Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

PROPULSION AND INDUSTRIAL NICKEL-METAL HYDRIDE BATTERIES 30.7
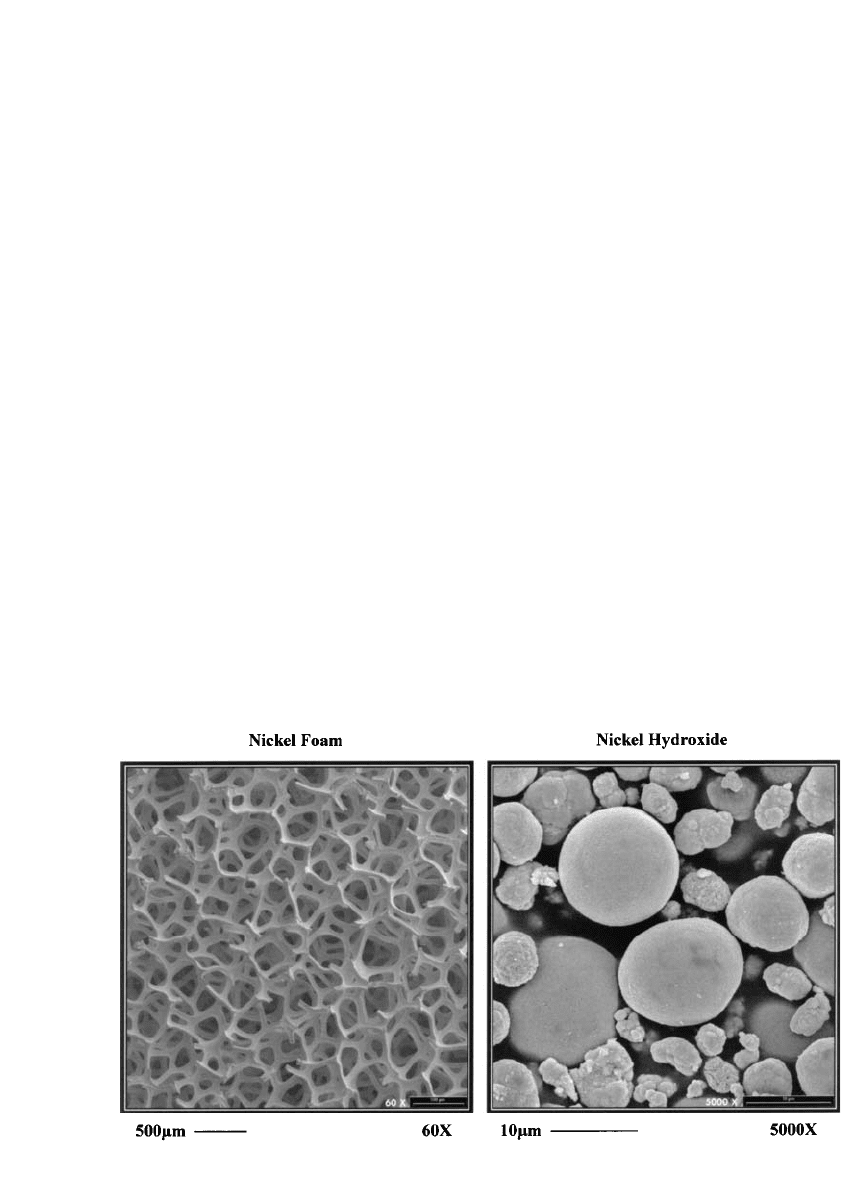
FIGURE 30.4 SEM micrographs of positive electrode nickel foam substrate and high density spherical nickel
hydroxide.
Important design variables in the manufacture of sintered nickel hydroxide electrodes in-
clude:
•
Strength and pore diameter of the filamentary nickel skeleton
•
Chemical composition of the nickel hydroxide active material
•
Active material loading
•
Level of harmful impurities (e.g. nitrate, carbonate)
The more common pasted nickel hydroxide positive electrode is typically produced by
mechanically pasting high density spherical nickel hydroxide into the pores of a foam metal
substrate (Fig. 30.4). The foam metal substrate is typically produced by coating polyurethane
foam with a layer of nickel either by electrodeposition or by chemical vapor deposition,
followed by a heat treatment process to remove the base polyurethane. The typical pore size
of the foam metal skeleton current collector is on the order of 200 to 400 microns. The
foam
8
is then physically loaded with nickel hydroxide having an average diameter of about
10 microns in a paste containing conductive cobalt oxides which form a conductive network
to compensate for the large distance from the nickel hydroxide to the metal current collector
and for the fact that the nickel hydroxide itself is relatively low in conductivity. A cross-
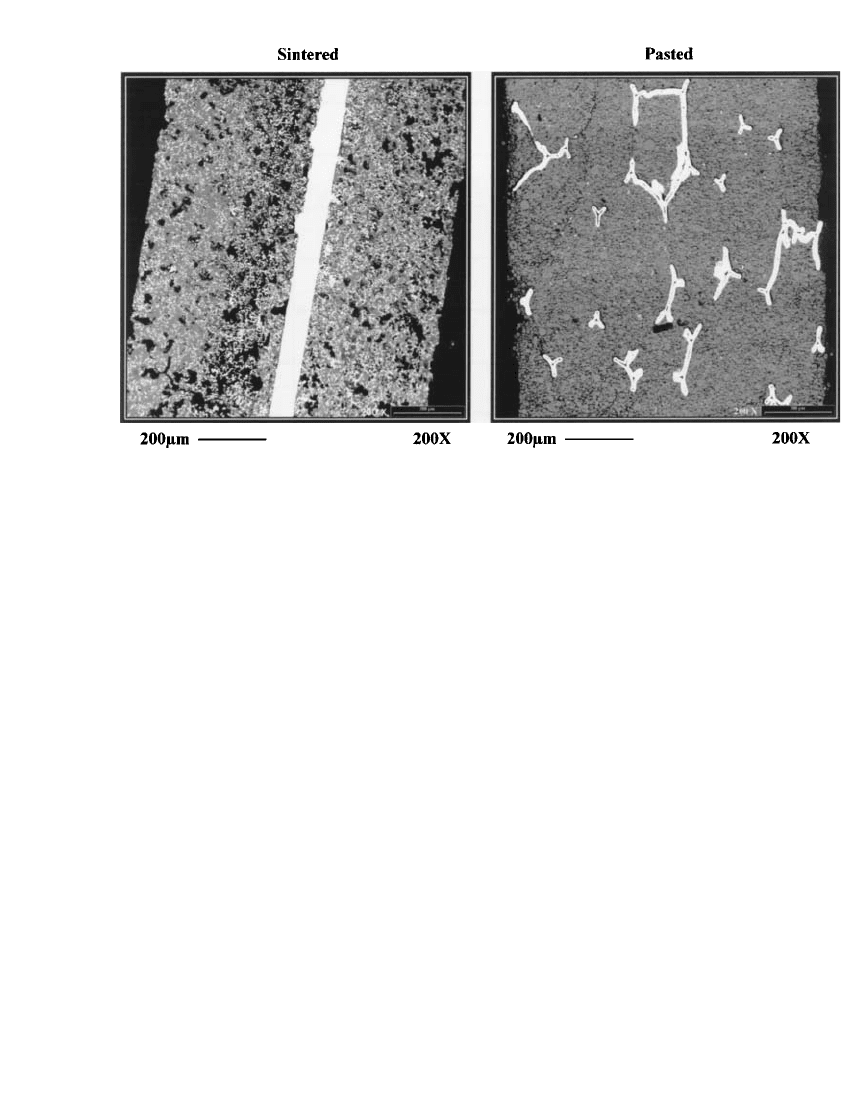
sectional comparison of sintered and pasted positive electrodes is presented in Fig. 30.5
under backscattered electron imaging where the bright areas indicate metallic nickel current
collection.
One aspect of sintered versus pasted nickel hydroxide technology is that sintered elec-
trodes require the battery manufacturer to make a significant capital investment in facilities
and equipment, and to have a great deal of expertise in processing. Pasted electrodes con-
versely place a great deal of emphasis on the expertise of the suppliers for both the nickel
foam substrate and for the high density spherical nickel hydroxide. Recent development of
the pasted electrode has brought about exceptional improvements in power and high rate
discharge capability to a level comparable to that of the sintered electrode, making the choice
of which electrode configuration one of ease of manufacture and cost; these are areas where
the pasted electrode is superior and this is the main explanation for the predominance of
this process.
30.8 CHAPTER THIRTY
FIGURE 30.5 SEM micrographs under BEI imaging where bright areas indicate nickel metal current col-
lection illustrating difference in active material distance to current collector for pasted and sintered positive
electrodes.
30.4.4 Energy Versus Power Design Tradeoffs
As with most other battery technologies, NiMH batteries can be designed to emphasize
energy, power, or some combination of the two. The application itself may make the choice
apparent, but perhaps not for obvious reasons. In electric vehicle applications, a certain
threshold power such as 200 W /kg is required for adequate vehicle performance, and once
the power requirement is met competing designs usually provide a specific energy in the
range of 62 to 80 Wh/ kg. The motivation for the higher energy is of course longer vehicle
range, but also cost. The typical figure of merit for electric vehicle battery cost is $/ kWh,
where the USABC goal of $150 per kWh has proven to be one of the most challenging
development targets. NiMH cost is mainly controlled by raw material amounts, costs and
utilization rather than processing labor, assembly, packaging, etc. Consequently, an important
development activity for many NiMH manufacturers is to get a higher utilization of less
expensive active materials on a mAh/ g level basis and therefore reduce manufacturing costs.
For HEV applications, specific energy is of far less importance since the battery has a
vastly different function than in pure electric vehicles. For HEVs, the main purpose of the
battery is to accept and utilize the energy from regenerative braking and assist in acceleration.
The battery is commonly exposed to very high current pulses during both charge and dis-
charge, but energy extraction is usually limited to relatively low depths of discharge. The
crucial requirement for the NiMH HEV battery is therefore specific power, and the USABC/
PNGV (Partnership for a New Generation of Vehicles) goal for battery developers is greater
than 1000 W/ kg. NiMH batteries available today are in the 500 to 800 W/ kg specific power
range, with development reports approaching 1000 W /kg. Besides performance required for
HEV operation, a crucial figure of merit for NiMH battery developers is power cost in
$/ kW, and the PNGV goal is below $12 per kW. In this case, specific energy for a typical
HEV battery may be in the 32 to 56 Wh /kg range combined with specific power in the 500
to 800 W/kg range.

PROPULSION AND INDUSTRIAL NICKEL-METAL HYDRIDE BATTERIES 30.9
30.4.5 Metal Hydride Alloy
NiMH batteries are an unusual battery technology in that the metal hydride active material
is an engineered alloy made up of many different elements and that the MH alloy formulas
vary to a significant degree.
9,10
As stated previously, the active material in the negative
electrode is either of the disordered AB
5
(LaCePrNdNiCoMnAl) or disordered AB
2
(VTi-
ZrNiCrCoMnAlSn) type.
11,12,13,14
AB
5
type alloys are more common, despite significantly
lower hydrogen storage capacity as compared to AB
2
(320 vs. 440 mAh /g). The advantages
of the AB
5
alloys include low raw material cost, ease of activation and formation, flexibility
in electrode processing and high discharge rate capability. On the other hand, there has been
significant ongoing development to improve the properties of AB
2
materials to take advantage
of their inherently higher energy, which is especially important to reduce cost.
Electrochemical utilization of metal hydride materials as anodes in NiMH batteries re-
quires meeting a demanding list of performance attributes including hydrogen storage ca-
pacity, suitable metal-to-hydrogen bond strength, acceptable catalytic activity and discharge
kinetics, and acceptable oxidation/corrosion resistance to allow for long cycle life. Multi-
element, multiphase, disordered alloys of the LaNi
5
and VTiZrNiCr type are attractive de-
velopment candidates for atomic engineering due to a broad range of elemental addition and
substitution, availability of alternate crystallographic phases which form the matrix for chem-
ical modification, and a tolerance for non-stoichiometric formulas. Through the introduction
of modifier elements, ease of activation and formation have been achieved. Special processing
steps, such as alloy melting and size reduction, suitable for these metallurgically challenging
materials have also been developed.
The metal hydride active material and construction also have similar special design op-
tions. The active materials may be adjusted to influence one or more of the factor of capacity,
power, and/or cycle life. For the AB
5
system, typical formulas include:
•
La
5.7
Ce
8.0
Pr
0.8
Nd
2.3
Ni
59.2
Co
12.2
Mn
6.8
Al
5.2
(atomic percent, at.%)
•
La
10.5
Ce
4.3
Pr
0.5
Nd
1.3
Ni
60.1
Co
12.7
Mn
5.9
Al
4.7
While the capacity of various AB
5
alloys is usually around 290 to 320 mAh/g, other
overall performance attributes can be greatly influenced. It is common for the ratio of La/
Ce to be reversed to emphasize cycle life and power. The total amount of Co, Mn and Al
significantly affect ease of activation and formation but increased cobalt has cost implica-
tions. After production of the AB
5
alloy ingot, it is common to further refine the micro-
structure of the material by a post annealing treatment of about 1000
⬚C for 10 hours. The
annealing treatment can have a significant effect on capacity, discharge rate and cycle life
by adjusting crystallite size and grain boundaries, as well as eliminating unwanted phases
precipitated during ingot melting and casting. Further, special processing methods such as
melt spinning and other rapid solidification techniques can also promote cycle life, although
these processing methods are more expensive and may involve a tradeoff with discharge rate
capability.
15
Commercial AB
5
alloys have a predominantly CaCu
5
crystallographic structure. However,
within that structure, there are a range of lattice constants brought about by compositional
disorder
16
within the material which are important for catalysis, storage capacity and stability
to the alkaline environment and embrittlement. These materials also precipitate a nickel-
cobalt phase which is important for high rate discharge.
17,18
AB
2
alloys also have formula and processing choices. Popular AB
2
alloy formulas (at.%)
include:
•
V
18
Ti
15
Zr
18
Ni
29
Cr
5
Co
7
Mn
8
•
V
5
Ti
9
Zr
26.7
Ni
38
Cr
5
Mn
16
Sn
0.3
•
V
5
Ti
9
Zr
26.2
Ni
38
Cr
3.5
Co
1.5
Mn
15.6
Al
0.4
Sn
0.8

30.10 CHAPTER THIRTY
Alloy capacity may range from 385 to 450 mAh /g. Higher vanadium content alloys may
suffer from higher rates of self discharge due to the solubility of vanadium oxide and its
consequent ability to form a special type of redox shuttle. The concentration of Co, Mn, Al
and Sn are important for ease of activation and formation and for long cycle life. The ratio
of hexagonal to cubic phase is important to emphasize capacity or power.
For both AB
5
and AB
2
metal hydride alloys, the metal/electrolyte surface oxide interface
is a crucial factor in discharge rate capability and cycle life stability. Original LaNi
5
and
TiNi
19
alloys extensively studied in the 1970s and 1980s for NiMH battery applications were
never commercialized due to poor discharge rate and cycle life capability.
20,21
Lack of cat-
alytic activity at the surface oxide limits discharge and lack of sufficient oxidation/ corrosion
resistance is a critical obstacle to long cycle life. The complicated chemical formulas and
microstructures of present disordered AB
5
and AB
2
alloys extend to the surface oxide. In
the oxide, important factors include thickness, microporosity and catalytic activity. Of crucial
importance to discharge rate was the discovery that ultrafine metallic nickel particles having
a size on the order of 50 to 70 Angstroms or less dispersed within the oxide are excellent
catalysts for the reaction of hydrogen and hydroxyl ions.
22
The other critical design factor within the surface oxide is to achieve a balance between
surface oxide passivation and corrosion. Porosity with the oxide is important to allow ionic
access to the metallic catalysts and therefore promote high rate discharge while passivation
of the oxide is problematic for high rate discharge and cycle life, unrestrained corrosion is
equally destructive. Oxidation and corrosion of the anode metals consume electrolyte, change
the state of charge balance and create corrosion products which are sometimes soluble and
capable of poisoning the positive electrode. Establishing a balance between passivation and
corrosion for stability is a primary function of compositional and structural disorder.
The construction of the metal hydride electrode is quite versatile, in part because the
metal hydride active material powder itself is conductive. Therefore, the choice of substrate
is partially current collector and partially as a carrier for the application of powder. While
metal hydride electrodes have used substrates of foam, fiber mat, and wire mesh all com-
mercially, two substrates dominate: expanded metal and perforated sheet. The choice of
substrate is mostly influenced by cost, method of connecting a current collecting tab, and
statistical likelihood of a short circuit. For all of these factors, perforated sheet has emerged
as the leading substrate. Most manufacturers find that a reduced incidence of sharp edges
and metallic burrs occur with perforated sheet and therefore a parts per million level of short
circuit defects in cell assembly can be achieved. It is common for the perforated hole patterns
to leave solid material at the edge of the substrate to facilitate welding and edge welding
for high power cell designs.
Substrate material has been dominated by nickel and nickel-plated steel. For cost reasons,
nickel-plated steel is preferred. Further, the metal hydride electrode is unlikely to undergo
the low voltage condition due to over discharge because hydrogen gas evolved at the nickel
hydroxide electrode during reversal can recombine at the metal hydride electrode to maintain
state of charge, except under highly abusive conditions. Consequently, corrosion of iron that
poisons the nickel hydroxide is of less concern in a NiMH battery. Taking advantage of the
metal hydride electrode voltage protection against corrosion has led to the emergence of a
copper substrate for both cost reduction and higher conductivity, vital for high power appli-
cations such as HEVs and power tools.

PROPULSION AND INDUSTRIAL NICKEL-METAL HYDRIDE BATTERIES 30.11
30.4.6 Nickel Hydroxide
Nickel hydroxide for use in NiMH batteries is fundamentally the same as that used in nickel
cadmium and nickel-iron batteries, and from a simple viewpoint, the basic compound is the
same as that used by Thomas Edison 100 years ago. Today’s high performance nickel hy-
droxide is much more complicated and continues to improve in capacity and utilization,
power and discharge rate capability, cycle life, high temperature charging efficiency, simpli-
fied manufacturing processes and cost.
As mentioned previously, one type of nickel hydroxide is by far the most common—a
high density spherical type for use in pasted electrodes which became commercial around
1990.
23,24
High density spherical nickel hydroxide is made in a precipitation process where
metal salts such as nickel sulfate are reacted with caustic such as NaOH in the presence of
ammonia. The nickel source may have additives such as cobalt and zinc to enhance per-
formance. The important physical parameters within this type of nickel hydroxide are:
•
Chemical formula. Common compositions are Ni
94
Co
3
Zn
3
(wt%) where cobalt is copre-
cipitated for the purpose of conductivity enhancement and both cobalt and zinc have the
function of oxygen overvoltage adjustment and microstructure refinement.
•
Tap density. Usually around 2.2 g/ cc, tap density is a measure of the dry nickel hydroxide
powder packing efficiency and influences the amount of active material which can be
loaded into the pores of the nickel foam current collector.
•
Particle size, usually having an average particle size of about 10 microns.
•
Surface area. Measured by the BET method, surface area refers not to the geometric area
of each nickel hydroxide sphere, but rather to the total surface area of each particle which
contribute to the charge/ discharge reactions and can thus affect utilization and high rate
discharge capability. A typical BET surface area for high-density spherical nickel hydrox-
ide is about 10 m
2
/g.
•
Crystallinity. Each nickel hydroxide sphere has an extremely high surface area correspond-
ing to the nickel hydroxide crystallites themselves. Crystallinity is measured by X-ray
diffraction, where the full width at half maximum (FWHM) of a reflection such as the
⬍101⬎ plane may yield a typical crystallite size of about 110 angstroms.
A variety of other factors contribute to performance, including impurities from processing
such as residual sulfates, nitrates, sodium sulfate, etc.
The nickel hydroxide active material and electrode formula can be formulated for specific
applications. For operation at 65
⬚C, some manufacturers may add Ca(OH)
2
, CaF
2
,orY
2
O
3
to the paste formula to inhibit premature oxygen evolution on charge.
25
Other paste formula
modifications may be an adjustment in the type and quantity of conductive network additives
such as cobalt metal and cobalt monoxide.
26,27,28
When long-term storage over several years
is required, it is common to increase the levels of cobalt additives to combat breakdown of
the conductive network and isolation of the active material. For ultra-high power discharge,
it is also possible to add metallic nickel fibers to the paste formula to enhance conductivity.
In the case of calcium additives, the additive may cause a loss in power and/ or cycle life.
Cobalt metal and cobalt monoxide are relatively expensive materials, and increased use has
cost implications. Addition of metallic nickel fibers lowers the amount of active material
and consequently capacity and specific energy are reduced.
The nickel hydroxide active material itself is most commonly a NiCoZn triprecipitate.
However, the amounts of cobalt and zinc which are usually about 1 to 5% each can be
adjusted for conductivity and oxygen overvoltage with some tradeoffs in terms of active
material capacity and cost. Other more complicated multi-element precipitates such as
NiCoZnCaMg offer higher capacity, cycle life and high temperature performance, but cannot
be manufactured by conventional precipitation processes. Another common active material
choice is ‘‘cobalt-coated nickel hydroxide.’’ Usually, the paste additives are finely divided

30.12 CHAPTER THIRTY
cobalt metal and cobalt monoxide that will dissolve and reprecipitate on the nickel hydroxide
active material surface. However, the coating may not be uniform and complete in coverage.
Consequently, although more expensive and lower in capacity, the nickel hydroxide itself
may be coated with cobalt hydroxide by the active material manufacturer. It has been reported
the cobalt-coated materials offer increased utilization and high rate discharge performance.
29
In addition to the nickel hydroxide active material and paste formula, the NiMH battery
manufacturer may modify the conductive skeleton current collector, which is most commonly
nickel foam. The pore size may be decreased from about 400 microns to 200 microns for
better conductivity. The density of the foam may also be adjusted to promote conductivity
and power versus capacity and utilization.
30.4.7 Electrolyte
The electrolyte in NiMH batteries of all types is normally a about 30% potassium hydroxide
in water, providing high conductivity over a wide temperature range. It is most common for
the KOH/H
2
O electrolyte to have a lithium hydroxide additive at a concentration of about
17 grams per liter to promote improved charging efficiency at the nickel hydroxide electrode
by suppressing oxygen evolution, the competing reaction to charge acceptance.
An important feature of the electrolyte is related to fill fraction. Essentially all NiMH
batteries are of the sealed, starved electrolyte design. As with NiCd batteries, the electrodes
are nearly saturated with electrolyte, while the separator is only partially saturated to allow
for rapid gas transport and recombination.
Special electrolytes are also used in NiMH batteries to enhance high temperature opera-
tion. Instead of binary KOH/LiOH electrolytes, it is also possible to substitute a portion of
the KOH with NaOH. The ternary KOH/ NaOH/ LiOH electrolyte is used at a high concen-
tration of about 6 Molar, but the contribution of NaOH promotes high temperature operation
charging efficiency although it is typical for this electrolyte to decrease cycle life by increased
corrosion of the metal hydride active materials.
30.4.8 Separator
The primary function of the separator is to prevent electrical contact between the positive
and negative electrodes, while holding the electrolyte necessary for ionic transport. Original
separators for NiMH batteries were standard NiCd and NiH
2
separator materials. However,
NiMH batteries were more sensitive to self discharge, especially when conventional nylon
separators were used.
30
The presence of oxygen and hydrogen gas cause the polyamide
materials to decompose, producing corrosion products which poison the nickel hydroxide,
promoting premature oxygen evolution and also forming compounds capable of a redox
shuttle between the two electrodes which further increases the rate of self discharge.
Consequently, there was a need for a more stable NiMH separator material to reduce self
discharge while still retaining electrolyte crucial for maintaining cycle life. NiMH batteries
now have widespread use of what is termed ‘‘permanently wettable polypropylene.’’ In fact,
the polypropylene is a composite of polypropylene and polyethylene fibers where the base
composite is hardly wettable to the KOH electrolyte without surface treatment.
Surface treatments involve the grafting of a chemical such as acrylic acid to the base
fibers to impart wettability and accomplished using a variety of techniques such as UV or
cobalt radiation.
31
Another method of imparting wettability to the polypropylene is a sulfo-
nation treatment where the base fiber material is exposed to fuming sulfuric acid. The sep-
arator surface is designed to be made hydrophilic to the electrolyte.

PROPULSION AND INDUSTRIAL NICKEL-METAL HYDRIDE BATTERIES 30.13
The separator has a crucial role relative to cycle life. In the starved electrolyte design, it
is a common design principle to saturate the electrodes with electrolyte at the assembly
stage. The separator is designed to have a high electrolyte fill fraction in order to hold as
much electrolyte as possible but not be overfilled so as to inhibit gas recombination. To the
battery manufacturer, this has the implication that during the first few charge/discharge
cycles (‘‘formation’’) when the electrodes have not yet absorbed all of the intended electro-
lyte, charging must be initiated carefully to avoid venting.
The electrolyte design concept relates to capillary theory that the electrolyte will migrate
to the smallest pores. In the NiMH battery, this translates to the nickel hydroxide positive
electrode having the smallest pores, followed by the metal hydride negative electrode and
finally the separator. At cell assembly, it is common for the separator to be about 90% filled,
and then reduced to about 70% during the cell formation process of the first few charge/
discharge cycles as both the positive and negative electrode expand and contract opening
interior regions for electrolyte absorption. This process continues to some degree over many
hundreds of charge/ discharge cycles, where NiMH cell failure is commonly when the
separator fill fraction has been reduced to about 10 to 15% of original. Consequently, sep-
arators which can absorb larger quantities of electrolyte at cell assembly, have small pores
able to retain electrolyte, and maintain surface wettability are highly desirable. Inspection of
the separator in failed batteries shows even these types of separators undergo some degra-
dation and loss of electrolyte absorption ability, although certainly not to the same degree
as earlier separators.
30.4.9 Monoblock Construction
NiMH technology is especially well suited for monoblock construction due to its high tol-
erance to overcharge and overdischarge. A monoblock design reduces cost by having a
common pressure vessel construction and far fewer cell parts; a single vent assembly and
shared hardware can be used in multicell modules. Further attributes of monoblock construc-
tion include reduced volume since interior cell walls can be shared, and flexible choices of
liquid or air cooling. A water cooled 12 V, 12 Ah plastic monoblock NiMH battery is shown
in Fig. 30.6, and has the following characteristics:
Number of cells 10
Nominal voltage 12 V
Rated capacity 12 Ah
Weight 3.2 kg
Volume 2.2 L
Rated energy 150 Wh
Specific energy 48 Wh/kg
Energy density 71 Wh/L
Peak power 1.76 kW
Specific power 550 W/kg
Power density 800 W/L
Issues of monoblock construction include selection of the plastic casing material to avoid
gas permeation, the need for individual cells to have well matched capacity and impedance
to avoid cell-to-cell imbalance. Further, monoblock construction must recognize that elec-
trodes within each cell expand and contract during charge and discharge and that the ensuing
swelling of the electrode stack must be compensated for in the mechanical design and loading
of the monoblock.

30.14 CHAPTER THIRTY
FIGURE 30.6 GM Ovonic 12 Ah, 12 V NiMH
watercooled plastic monoblock.
30.5 EV BATTERY PACKS
30.5.1 USABC Performance Targets
The USABC technical performance requirements are listed in Table 30.1, with the perform-
ance of NiMH batteries against these requirements.
In 1991, there were only two industrial size rechargeable batteries that could be used for
electric vehicles; lead-acid and nickel-cadmium. Lead acid batteries simply do not have
sufficient specific energy and cycle life to be viable for EV use. Industrial NiCd, while
somewhat higher in specific energy and in cycle life, was still not high enough in energy
and faced the environmental issues of cadmium use, operation in a flooded electrolyte con-
figuration requiring maintenance, and memory effect.
Essentially, an advanced battery technology for EV application must satisfy all minimum
performance requirements and then compete on energy and cost. NiMH EV batteries dem-
onstrate:
•
High energy: Commercial electric vehicles achieve over 200 mile range when using 70
Wh/ kg specific energy NiMH. Prototype vehicles using NiMH batteries have demonstrated
a 350 mile range.
•
High power: Commercial electric vehicles using 220 W/ kg specific power NiMH batteries
have demonstrated acceleration comparable to ICE powered vehicles. Advanced EVs using
NiMH routinely set race and demonstration range and speed records.
•
Flexible packaging: EV NiMH batteries operate well in 320 V AC propulsion or 180 V
DC propulsion systems and have a wide variety of cell capacities (Fig. 30.7). The volu-
metric performance of NiMH is high, offering vehicle designers a small size battery pack-
age.
•
Long life (600 to 1200 cycles to 80% DOD)
•
Wide operating temperature range (⫺30⬚Cto⫹65⬚C)
•
Fast charge and simple normal charging
•
Maintenance free operation
PROPULSION AND INDUSTRIAL NICKEL-METAL HYDRIDE BATTERIES 30.15
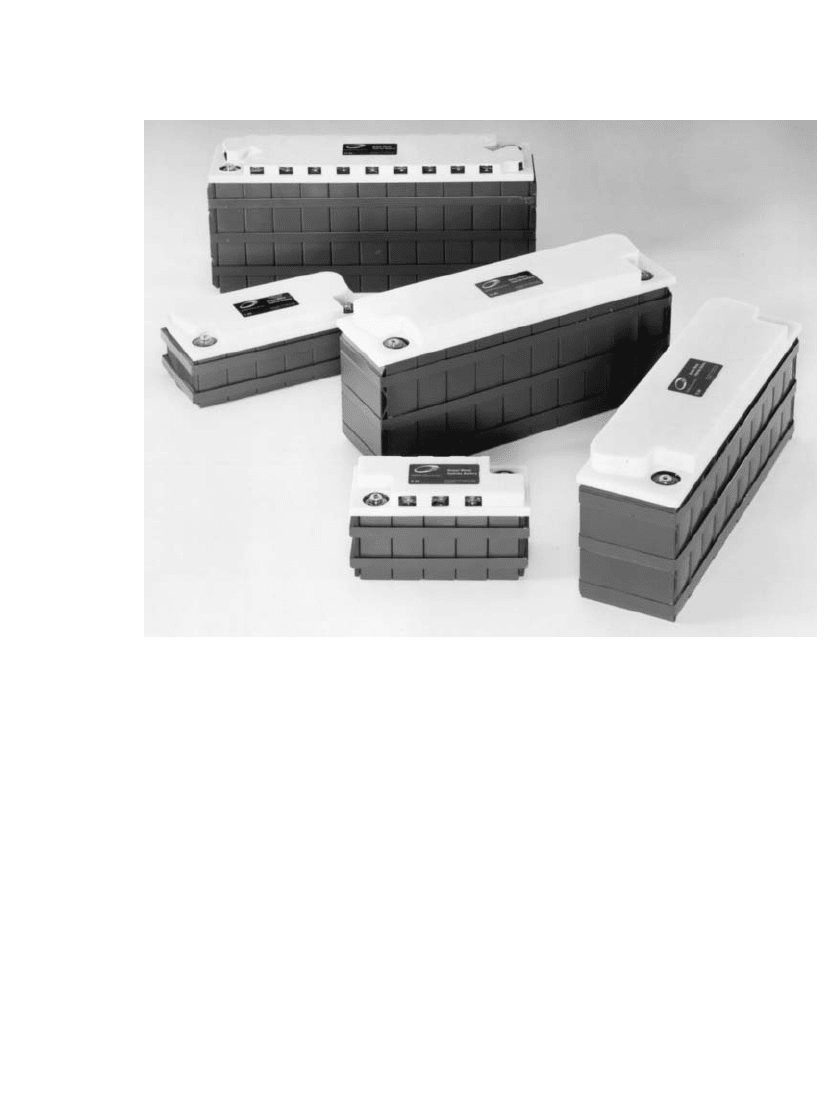
FIGURE 30.7 GM Ovonic ‘‘Family of Batteries’’ NiMH modules of varying capacity and dimensions
(12-100 Ah).
The main development activity for NiMH EV batteries has been to reduce cost. Because
only prototype production volumes exist to date, initial cost of manufacture for NiMH has
been high. Significant investment has been made to decrease cost, with highlights including:
•
Pilot manufacturing capability. Increased production rates allows reduced overhead and
labor, and the incorporation of automated and semi-automated manufacturing.
•
Increased specific energy. By raising specific energy from 56 to 80 Wh/kg through in-
creased utilization of active materials, the battery cost in $/kWh has decreased significantly
(
⬎$1000/ kWh to ⬍$350/ kWh, even at relatively low production volumes).
•
Low cost nickel hydroxide. From 1995, where high density spherical nickel hydroxide
prices were around $30 per kg, new low cost suppliers and manufacturing processes have
allowed nickel hydroxide prices to drop below $10 per kg.
•
Low cost metal hydride materials. MH development has included improved efficiency melt/
cast processes, scrap recycle, elimination of costly sintering, versatile use of inexpensive
substrate materials and significantly reduced battery formation.

30.16 CHAPTER THIRTY
30.6 HEV BATTERY PACKS
30.6.1 PNGV Performance Targets
The Partnership for the Next Generation Vehicles (PNGV) was established to enable auto-
makers and the government to meet an equivalent fuel economy of 80 miles per gallon of
gasoline. For many car manufacturers, development efforts focused on hybrid electric ve-
hicles of a variety of concepts.
32
HEVs provide many of the advantages of electric vehicles
without the need to recharge the battery which is carried out on-board the vehicle by a
combustion engine. There are basically two major types of HEVs based on how the inter-
action between the internal combustion engine (ICE) and the electric drive are incorporated
into the system. In a series design, the ICE is connected to a generator to provide electricity
for the battery pack and the electric drive motor. In a parallel design, the ICE and the electric
motor drive the wheels. In both cases, the electric motor can also be used to recharge the
battery. There are a variety of options within each battery approach such as range extender,
power assist, or dual mode. It is common for an HEV to be described as a 70:30 or 90:10
vehicle where the ratio is the amount of power supplied by the ICE compared to that of the
electric drive and battery.
The various specific operating modes for each type of vehicle make the size of the battery
extremely wide ranging, anywhere from 0.9 to 5 kWh. For hybrid vehicles, the smallest cell
size used is 6.5 Ah NiMH D cells and the largest HEV NiMH cell size is about 60 Ah. In
the Honda Insight HEV, the NiMH battery is 900 Wh and consists of 6.5 Ah D cells at 144
V. In contrast, the Toyota Prius HEV is 1.8 kWh and consists of 6.5 Ah D cells at 288 V.
In the General Motors Precept HEV, designed as a 50:50 ICE to electric drive, a 4.2 kWh
NiMH battery consisting of twenty-eight 12 V modules having a capacity of 12 Ah is
employed.
HEV battery requirements include:
•
Power density ⬎ 1000 W/ L
•
Specific power ⬎ 500 W/kg up to ⬎1000 W /kg
•
Specific energy ⬎ 50 Wh /kg
•
Regenerative braking charge ⬎ 500 W/kg
•
HEV cycle efficiency ⬎ 85%
•
Long life
•
Low cost
The crucial targets for HEV as compared to EV goals is a much greater emphasis on power,
both on charge and on discharge, and less emphasis on energy. Cycle life is measured at
low depth of discharge (
⬃ 2-5% DOD) compared to 80% DOD for an EV. Even cost is
calculated on available power in $ /kW. Car makers essentially want to use the smallest
energy battery capable of supplying the required power. A figure of merit target from the
HEV car makers is a power to energy ratio of 40. Present NiMH HEV batteries have attained
P/ E ratios of about 25. Performance specifications for several NiMH HEV designs are pre-
sented in Table 30.2.
