Lloyd L. Handbook of Industrial Catalysts
Подождите немного. Документ загружается.

218
Figure
Exxon
M
TAB
Year
1938
1946
1957
1978
1986
1993
1998
Chapter 6
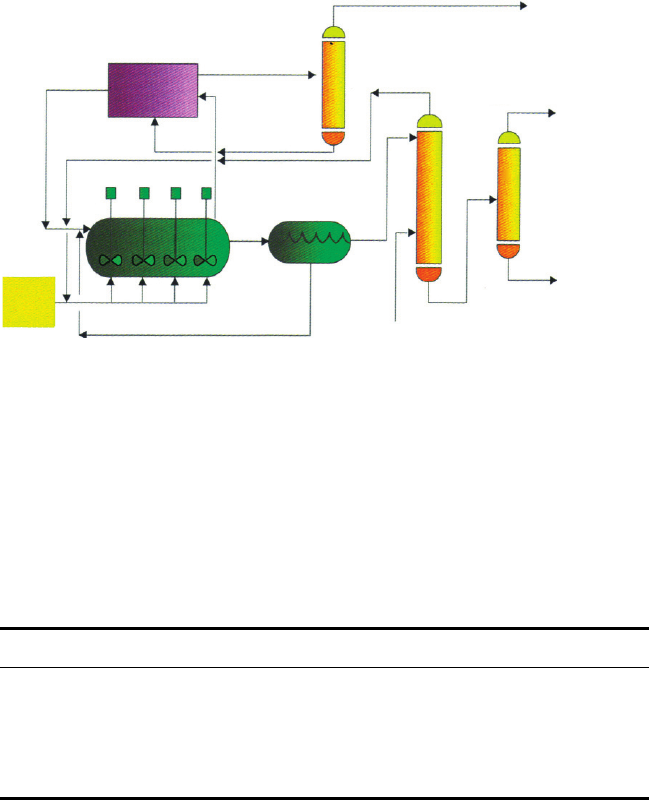
6.2. Outline flo
w
M
obil Research
a
L
E 6.7. Import
a
w
sheet of alkyl
a
a
nd Engineerin
g
a
nce of the H
2
S
O
% H
2
10
0
8
0
7
5
6
0
5
0
4
5
5
0
a
tion plant. Rep
r
g
Company.
O
4
/HF Alkylatio
n
SO
4
0
0
5
0
0
5
0
r
oduced with pe
r
n
Processes in t
h
% HF
—
20
25
40
50
55
50
r
mission from
h
e United States
Total productio
n
(million bpd)
Small
0.17
0.27
1.00
1.05
1.07
1.10
.
n
compressor
recycle
refrigerant
recycle acid
makeup
isobutane
alkylate
product
butane
product
propane
product
olefin
feed
recycle isobutane
reactor
settler
deisobutanizer
depropanizer
debutanizer

Refinery Catalysts 219
TABLE 6.8. Sulfuric Acid and Hydrogen Fluoride Alkylation.
6.3.1. Liquid Acid Processes
All commercial plants operate with a high proportion of isobutane in the cir-
culating gas and conversion depends on the residence time of the gas in the reac-
tor. Typical feeds include olefins derived from catalytic cracking and, more re-
cently, the refnates from MTBE or TAME units. Typical operating conditions
are summarized in Table 6.8. Some of the acid catalyst must be removed on a
continuous basis for recovery as it becomes diluted with inactive hydrocarbons.
Sulfuric acid must be reused in other processes or recovered off-site for resale, a
process which costs more than fresh acid. Hydrogen fluoride is usually recov-
ered on site and reused.
10
6.3.2. The Mechanism of Alkylation with an Acid Catalyst
The generally accepted mechanism for the alkylation reaction is as follows:
• Initiation. Butylene combines with a hydrogen ion to form a secondary
butyl carbonium ion:
+
CH
3
CH=CHCH
3
+ H
+
→ CH
3
CH
2
CHCH
3
(6.2)
• Isomerism. The secondary butyl carbenium ion can then either isomerize
to form a tertiary butyl carbonium ion:
CH
3
+
|
CH
3
CH
2
CHCH
3
→ CH
3
C
+
(6.3)
|
CH
3
H
2
SO
4
HF
Temperature (◦C) 5–10 21–38
Isobutane/olefin feed ratio (vol) 5–8 10–14
Olefin space velocity (vol olefin/vol acid) 0.2–0.3
Contact time minutes 20–30 10–15
Acid strength (%) 90–92 85–95
Acid in emulsion (%) 50–60 25–60
Acid used (lb barrel
−1
)
13–30 0.1–0.3
Alkylate from C
4
feed (%):
Trimethyl pentane 73
Dimethyl hexane 10
Heavy alkylate C
9
+
8
Light ends C
7
−
8
220 Chapter 6
or react with an isobutane molecule to give a butane molecule and a ter-
tiary butyl carbonium ion:
CH
3
CH
3
+
| |
CH
3
CH
2
CHCH
3
+ CH
3
CH → CH
3
CH
2
CH
2
CH
3
+ CH
3
C
+
(6.4)
| |
CH
3
CH
3
• Addition. The tertiary butyl carbonium ion reacts with a butylene mole-
cule to form branched C
8
+
trimethyl pentene carbonium ions:
CH
3
CH
3
| |
CH
3
C
+
+ CH
2
=CH
2
CH
2
CH
3
→ CH
3
C CH
+
CHCH
2
CH
3
(6.5)
| |
CH
3
CH
3
• Propagation. The C
+
carbonium ion reacts with an isobutane molecule by
hydrogen transfer to give the C
8
paraffin and to generate a tertiary C
4
+
carbonium ion that continues the sequence of alkylation reactions.
A number of side reactions can also take place such as:
• The decomposition of a C
8
+
carbonium ion to the C
8
olefin and a hydro-
gen ion. This is likely at either a low isobutane/olefin ratio or low acid
strength.
• Reaction of a C
8
+
carbonium ion with another C
4
olefin molecule to form
a C
12
+
carbonium ion. This can react with an isobutane molecule to give a
C
12
paraffin, but in the presence of excess olefin, further chain growth can
take place.
6.3.3. Liquid Acid Operating Conditions
During operation hydrocarbons are emulsified in acid, ensuring that the olefin is
not in excess, and reacts quickly. Operating pressure is adjusted depending on
the boiling point of the hydrocarbon mixture and the need to dissolve the hydro-
carbons. The space velocity of the mixed olefins and the catalyst is regulated,
depending on the octane level of the product needed and operating temperature.
Octane levels increase if the acid strength is low, which means that more spent
acid has to be removed from the system. In sulfuric acid alkylation the optimum
acid strength decreases with propylene, butylene, and pentylene feeds.
Refinery Catalysts 221
6.3.4. Processes Using Solid-State Acid Catalysts
Several solid acid catalysts were tested in the laboratory for the alkylation of
ethylene with isobutane following the introduction of the sulfuric acid alkylation
process.
11
These were mostly derived from aluminum chloride or boron trifluo-
ride and were never used in the full-scale production of alkylates.
Operators have always hoped that a successful solid acid catalyst would be
discovered because of the problems and hazards involved in handling large
quantities of sulfuric and hydrofluoric acids. This situation reached a climax in
1990, when California refineries were faced with the possibility of having to
close down units using HF catalysts. From about that time, potential solid-state
acid catalysts were investigated by catalyst producers and several have been
tested in pilot units.
12
In the meantime, although modifications were introduced
to HF units and additives were used to reduce the volatility of the acid, the pro-
cess has been less popular than the sulfuric acid process.
13
The short-term alter-
native of converting HF units to the use of sulfuric acid was much more expen-
sive than the use of additives.
Suitable solid acids have been difficult to find. Those used for benzene al-
kylation are not sufficiently acidic for economic operation and, also, the olefin
can polymerize on the catalyst surface. This blocks the active sites and prevents
adequate hydrogen transfer. Frequent regeneration of the catalyst is not econom-
ic. The use of antimony pentafluoride slurried with the hydrocarbons is one of
the options that has been investigated in recent years.
12
The best solution appears to be the use of an almost insoluble liquid catalyst
held within the pores of a suitable inert support. Supported liquid catalysts are
well known and can be used with a continuous catalytic regeneration system
similar to that developed for catalytic reforming processes. Haldor Topsøe has
successfully tested trifluoromethane sulfonic acid in this way since 1993 with a
variety of olefin feeds.
14
No formal regeneration was necessary apart from peri-
odic removal of some catalyst for reimpregnation and the recovery of dissolved
acid from the alkylate. Both catalyst and support are, therefore, recirculated. The
small quantity of polymeric by-products formed (acid soluble oil) appears to be
less than that formed in the sulfuric acid process, but slightly more than in the
HF process.
6.4. HYDROTREATING
Sulfur impurities in products manufactured from crude oil products are undesir-
able because hydrogen sulfide, sulfur dioxide, etc., formed during product use.
For many years it was possible to obtain acceptable quality gasoline and kero-
sene by selecting low-sulfur, or sweet, crude oils. Sour crude oils contain dis-
222 Chapter 6
solved hydrogen sulfide, mercaptans, organic sulfides, thiophenes, and elemental
sulfur in varying amounts. These could be sweetened by a number of chemical
processes. High-sulfur crude oils were more difficult to desulfurize and the
chemical and solvent extraction processes were combined with or replaced by
relatively cheap and more efficient catalytic processes that could also remove
gum-forming compounds from cracked gasolines.
For a short time from 1946 bauxite or fuller’s earth was used without the
addition of hydrogen.
15
It was found that sulfides and mercaptans reacted with
impurities in the bauxite and that this together with some mild cracking of hy-
drocarbons produced sufficient hydrogen to hydrogenate thiophenes. It was soon
realized that the catalytic desulfurization was actually a mild, selective hydro-
genation process that did not saturate aromatics.
16
During the 1950s, co-
balt/molybdate catalysts supported on bauxite or Fuller’s earth were used. These
were similar to the catalysts developed for coal hydrogenation and which were
also used to desulfurize steam reforming feeds. The new catalysts were most
effective when hydrogen was added to the feed. This also had the effect of re-
ducing the deposition of carbon, and allowed for longer operating cycles before
regeneration was necessary. More effective cobalt/molybdate catalysts were
developed using γ-alumina as support. The activation step for the catalyst in-
volved the formation of metal sulfides, and when the catalyst was pre-sulfided
before use, it was found that light distillates, kerosene and even crude oils could
be treated effectively with these catalysts.
18
Operating conditions depended on
the boiling range of the fraction being treated. Catalyst temperature was usually
limited to about 400
0
C in order to avoid excessive carbon deposition while total
pressure was increased from 300–500 psig for low-boiling distillates and up to
700–1000 psig for higher-boiling or cracked feeds. Liquid space velocity was
usually up to 8 h
-1
, with a hydrogenn/oil ratio of about 1000 scf of hydrogen per
barrel of feed for low-sulfur distillates. Lower space velocities, in the range from
0.5–3 h
-1
, with hydrogen/oil ratios up to 10,000 scf per barrel, needed to be used
for higher-boiling residues. In the hydrotreating of heavy feeds, more carbon
was deposited by thermal cracking than in the hydrotreating of lighter feeds.
Catalyst regeneration was required after operation for less than 24h.
The use of hydrodesulfurization became more widespread as catalytic naph-
tha reforming processes were introduced. The operation of platinum catalysts
needed an increasingly strict sulfur specification for the naphtha, and as a bonus,
the cheap by-product hydrogen from the reforming process could be used to
hydrotreat other refinery product streams.
By the early 1960s, hydrotreating capacity in the United States had in-
creased to 2.5 million bpd, with catalyst lifetimes averaging about 5 years. The
use of hydrotreating was extended to kerosene, gas oil, and vacuum gas oils as
government regulations on sulfur emissions became more stringent and as better
cobalt molybdate catalysts became available. By the late 1970s, when atmos-
pheric and vacuum residues were also being desulfurized, US hydrotreating ca-
Refinery Catalysts 223
pacity had increased to about eight million bpd. By 1999 US capacity exceeded
10 million bpd, with more than 35 million bpd being treated worldwide.
6.4.1. What Is Hydrotreating?
The term hydrodesulfurization was used to describe processes that removed sul-
fur compounds from crude oil fractions by reaction with hydrogen. As the pro-
cesses evolved to include nitrogen and oxygen removal, together with the hy-
drogenation of aromatics and olefins, the group of processes became known as
hydrotreating. Hydrotreatment simply results in the conversion of organic sul-
fur, nitrogen, and oxygen compounds to hydrocarbons and hydrogen sulfide,
ammonia, or water, respectively. At the same time olefins and aromatics may be
converted to saturated hydrocarbons without any cracking of the hydrocarbons.
When high-boiling crude oil fractions are hydrotreated under more severe condi-
tions a proportion of the heavy molecules may crack as impurities are removed.
For this reason it is now common to refer to processes that crack a relatively
small proportion of the light feeds while sulfur or other impurities are removed
as hydrorefining.
Deliberate reduction in the molecular weight of heavy feeds was introduced
with the hydrocracking process (Section 6.5). This operates at high pressure, and
heavy gas oils and residual fractions are converted to gasoline or other desirable
products. Obviously there can be an overlap in defining processes where some
precracking is an advantage to refineries. When an overall description is re-
quired, the term hydroprocessing is often used.
6.4.2. Hydrotreating Processes
In the early hydrotreating processes, sulfur compounds were removed from the
light hydrocarbon fractions used in gasoline by hydrogenation over co-
balt/molybdate catalysts to produce hydrogen sulfide and a saturated hydrocar-
bon. Around the same time, it was found that nickel/molybdate catalysts were
more active for the hydrogenation of nitrogen compounds to ammonia and a
hydrocarbon while also giving some saturation of olefins and aromatics.
In modern refineries both cobalt/molybdate and nickel/molybdate catalysts
are now widely used in the purification of various crude oil fractions. These in-
clude:
• Straight-run naphthas, used as feedstock for catalytic reforming and
steam reforming processes. They must contain less than 1 ppm of sulfur
and nitrogen to avoid poisoning platinum or nickel catalysts.
• Cracked gasoline, to hydrogenate undesirable sulfur and nitrogen com-
pounds as well as olefins.
224 Chapter 6
• Middle distillates such as diesel fuel, kerosene, jet fuel, domestic heating
oil, and other gas oils, to remove sulfur for environmental reasons. Hy-
drotreating is also used to increase the smoke point or cetane number by
hydrogenating aromatic components.
• Vacuum gas oils, used as catalytic cracker or hydrocracker feeds, to re-
move sulfur, nitrogen, and metal impurities.
• Atmospheric and vacuum residues, to remove as much sulfur as possible
to provide low-sulfur fuel oils. It is also used to hydrogenate asphaltenes
and porphyrins to reduce both Conradson carbon and metal contents.
As the boiling point and the specific gravity of the fractions increase, more
severe hydrotreating operating conditions are needed. A lower space velocity
and more extensive hydrogen recycle are needed to limit deactivation of the cat-
alyst by deposition of coke. The catalyst must be regenerated after shorter inter-
vals and discarded more often than when using light fractions.
A very brief summary of typical operating conditions is shown in Table 6.9
although these can vary significantly for different crude oils and blends contain-
ing cracked fractions.
6.4.2.1. Catalyst Production and Operation
Hydrotreating catalysts are usually produced by impregnating preformed γ-
alumina supports with aqueous solutions of ammonium molybdate. Particles are
then dried and calcined. Molybdenum oxide forms a uniform surface layer on
the alumina by reaction with the surface hydroxyl groups. It is important that the
surface area of the alumina is consistent with the amount of molybdenum oxide
required for the hydrotreating catalyst specification (typically 1 wt% MoO
3
≡ 12
m
2
g
-1
area).
19
Table 6.9. Typical Operating Conditions.
Fraction
Naphtha Gas oil Vacuum gas oil Atmospheric residue
Boiling Range (
0
C) 66–200 240–380 350–560 560+
Liquid space velocity (h
−1
)
6–10 2–4 1–2 0.2–0.5
Hydrogen/oil ratio 60 240 350 7500
Temperature (
0
C)
280–320 340–360 360–400 400–420
Pressure (atm) 10–25 20–40 30–60 80–100
Regeneration interval 1–3 years 1–2 years 1 year 0.5–1 year
Sulfur content feed (%) 0.05–0.15 1–2 2–3 4–5
Sulfur content product (%) < 1 ppm 0.1–0.3 0.2–0.4 0.6–0.8
Nitrogen removal (%) 99 45 40–45 45
Refinery Catalysts 225
The molybdenum-treated alumina is then impregnated with either cobalt or
nickel nitrate solutions and again dried and calcined. Several reactions can take
place during the calcination step. At temperatures in the range 400–500
0
C, co-
balt and nickel nitrates decompose to form oxides on the molybdena/alumina
catalyst surface. The deposition of cobalt and nickel in contact with the molyb-
denum on the catalyst surface is important in forming active sites. As the tem-
peratures are increased both cobalt and nickel oxides react to form aluminates
close to the alumina surface. Cobalt aluminate (CoAl
2
O
4
) produces the charac-
teristic blue color of cobalt/molybdate catalysts. The nickel and cobalt ions mi-
grate into the alumina particles as the temperature is increased to 650–700
0
C,
forming bulk aluminates. These are not active catalyst precursors and the tem-
perature of calcinations must be carefully limited to avoid their formation.
A typical support can be prepared by mixing boehmite [AlO(OH)] and wa-
ter to form a smooth paste, often adding a small volume of dilute nitric acid to
peptize the alumina to give increased strength. The paste is extruded to form
small-diameter, relatively long, spaghetti-like cylinders, rings, and other shapes,
or the boehmite can be granulated to produce spheres. The supports are dried
and then calcined at up to 600
0
C to produce γ-alumina. It is common practice to
add up to 1% of silica, often as montmorillonite or bentonite, to stabilize the γ-
alumina at high operating temperatures and to act as a lubricant during extru-
sion. Addition of phosphoric acid appears to produce phosphate groups on the
alumina surface that inhibit coke formation as well as to improve molybdenum
dispersion.
21
Finally, small amounts of potash can be added to reduce surface
acidity and to control coke formation. Typical catalyst specifications are given
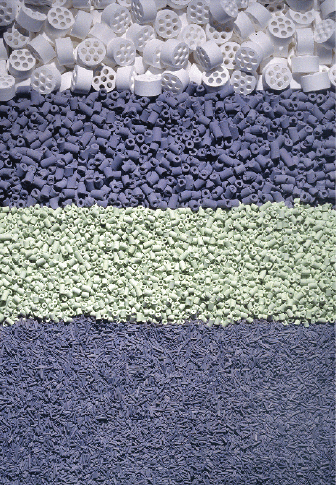
in Table 6.10 and some catalyst are shown in Figure 6.3.
When using heavy feeds, particularly residues, the catalyst pores at the top
of the bed soon become blocked with metals and high molecular-weight hydro-
carbons present in the feed. This reduces catalyst activity and the catalyst must
be regenerated. In an attempt to overcome the problem and to increase the cycle
time, so-called bimodal catalysts were introduced containing both large and
small pores. Small particles of cellulose, combustible polymers, or carbon were
mixed with the boehmite and burned out during the final calcination step of the
formation of the γ-alumina support. By using different techniques it was possi-
ble to generate pores shaped like bottles which could contain large molecules,
and large pores which gave access to small subsidiary pores. These special sup-
ports extended the operating cycle before the catalyst had to be regenerated or
discarded.
6.4.2.2. Catalyst Handling
The catalyst is supported by a layer of relatively large inert spheres, and must be
packed carefully into the reactor. Dense-loading procedures to increase the
packing density of the catalyst are now available that use a rotating disk at the
226
end of
dates,
a
An ad
v
flat as
l
izontal
l
resulti
n
catalys
pletely
uids t
h
p
lane
p
differe
n
idea is
ids an
d
shapes
A
distrib
u
Chapter 6
Fi
g
R
e
T
o
a hose to dis
t
a
nd trilobe sh
a
v
antage of the
l
oading proce
e
l
y and this im
p
n
g in better c
o
t is packed
m
flat, but usua
l
h
en run prefer
e
p
erformance.
22
n
t catalyst typ
to have large
d
d
impurities in
and activities
layer of cera
m
u
tion of the fe
e
g
ure 6.3. Ty
p
e
produced wit
h
o
psøe A/S.
t
ribute the cat
a
a
pes can be in
c
dense-loadin
g
e
ds. Catalyst p
a
p
roves the dist
r
o
ntrol of temp
e
m
anually into
a
l
ly has rather
a
e
ntially down
s
In the design
es in a two-o
r
d
iameter parti
c
the feed. Diff
e
can also be us
e
m
ic balls is pla
c
e
d and to catc
h
p
ical hydrotreat
i
h
permission
f
a
lyst. The pac
k
c
reased by 8
%
g
technique is
t
a
rticles, which
r
ibution of liq
u
e
rature distrib
u
a
converter, t
h
a
convex or c
o
s
ome channels
f
or modern hy
r
three-
b
ed re
a
c
les at the top
e
rent catalysts
e
d.
c
ed on top of t
h
h
some of the s
o
i
ng catalysts.
f
rom Haldor
k
ing density
o
%
, 12%, and 2
0
t
hat the cataly
s
are very long
u
id residue th
r
u
tion and con
v
h
e surface lay
e
o
ncave orienta
t
, resulting in
p
y
drotreating pr
o
a
ctor system
m
of the reactor
having differ
e
h
e catalyst be
d
o
lid particles i
n
o
f spheres, ext
r
0
%, respectiv
e
s
t layer is alw
a
and thin, lie h
o
r
oughout the b
e
n
version. Whe
n
e
r is rarely co
m
t
ion. Process l
p
oor catalyst
a
o
cesses, up to
s
m
ay be used.
T
to cope with s
e
nt compositio
n
d
to help impr
o
n
troduced by t
h
r
u-
e
ly.
a
ys
o
r-
e
d,
n
a
m
-
iq-
a
nd
s
ix
T
he
ol-
n
s,
o
ve
h
e
Refinery Catalysts 227
Table 6.10. Typical Hydrotreating Catalyst Specification.
flow of the reactants. In older plants, wire mesh baskets were often placed on top
of the catalyst bed and filled with pebbles. These were part of the vessel design
to catch solids and to distribute liquid feed, and were known as trash baskets.
Although still in use, better filtering of the feed and graded catalyst layers with
the larger catalyst particles at the top of the beds have largely replaced the use of
baskets.
6.4.2.3. Activating the Catalyst
Hydrotreating catalyst is activated by presulfiding with hydrogen sulfide. This is
achieved by circulating sulfur compounds in a stream of hydrogen at tempera-
tures too low to reduce the oxides to metals. Small molybdenum disulfide plate-
lets (truncated hexagons) that incorporate cobalt or nickel ions at the plate edges
are formed to produce the active CoMoS or NiMoS sites. The surface area of the
alumina support is critical to achieve the appropriate distribution of molyb-
denum, and the optimum ratio of nickel or cobalt to molybdenum is approxi-
mately 1:4. In the case of cobalt molybdate, if the atomic ratio exceeds 30:1,
cobalt sulfide (Co
9
S
8
) crystals form on the molybdenum disulfide in preference
Cobalt molybdate Nickel molybdate
Composition
Cobalt oxide % 4–5 —
Nickel oxide % — 4–6
Molybdenum oxide % 12–20 15–20
Phosphorus % Up to 2 Up to 2
Alumina Balance Balance
Impurities SO
4
, Na
2
O, Fe
2
O
3
1%, 0.06%, 0.6%,
respectively
1%, 0.06%, 0.6%,
respectively
Properties
Loss on ignition 500◦C (wt%) 2–4 2–4
Bulk density (kg liter
−1
) 0.5–0.7 0.5–0.7
Surface area (m
2
g
−1
) 200–300 200–300
Pore volume (ml g
−1
) 0.5–0.7 0.5–0.7
Mean pore radius (nm) 4.5–5.0 4.5–5.0
Shapes Size (mm)
Extrudates 1.6, 1.3, 0.8 diameter × 3–5 long
Rings 5 × 5, 3 × 3
Trilobes 1.6, 1.3 × 5
Quadrilobes 1.6, 1.3 × 5
Used catalyst Before regeneration After regeneration
Sulfur (wt%) Up to 10 < 0.5
Carbon (wt%) 8–12 0.1–0.3
Loss on ignition (wt%) 17–20 < 0.5
