, that such names are exempt from the relevant protective laws and regulations and
therefore free for general use.
Please note: All rights pertaining to the software (program and this book) are owned exclusively by
the authors, Springer-Verlag, and the European Communities. The software is protected by copyright.
The authors, Springer-Verlag and the European Communities accept no legal responsibility for any dam-
age caused by improper use of the instructions and programs contained in this book and the CD-ROM.
Although the software has been tested with extreme care, errors in the software cannot be excluded. De-
compiling, disassembling, reverse engineering or in any way changing the program is expressly forbidden.
Cover design: Erich Kirchner, Heidelberg
Typesetting: Typeset in Word by the authors and edited by PublicationService Gisela Koch, Wiesenbach,
using a modified Springer L
A
T
E
X macro-package.
Printed on acid-free paper 57/3141/Ko–543210

Contents
radioactivity
1. Origins and Discovery ............................................... 1
The Greek Elements and the Indivisible Atom ......................... 1
Twentieth Century Science and the Multi-Corpuscular Atom ............ 4
Discovery of Radioactivity ......................................... 5
Structure of the Atom: Kelvin-Thomson “Plum Pudding” Atom .......... 6
Bohr-Sommerfeld Model of the Atom ............................... 8
The Neutron ..................................................... 11
Wave/Particle Duality: The de Broglie Relation and Wave Mechanics ..... 12
Classification of the Elements ...................................... 14
Synthesis of the Elements in Nature ................................. 15
2. Nuclear Energetics .................................................. 19
Nuclear Units – Atomic Mass Unit and the Electron Volt ................ 19
Nuclear and Atomic Masses ........................................ 19
Atomic and Molecular Weights ..................................... 20
Avogadro’s Number............................................... 21
Mass of an Atom and Atomic Number Density ........................ 22
Relativistic Mechanics and Einstein’s Mass/Energy Equivalence ......... 23
Binding Energy of the Nucleus ..................................... 24
Nucleon Separation Energy ........................................ 26
Q-Value for a Reaction ............................................ 28
Energy Level Diagrams ........................................... 29
Nuclear Spin and Parity ........................................... 30
Nuclear Isomerism ............................................... 31
Nuclide Charts ................................................... 31
3. Radioactivity and Nuclear Reactions ................................. 43
Simple Radioactive Decay: Half-life and Decay Constant ............... 44
Activity ......................................................... 44
Average (Mean) Lifetime .......................................... 45
Branching Ratios and Number of Decay Modes ....................... 45
Decay Chains .................................................... 46
Radioactive Equilibria............................................. 49

VI Contents
Decay Energy.................................................... 52
Nuclear Reactions ................................................ 53
Cross-sections ................................................... 54
Neutron Reactions ................................................ 56
Burnout Time .................................................... 56
4. Types of Radioactive Decay .......................................... 59
Decay Modes .................................................... 59
Alpha (α) Decay ................................................. 62
Beta-minus (β
−
) Decay ........................................... 64
Gamma Emission and Isomeric Transition (IT) ........................ 67
Internal Conversion (IC) ........................................... 68
Beta-plus (β
+
) Decay (Positron Emission) ............................ 69
Electron Capture (ε orec).......................................... 71
Spontaneous Fission (SF) .......................................... 73
Proton Decay .................................................... 77
Special Beta-Decay Processes ...................................... 79
Heavy-Ion or Cluster Radioactivity .................................. 81
Magic Radioactivity .............................................. 82
Decay of Bare Nuclei – Bound Beta Decay ........................... 83
Halo Nuclides ................................................... 86
radionuclides
5. Transmutation Research ............................................ 89
How Constant is the Decay Constant? ............................... 89
Natural Transmutation by Radioactive Decay ......................... 90
Synthesis of Superheavy Elements by Transmutation ................... 91
Laser Transmutation .............................................. 92
6. Archaeology and Dating ............................................ 105
Radioactive Dating ...............................................105
An Astrophysical Clock ...........................................108
Age of the Earth ..................................................109
Prehistoric Cave Art at Altamira, Northern Spain ......................111
The Age of Groundwater – The Oasis Ballad Seet......................112
Nuclear Forensic Science – Age of Plutonium Particles .................114
7. Radioisotopes in Medicine .......................................... 117
Imaging ........................................................117
External Beam Radiotherapy .......................................119
Brachytherapy ...................................................119
Immunotherapy ..................................................119
Ion Beam Therapy ................................................119
Boron Neutron Capture Therapy ....................................120
Radioactive “Bullets” – Alpha-Immunotherapy ........................121

Contents VII
8. Scientific and Industrial Applications ................................125
Radioisotope Tracers ..............................................126
Radiography and Gauging .........................................128
Radiation Processing ..............................................133
Nuclear Batteries .................................................135
radiation
9. Radiation and the Environment ..................................... 139
Biological Effects of Ionising Radiation ..............................139
Radiotoxicity and Annual Limits of Intake ............................142
Radiation Hormesis and the Linear Non-Threshold (LNT) Model ........144
High Background Radiation Areas Around the World ...................146
Radon: A Test for the LNT Hypothesis? ..............................147
Radiation Exposure in High-Flying Aircraft ..........................149
Conan the Bacterium..............................................152
Packaging and Transport of Radioactive Materials .....................153
Nuclear Waste Disposal ...........................................155
Nuclear Tests in the South Pacific ...................................158
The Chernobyl Accident ...........................................161
The Goiˆania Radiation Incident – a Benchmark for Radiological
Dispersion Devices (RDDs) ...................................162
References ............................................................ 167
Recommended Reading and Weblinks .................................. 175
Reading ........................................................175
Weblinks ........................................................176
Glossary .............................................................. 177
Appendices ............................................................189
Appendix A: Physical Constants, Conversion Factors, Prefixes,
Greek Alphabet ..............................................189
Appendix B: Table of the Elements ..................................191
Appendix C: Properties of the Elements ..............................194
Appendix D: Table of Atomic Masses ................................197
Appendix E: Universal Nuclide Chart ................................229
Computer System Requirements ................................229
User Interface ...............................................230
Radioactive Decay Chain Simulator .............................233
Neutron Reaction Path Simulator ...............................236
Appendix F: Periodic Table of the Elements ..........................239
Appendix G: Karlsruhe Chart of the Nuclides – Karlsruher Nuklidkarte . . . 243
Index ................................................................. 249

1. Origins and Discovery
The Greek Elements and the Indivisible Atom
Around 2500 years ago, most people believed in a world ruled by gods with supernat-
ural powers. All the more surprising then to realise that there was a group of Greek
thinkers seeking a rational explanation of the observable world in terms of natural
causes and effects. This “natural” philosophy, although different from the scientific
enquiry we know today, was based on reason and contained the principal ingredients
of the scientific approach [1] i.e.
• The identification of the universal and essential
• The belief that nature can be explained in terms of simple “elements” and their
interactions
The first school of rational thought is believed to be the Milesian school based in
Miletus in Ionia in the 6th and 5th centuries B. C. At this time Miletus was the most
important city on the Aegean Sea. It was in this early school that Thales, Anaximenes,
and Anaximander were searching for rational and natural causes in terms of a “pri-
mary reality” or “primordial substance”. These early philosophers differed, however,
in the choice of what the substance should be. Thales believed water was primordial,
Anaximenes chose air, and Anaximander apeiron – an indefinite substance. They
each had their own views on how the simple elements could transform to give rise to
the diversity of natural objects. Thales believed in an unknown process. Anaximenes
proposed a process of rarefaction and condensation, and Anaximander a growth or
push as the driving force.
Since none of these single element theories were entirely satisfactory, more so-
phisticated approaches with two-elements theories (Xenophanes and Parmenides),
four-element (Empedocles of Agrigentum), or indeed infinite-element (Anaxagoras
of Clazomenae) were proposed.
The contribution made by Empedocles is particularly noteworthy. In addition to
postulating that there are four primordial substances, he postulated that these primary
substances are made of small, homogeneous, changeless, indivisible particles and
that all material bodies are formed by particles of one substance penetrating into
particles of another. Clearly, one can identify the basic elements of modern atomic
theory in these ideas which were to survive for more than two thousand years until
the developments of modern chemistry to be established by Priestley, Lavoisier, and
Cavendish in the eighteenth century.
2 1. Origins and Discovery
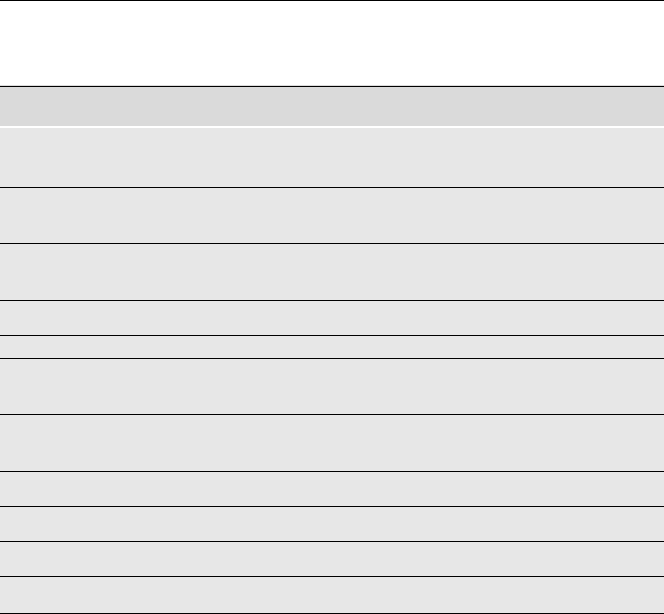
Table 1.1. Primordial substances of the early Greeks – Promoters of single and multiple
element theories
Element(s) Promotor Period
Water Thales 7th B.C.
(founder of the Milesian school)
Apeiron Anaximander 7th B.C.
(an invisible, infinite substance) (pupil and successor of Thales)
Air Anaximenes 6th B.C.
(successor to Anaximander)
Fire Heraclitus of Ephesus 6th B.C.
Earth + Water Xenophanes of Colophon 6th B.C.
(founder of the Eleatic School)
Fire + Earth Parmenides (prominent member 6th B.C.
of the Eleatic school )
Water + Air + Fire + Earth Empedocles of Agrigentum 5th B.C.
Infinite number of elements Anaxagoras of Clazomenae 5th B.C.
Geometrical triangles Plato 4th B.C.
Warm, cold, dry, moist Aristotle 3rd B.C.
Pythagoras
In stark contrast to the Milesian school, Pythagoras held that the basis of reality is
to be found in numbers rather than in fire, earth, water or air. For the Pythagoreans,
number is the primordial element. In addition, these numbers were closely related
to the simple geometric objects i.e. point: 1, line: 2, plane: 3, volume: 4. Since
everything has a shape, all objects can be broken down into geometric shapes and
hence to numbers. The Pythagoreans did not reject the elements of fire, water, earth,
air and the ether. They regarded these as perceptible elements, constructed from
geometric objects, i.e. the earth is a cube, fire is tetrahedron, air is octahedron, water
icosahedron and the ether is duodecahedron.
The Atomist of Antiquity
The theory of atomism, where atoms are postulated as the “elements”, follows the
philosophies of the Milesian school and the Pythagoreans. The leading proponent,
Leucippus, was contempory with the Eleatic school and Empedocles. Atomism was
an attempt to address the problem of coming into being and of change. Fundamental
to atomism is the idea of two basic elements: corpuscles and void. In particular, the
idea of void being primordial was central to the atomists.

The Greek Elements and the Indivisible Atom 3
The atoms are compact and full and are in constant motion. The atoms of Leu-
cippus and Democritus differed from each other by their size and shape. Fire, water,
air and earth, are organisations of certain atoms. The theory of atomism reached its
pinnacle one century later with the work of Epicurus who introduced the notion of
weight in addition to size and shape and the idea that in void, all atoms moved with
the same speed.
Plato
Although Plato’s philosophy was based on Empedocles’s theory of the four primor-
dial substances – fire, earth, air, and water – he did invoke some of the ideas of
Pythagoras through the attribution of geometric shape to each of the elements i.e. the
introduction of geometric atoms. The attribution of the elements with polyhedra was
as follows: fire (tetrahedron), earth (cube), air (octahedron) and water (icosahedron –
20 faces). These geometrical objects could all be constructed from Plato’s polyhedra
atoms based on two types of primordial triangles. Of particular interest in Plato’s the-
ory is the fact that through dissociation and recombination of these primary triangles
to form new objects, the idea of transmutation of elements arises naturally.
Aristotle
In contrast to Plato, Aristotle did not believe in atomism. Instead of primordial ele-
ments, he introduced the idea of primordial qualities of warm, cold, dry, moist. The
four primordial substances derive from these qualities i.e.
warm + dry = fire
warm + moist = air
cold + dry = earth
cold + moist = water
By interchanging qualities, transmutation of elements again arose naturally. By in-
terchanging, for example, dry with moist, the element fire could be transmuted into
air. Chemical reactions were seen as combinations and separations of elements.
Modern Atomism
These ideas of the Greeks were to persist for more than two thousand years until
the introduction of scientific atomism which can be traced to the 18th Century.
Through the work of Lavoisier, Cavendish and Priestly, it was shown that the idea
of four elements was incorrect through the demonstration that water and air are
compounds. The discovery by Proust of the laws governing the combinations of
chemical elements and by Dalton of the law of simple multiple proportions cemented
these ideas further. In particular, it was the work of Dalton which forms the basis
of modern-day quantitative theory of matter. Whereas Dalton maintained that atoms
are indivisible and indestructible, he advanced our ideas through postulating:
• All atoms of a elements are similar and have the same weight
• Atoms of different elements have different weights
