Lloyd L. Handbook of Industrial Catalysts
Подождите немного. Документ загружается.

228 Chapter 6
to the active mixed sulfide. The subsurface cobalt/aluminate does not sulfide.
The same mechanism probably applies to nickel/molybate and the metal ratio
should be controlled to avoid the formation of nickel sulfide (Ni
3
S
2
).
Catalyst can be presulfided in the reactor in a controlled manner using the
following procedure:
• The reactor is freed from moisture by purging with dry air, before purg-
ing with nitrogen to remove oxygen. Hydrogen is then circulated at the
operating pressure of the process and at 175
0
C.
• Feed containing 1–2% sulfur is circulated and the temperature is in-
creased to 200–250
0
C to start the sulfiding process.
• Some of the sulfur in the feed is incorporated into the catalyst as metal
sulfides. The level of sulfur in the feed leaving the catalyst bed reaches a
constant level when this stage has come to quasi-equilibrium. The tem-
perature is then increased to 300
0
C and the process repeated, giving fur-
ther sulfiding of the catalyst. Normal operation can be started after this
stage.
Presulfiding normally takes 10–20 h to complete. Sulfur uptake depends on
the metal oxide content of the catalyst used but is normally in the range 7–10%.
Good gas distribution through the catalyst bed is very important to sulfide even-
ly all of the catalyst. Pre-sulfurised catalysts were introduced to avoid problems
associated with poor gas distribution and temperature control. They also had the
added bonus of a quicker and simpler start-up procedure. The early pre-
sulfurised catalysts were prepared by treating green catalyst with elemental sul-
fur to fill the pores within the catalyst particle. The active sulfides were formed
subsequently, when the catalyst was heated in a stream of hydrogen in the reac-
tor. This procedure led to a significant temperature rise during start-up, which
resulted in the vaporization and subsequent deposition of sulfur in downstream
equipment.
Improved pre-sulfurised catalysts containing polysulfides or metal oxysul-
fides were later developed to overcome these problems.
24
Polysulfides still react
rapidly with hydrogen during conversion of the cobalt, nickel, and molybdenum
oxides to sulfides and produce a temperature rise in the catalyst bed. On the oth-
er hand, metal oxysulfides react slowly over a much wider temperature range
and do not produce an exotherm.
Presulfurized catalysts can be commissioned satisfactorily with a flow of
hydrogen in the temperature range 150–300
0
C. There is no significant tempera-
ture rise in the catalyst bed and water evolves gradually between 200–300
0
C.
Refinery Catalysts 229
6.4.2.4. Catalyst Operation
The early processes used to hydrotreat naphthas only required single beds, but
modern plants treating heavier feeds are more complicated. Several catalyst beds
with a variety of catalysts are usually required.
25
Interbed cooling with cold gas
quench or liquid feed may also be necessary. Hydrotreating reactions are exo-
thermic and cooling is essential to avoid catalyst damage.
Different catalysts are used in separate beds, because several different reac-
tions are taking place at the same time. Top beds operate as guards that remove
metals from vacuum gas oils and residues, as well as promoting sulfur and ni-
trogen removal reactions. Guard catalysts consist of large particles which have
large pores to absorb metal porphyrins and Conradson carbon. The large particle
size is needed to limit any increase in pressure drop caused by trapped impuri-
ties. There is usually an improvement in hydrotreating performance if the mo-
lybdenum and nickel content of these catalysts are high. Pressure drop is still the
most significant operating problem.
A selection of the different sulfurous and nitrogenous impurities in a typical
feed and the reaction sequences involved in their removal is shown in Table
6.11.
Coke is gradually deposited onto the catalyst surface during operation, even
when using naphtha and middle distillates as feed, and this leads to a reduction
in catalyst activity and an increase in pressure drop throughout the reactor. The
plant may need to be shut down for catalyst regeneration to restore activity and
to reduce pressure drop. Some liquid feeds do not vaporize completelyunder
operating conditions and uneven liquid distribution through the beds also leads
to poor performance.
6.4.2.5. Catalyst Regeneration
A typical procedure to regenerate a hydrotreating catalyst in the reactor is as
follows:
• Isolate feed to the reactor and purge all volatile organic material from the
catalyst with hydrogen.
• Purge hydrogen from the reactor with steam and increase temperature to
350
0
C.
• Add air to the steam, keeping the maximum bed temperature to 400
◦
C.
There is a localized hot zone passing through the bed as the residual car-
bon is burnt to carbon dioxide, and as the hot spot passes through the bed,
increase the temperature to 450
0
C.
• Maintain temperature at 450
0
C until no carbon dioxide is detected at the
bed outlet and then purge residual oxygen from the reactor with steam.
230 Chapter 6
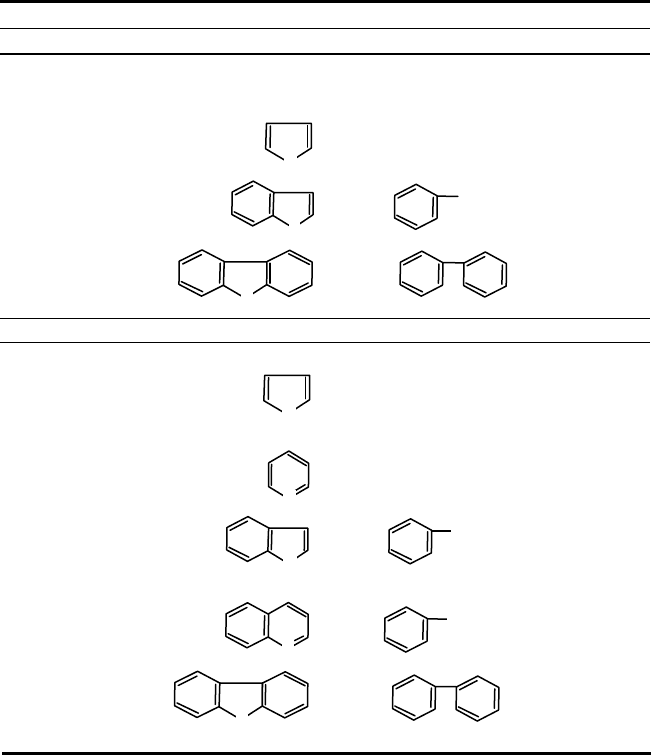
TABLE 6.11. Hydrotreating Reactions.
Impurity
Hydrodesulfurization (HDS)
Mercaptans
RSH + H
2
→ RH + H
2
S
Sulfides
RSR + 2 H
2
→ 2 RH + H
2
S
Disulfides
RSSH + 3 H
2
→ 2 RH + 2 H
2
S
Thiophene
+ 4 H
2
→ C
4
H
10
+ H
2
S
Benzothiophene
+ 3 H
2
→ + H
2
S
Dibenzothiophene
+ 2 H
2
→ + H
2
S
Hydrodenitrogenation (HDN)
Amines
RNH
2
+ 2 H
2
→ RH + NH
3
Pyrrole
+ 4 H
2
→ C
4
H
10
+ NH
3
Pyridine
+ 5 H
2
→ C
5
H
12
+ NH
3
Indole
+ 4 H
2
→ + NH
3
Quinoline
+ 4 H
2
→ + NH
3
Carbazole
+ 4 H
2
→ + NH
3
Note: Olefins are also saturated during its hydrotreating process.
The catalyst can be reused following regeneration provided that it has not
been deactivated by metals such as vanadium, nickel, sodium from the feed or
iron scale. Deactivated catalysts can contain between 10 and 30% of metal de-
posits, which may be recovered for economic or environmental reasons. Oxida-
tion of cobalt or nickel sulfides to sulfate, during in situ regeneration, can gradu-
ally lead to deactivation of the catalyst.
S
S
CH
2
CH
3
S
H
N
N
N
H
CH
2
CH
3
N
CH
2
CH
2
CH
3
N
H
Refinery Catalysts 231
With the large volumes of catalyst now used it has become usual to regen-
erate catalyst externally in special rotary kilns to save time and energy.
26
In situ
regeneration can often take up to 20 days. External regeneration can be quicker
and gives better temperature control and gas circulation, so that regenerated cat-
alyst can be cleaner and almost as active as new catalyst. At the same time, dis-
charged catalyst can be separated into cobalt or nickel types and different sized
particles.
Apart from the possible need to hold a spare catalyst charge, external regen-
eration is more economic. However, as most refineries operate several hy-
drotreating units, it is usual to cycle regenerated catalysts from the less severe
naphtha hydrotreating to middle distillate and finally to residue treatments.
External regeneration in the rotary kiln requires two stages:
• Sulfur is removed from the catalyst at 200–250
0
C by gentle oxidation of
sulfides to sulfur dioxide in a flow of air. Temperature control is im-
portant to avoid complete oxidation of sulfide to sulfate at high tempera-
tures.
• Carbon can then be oxidized in air at 400
0
C, taking care to limit the oxy-
gen content of the hot gas and avoid temperature runaway, which would
deactivate catalyst.
The operating cycle time before regeneration is needed varies considerably
depending on the quality of the feed being used, as shown in Table 6.9. The type
and size of catalyst is also important. For example, a naphtha hydrotreater can
probably operate for at least two years before regeneration, whereas diesel oil
and residue hydrotreaters only operate for a maximum of about 350 and 250
days, respectively.
6.5. HYDROCRACKING
Hydrocracking is a process for the conversion of heavy gas oils, including low-
value fractions not suitable for catalytic cracking, into light gases, gasoline, jet
fuel, and diesel oil. The process operates at a high hydrogen pressure (60–200
atm) and moderate temperatures in the range 350–450
0
C. Dual-function cata-
lysts are used to combine the hydrogenation activity of hydrotreating catalysts
with an acidic cracking support.
The process was introduced in modern refineries by the Union Oil Company
(Unocal) in 1964,
27
but dates back to the 1920s when coal hydrogenation pro-
cesses were developed in Germany and the United Kingdom. Gas oil was hy-
drocracked by Standard Oil at their Baton Rouge Refinery, during the 1930s
using the German catalysts. The process produced aviation gasoline during
World War II in both the United States and the United Kingdom.
232 Chapter 6
The nickel catalysts supported on silica/alumina introduced by Chevron
28
in
the low-temperature isocracking process in 1959 were used to upgrade distillates
and to produce low-molecular-weight isoparaffins for high-octane gasoline.
When the Union Oil Company used a more acidic zeolite support in 1964 the
process became more versatile and a wider range of high-boiling-point feeds
including middle distillates could be converted to high-quality products.
29
Or-
ganic nitrogen and sulfur compounds present in these feeds were also hydrogen-
ated, forming ammonia and hydrogen sulfide, and removed from the products.
Since the 1970s there has been a continuous improvement to the process and
catalysts, as lower-quality feeds have been upgraded.
6.5.1. Hydrocracking Processes
Low-value distillates, including heavy-cycle oils from FCC units, thermal and
coker gas oils, and other heavy-vacuum gas oils, are cracked to produce naph-
tha, jet fuel, and diesel oils. The reaction mechanism is the same as in catalytic
cracking and some aromatic products are also hydrogenated.
There are two steps in the hydrocracking process. Following hydrotreatment
to remove organic nitrogen and sulfur compounds, the feed is cracked. The main
hydrocracking reactions in the second step which proceed via a carbenium ion
mechanism are as follows:
• Dehydrogenation of long-chain paraffins; isomerization and cracking of
the resulting olefins at the third carbon atom; hydrogenation of olefins to
isoparaffins.
• Stepwise ring hydrogenation and hydrocracking of polycyclic aromatics.
• Dealkylation of alkyl aromatics and naphthenes.
• Saturation and cracking of aromatics.
• Hydrogenation of coke precursors formed during the hydrocracking reac-
tions.
The proportion of different products formed depends on the catalysts used.
Weak hydrogenation catalysts with a strongly acid support produce low-boiling
gasoline with a high iso-to-normal paraffin ratio. Catalysts with high hydrogena-
tion activity and low support acidity provide middle distillates. The hydrogen
added for the hydrogenation reactions suppresses coke formation, which would
quickly deactivate the catalyst. Catalysts operate for 2–6 years before being re-
generated or replaced.
Feed and hydrogen are circulated through the catalyst at the conditions se-
lected for the range of products required. Reaction temperatures in the range
250–500
0
C and pressures in the range 60–200 atm have been used. Hydrotreat-
ing and hydrocracking reactions are exothermic and the catalyst is loaded into
several beds so that temperature can be controlled by the addition of a cold hy-
drogen quench. Several different process designs have been used since 1959.
Refinery Catalysts 233
TABLE 6.12. Hydrocracking Processes.
Feed Aromatic cycle oil, heavy gas oil, thermal/coker
gas oil
Product LPG, gasoline, middle distillates
Single-stage process/with recycle:
One or two beds, relatively low sulfur and nitrogen in feed
Feed temperature (
0
C) 350–425
Pressure (atm) 60–200
Conversion (%) 40–70
Aromatics (vol%) ~15
Catalyst (sulfided) Hydrotreating plus ammonia tolerant hy-
drocracking types.
Two-stage process:
Two reactors, nitrogen and/or sulfur compounds can be removed between beds.
First stage:
Feed temperature (
0
C) 350–425
Pressure (atm) 60–200
Conversion (%) 40–50
Catalyst (sulfided) Early units: nickel molybdate/alumina;
high nitrogen conversion;
little cracking.
Later units: nickel tungstate/alumina;
High nitrogen conversion
up to 40% cracking.
Second stage:
Feed temperature (
0
C) ~260–375
Pressure (atm) 60–200
Conversion (%) 40–50 (better aromatic conversion than single
stage)
Aromatics (vol%) ~2
Catalyst Early units: sulfided nickel molybdate/silica
alumina catalysts.
Later units: Catalysts with high zeolite content.
These can be divided into two different groups,
27
and details are given in Table
6.12.
6.5.1.1. Single-Stage Processes
In the early hydrocrackers, the feed was hydrotreated in a separate unit to re-
move organic nitrogen compounds, which are catalyst poisons. A nick-
el/tungsten/silica/ alumina hydrocracking catalyst was then used in a single ves-
sel operating in the range 350–450
0
C. Conversion was in the range 40–70%, and
unconverted feed was recirculated after fractionation. These units operate with
relatively low levels of impurity in the feed, but recycle gas contains hydrogen
sulfide. However, early units still operating now use the new zeolite-based cata-
lysts to treat higher-boiling feeds.
234 Chapter 6
Design was improved by combining the two stages. Feed from the hy-
drotreating catalyst passed directly to the hydrocracking catalyst for conversion
and unconverted feed was recycled back to the hydrocracking section. Hy-
drotreating and hydrocracking catalysts were used so both feed and hydrogen
passed directly from one reactor to the other. This meant that after hydrotreat-
ment, the hydrogen still contained ammonia and hydrogen sulfide when it en-
tered the hydrocracker. This was usually acceptable because ammonia did not
poison the zeolite-based catalysts being used at the time.
6.5.1.2. Two-Stage Processes
In two-stage processes mainly hydrotreating and some cracking takes place in
the first reactor at 350–425
0
C. The cracked products are then fractionated before
the unconverted feed passes to the second reactor which contains hydrocracking
catalyst. Recycled gas is also scrubbed to remove ammonia and hydrogen sulfide
if palladium-based hydrocracking catalysts are used in the second reactor. Nick-
el molybdate or tungstate catalysts must be sulfided before use, so there is no
need to remove hydrogen sulfide from recycle hydrogen. Hydrocracking cata-
lysts can operate with or without sulfur at 260–375
0
C. Feeds with high nitrogen
content can therefore be hydrocracked at a lower second-stage temperature and
higher space velocity than in single-stage processes.
By using a high-activity hydrotreating catalyst in the first reactor, with a ze-
olite catalyst in the second, overall conversion can be increased to 50–80%. Un-
converted feed is still recycled, but a purge of about 5–10% may be needed to
remove accumulated polynuclear aromatics from the circulating feed. These can
cause deactivation of the catalyst, and foul the reactors. Typical compounds in-
clude coronene (seven fused rings) and ovalene (ten fused rings), both of which
are not very soluble. They are brightly colored and have been called the red
death!
30
6.5.1.3. Once-Through Process
More recently a very flexible hydrocracking process has been operated, based on
the single-stage process.
27
Conversion of the feed can range between 40–90%,
but unconverted feed is not recycled. Feeds including heavy-vacuum gas oils,
heavy-cycle oil, coker gas oil, and deasphalted oil are cracked to produce high-
quality middle distillates and naphtha for catalytic reformers. Residue from the
fractionator is used as catalytic cracker and ethylene plant feed, lube oil stocks,
or high-quality fuel oil. Organic nitrogen is almost completely removed from the
product and sulfur content is very low. Moderately active zeolite catalysts are
used.
Refinery Catalysts 235
6.5.2. Hydrocracking Catalysts
It is important that hydrocracking catalysts have both high cracking and hy-
drogenation activities. Typical catalysts are generally made with an acid support
containing either nickel/tungstate or palladium as the hydrogenation catalyst.
Typical examples are shown in Table 6.13. All nickel/molybdate and nick-
el/tungstate catalysts can only work effectively in the form of sulfides in hy-
drotreating and hydrocracking reactions. For this reason the hydrogen sulfide
content of recycle hydrogen must be controlled at an appropriate level. Ammo-
nia is normally scrubbed from recycle gas.
6.5.2.1. Acid Supports
The first acid support to be used in hydrocracking catalysts was an amorphous
silica/alumina, but following the successful application of zeolites in catalytic
cracking processes in 1964, this has generally been replaced by Y-zeolite. The
most important feature of the support, apart from the high acidity needed to
crack high-molecular-weight hydrocarbons, is high thermal stability to with-
stand the process conditions. Pore size is not as critical when using heavy feed
for hydrocracking as is the case for hydrotreating, provided that the surface area
is sufficiently high. Many different support formulations have been patented,
with many of them similar to FCC catalysts. All are produced as extrudates, held
together with binders such as peptized alumina.
TABLE 6.13. Hydrocracking Catalysts.
Design Composition
First stage/single stage
Catalyst 5–10% NiO/10–20% MoO
3
/1–2% P
2
O
5
Support Alumina containing varying amounts of silica
Porosity (cm
3
g
-1
) 0.4–0.6
Shape Extrudates
Rings
Second stage
Catalyst 5–10%NiO/10–20% MoO
3
5–10% NiO/10–20% WO
3
Palladium 0.5%
Support Amorphous silica/alumina 0–80%
Ultrastable Y-zeolite 20–80%
Mixed zeolite/silica/alumina (zeolite 0–100%)
Binders such as peptized alumina (0–20%)
Porosity (cm
3
g
-1
) 0.4–0.6
236 Chapter 6
Zeolites, often combined with amorphous alumina or silica/alumina and a
suitable binder, are now the most widely used acidic component to hydrocrack
heavy feeds. Hydrogen-exchanged and dealuminated Y-zeolites are normally
preferred. Zeolites are more active than amorphous materials because of their
uniform and acidic structure. Less coke is formed during operation, resulting in
less deactivation of the catalyst so that operational life can be longer than six
years. With nitrogen-free feed the zeolite is active at lower operating tempera-
tures and heavier feeds can be cracked.
Modification of the silica/alumina ratio of the Y-zeolite used in catalytic
cracking catalysts can also improve its hydrocracking performance. It was found
that dealuminated Y-zeolites give high diesel selectivity, whereas untreated Y-
zeolite provides a hydrocracking catalyst with high selectivity for gasoline.
A hydrocracking catalyst support can therefore be tailor-made depending on
how the catalyst is to be operated and the product type needed. To obtain maxi-
mum conversion to gasoline at the lowest operating temperature, up to 80% Y-
zeolite and about 20% of a peptized alumina binder is used as support. High
middle-distillate conversion is obtained at higher operating temperatures with a
support containing about 10% dealuminated Y-zeolite, 70% alumina or sili-
ca/alumina in varying proportions, and 20% of the binder. The appropriate Y-
zeolite and the proportion to be used in the catalyst can be selected for the prod-
ucts, as required.
6.5.2.2. Hydrogenation Catalysts
Sulfided nickel/molybdate catalysts supported on a suitable γ-alumina are used
for first-stage hydrocracking reactions and they have a higher nickel molybdate
content than typical hydrotreating catalysts. Sulfided nickel/tungstate or palladi-
um, supported on zeolite or silica/alumina, is used in the second hydrocracking
stage. Palladium catalysts are active if the feed contains residual sulfur com-
pounds but are not active for the hydrogenation of benzene rings. Hydrogen sul-
fide need not, therefore, be removed from the recirculating hydrogen unless it is
necessary to remove all aromatics.
6.5.2.3. Catalyst Preparation
Nickel/molybdate hydrocracking catalysts are made by impregnating preformed
supports with solutions of nickel and molybdenum salts.
31,32
The addition of
ammonia or phosphoric acid to the solutions before impregnation is claimed to
simplify the procedure and to improve activity of the catalyst.
Palladium catalysts are made by ion exchanging the zeolite with a solution
of palladium chloride containing ammonia followed by careful washing and
drying.
31,32
The tetramine complex can be decomposed in air at temperatures
above 800
0
C to produce palladium metal in the form of finely divided crystal-
Refinery Catalysts 237
lites. Direct reduction with hydrogen can lead to agglomeration of the palladium
crystals, which are inactive. The zeolite is extruded with a binder and any other
necessary component of the support.
6.5.2.4. Catalyst Activity
Catalysts are activated before use. Nickel catalysts are carefully presulfided ei-
ther before or after loading to the reactor with the same organic sulfur com-
pounds and procedures used for hydrotreating catalysts. Palladium catalysts are
briefly activated in hydrogen at about 350
0
C, taking care not to overheat the bed,
so that the crystallites do not sinter.
Silica/alumina hydrocracking catalysts are severely poisoned by ammonia.
The feed must therefore be hydrotreated and hydrogen recycle must be scrubbed
to eliminate as much organic nitrogen and ammonia as possible. Zeolites are
slightly less affected by typical ammonia levels and are widely used in the more
efficient two-reactor single-stage process.
27
Catalysts should, however, contain
more zeolite if the ammonia content is variable. The active metal content must
also be increased to achieve the same conversion in the presence of ammonia.
The cracking activity of the support and the hydrogenation activity of the metal
component must therefore be carefully optimized to obtain maximum conver-
sion with different operating conditions and feeds.
6.5.2.5. Catalyst Reactivation
A typical catalyst can operate for a number of years, and any slow loss of activi-
ty can be compensated for by a gradual increase in process operating tempera-
ture until the reactor temperature limit is reached. Regeneration is then possible,
either in the reactor or externally, by burning accumulated coke in dilute air.
This restores the activity of most nickel/molybdate or nickel/tungstate catalysts,
which must then be resulfided before further use.
Deactivated palladium supported on zeolite can also be regenerated, and the
catalyst must also be reactivated before being reused. This is because palladium
in the zeolite framework sinters. By treating the catalyst with an excess of am-
monium hydroxide solution, however, the agglomerated palladium dissolves to
form a tetramine complex. Calcination in air then decomposes the complex and
the original palladium distribution and activity are restored.
31
The first Y-zeolite hydrocracking catalysts contained residual sodium ions
in the sodalite cages that were mobile during operation and they entered the su-
percage. This led to a loss of cracking activity. Treatment of the zeolite with an
ammonium salt solution removed the mobile sodium ions and restored acidity.
The redistribution of palladium with ammonia solution could be combined with
an exchange of sodium ions to rejuvenate the catalyst in one step.
32
This was
done before reactivation by burning off the carbon deposits.
