Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

25.12 CHAPTER TWENTY-FIVE
TABLE 25.3 Typical Nickel-Iron Batteries
Nominal capacity, Ah 169 225 280 337 395 450 560 675
Nominal current, A:
5-h discharge 34 45 56 67 79 90 112 135
Cell weight, filled, kg 8.8 10.8 12.9 15.3 17.4 19.5 24.3 28.6
Installed weight, kg 9.8 12.0 14.3 16.9 19.3 21.7 26.5 31.2
Electrolyte (1.17 kg/ L), kg 1.8 2.2 2.6 3.0 3.4 3.8 4.9 5.9
Cell dimensions, mm*
Length 52 66 82 96 111 125 156 186
Width 130 130 130 130 130 130 135 135
Height 534 534 534 534 534 534 534 534
Battery dimensions, mm†:
Length:
2 cells — — — 265 295 321 343 343
3 cells — — — 376 421 460
4 cells 284 367 431 487
5 cells 346 448 545
6 cells 408 546
Width 161 161 161 161 161 161 197 228
Height 568 573 582 582 582 582 590 590
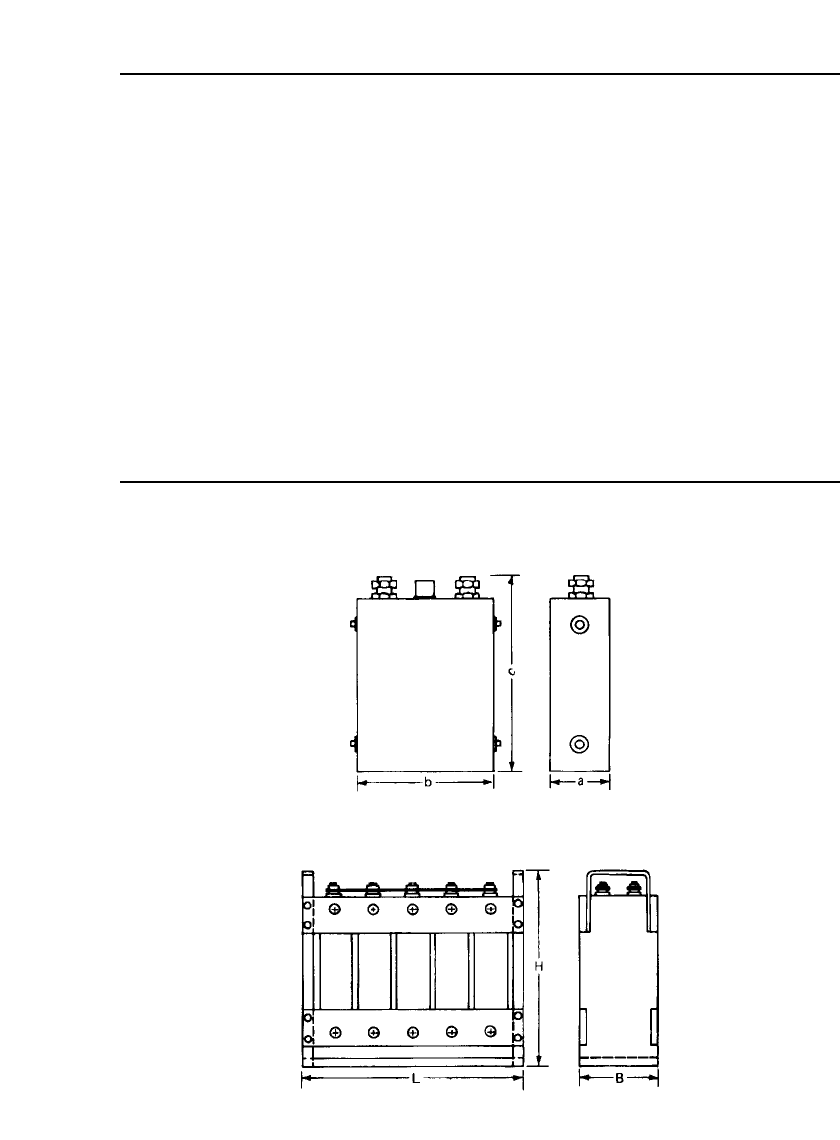
* See drawing (a).
† See drawing (b).
Source: Varta Batteries AG, Hanover, Germany.
(a) Cell showing dimensions used in Table 25.3.
(b) Multicell battery showing dimensions used in Table 25.3. Tolerances
are 5, 3, and 3 mm for dimensions L, B, and H, respectively.

IRON ELECTRODE BAT TERIES 25.13
25.3.4 Special Handling and Use of Nickel-Iron Batteries
The battery should be operated in a well-ventilated area to prevent the accumulation of
hydrogen. Under certain circumstances, hydrogen can be ignited by a spark to cause an
explosion with a resulting fire. In multicell batteries the usual precautions in dealing with
high voltages should be taken.
If the battery is to be out of service for more than a month, it should be stored in the
discharged condition. It should be discharged and short-circuited, then left in that condition
for the storage period. Filling caps must be kept closed. If this procedure is not followed,
several cycles are required to restore the capacity upon reactivation.
Constant-voltage charging is not recommended for conventional nickel-iron batteries. It
may lead to a thermal runaway condition which results in dangerous conditions and can
severely damage the battery. When the battery nears full charge, the gassing reactions pro-
duce heat and the temperature rises, lowering the internal resistance and the cell EMF.
Accordingly, the charge current increases under constant-voltage charge. This increased cur-
rent further increases the temperature, and a vicious cycle is started. A modified constant-
voltage charging with current limiting is, however, acceptable.
25.4 ADVANCED NICKEL-IRON BATTERIES
The desire to use the attractive features of the nickel-iron couple, such as ruggedness and
long life, in applications requiring high-rate performance and low manufacturing costs has
led to the development of advanced nickel-iron batteries with performance characteristics
suitable for electric automobiles and other mobile traction applications. The capability of
these batteries permits an electric vehicle a range of at least 150 km between charges,
acceleration rapid enough to merge into highway traffic, and a cycling life equivalent to 10
or more years of on-the-road service. The advanced battery utilizes sintered-fiber metal (steel
wool) plaques, impregnated with active material, for both the positive and the negative elec-
trodes. Nonwoven polypropylene sheets are used as separators between electrodes. The tech-
niques for making plaques, impregnation and activation, stacking, and assembly are all
amenable to high-volume production methods similar to those used in lead-acid battery
manufacture.
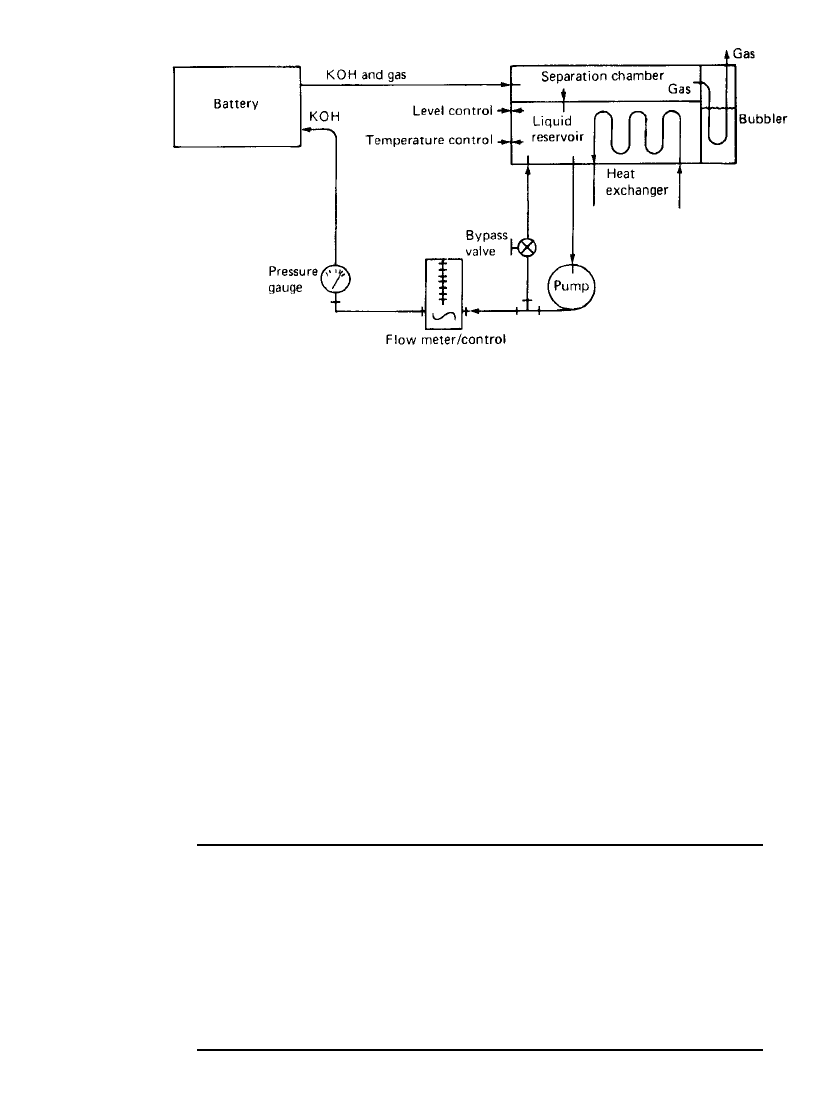
The battery system design incorporates an electrolyte management system to minimize
the maintenance problems associated with its widespread deployment in the public sector.
This system, shown schematically in Fig. 25.12, provides for semiautomatic watering of the
cells by utilizing a single-point watering port. The flow of electrolyte through the cells during
the charging cycle permits heat removal and effective management of gas evolved during
the charge. Uniform specific gravity for all cells is ensured and specific gravity maintenance
is easily achieved by use of the system.
Both the positive and the negative plates of the Westinghouse nickel-iron battery used
fiber metal plaques as the substrate. Two methods of active nickel impregnation were de-
veloped and used in demonstration batteries. An electroprecipitation process (EPP), devel-
oped in the mid-1960s, demonstrated good performance, ruggedness, and long cycle life.
The EPP deposits nickel hydroxide electrochemically into the porous substrate. Efficient use
of the nickel material is achieved, with active material utilization of 0.14 Ah /g of total
electrode. An alternate nickel electrode manufacturing process was also developed which
entails the preparation of a nickel hydroxide paste that is then loaded into the fiber metal
substrate by roll pasting methods. Pasted nickel electrodes demonstrated performance equiv-
alent to EPP electrodes (0.14 Ah /g of total electrode) while demonstrating a less expensive
manufacturing process. The iron electrodes were also produced by a pasting process. Iron
oxide, Fe
2
O
3
, was paste-loaded into the fiber metal electrode substrate and then furnace-
reduced in a hydrogen atmosphere. These electrodes demonstrated 0.26 Ah /g of total elec-
trode or, better, at C/3 discharge rates.
25.14 CHAPTER TWENTY-FIVE
FIGURE 25.12 Schematic of electrolyte circulation system.
The performance characteristics of the advanced nickel-iron batteries, as typified by the
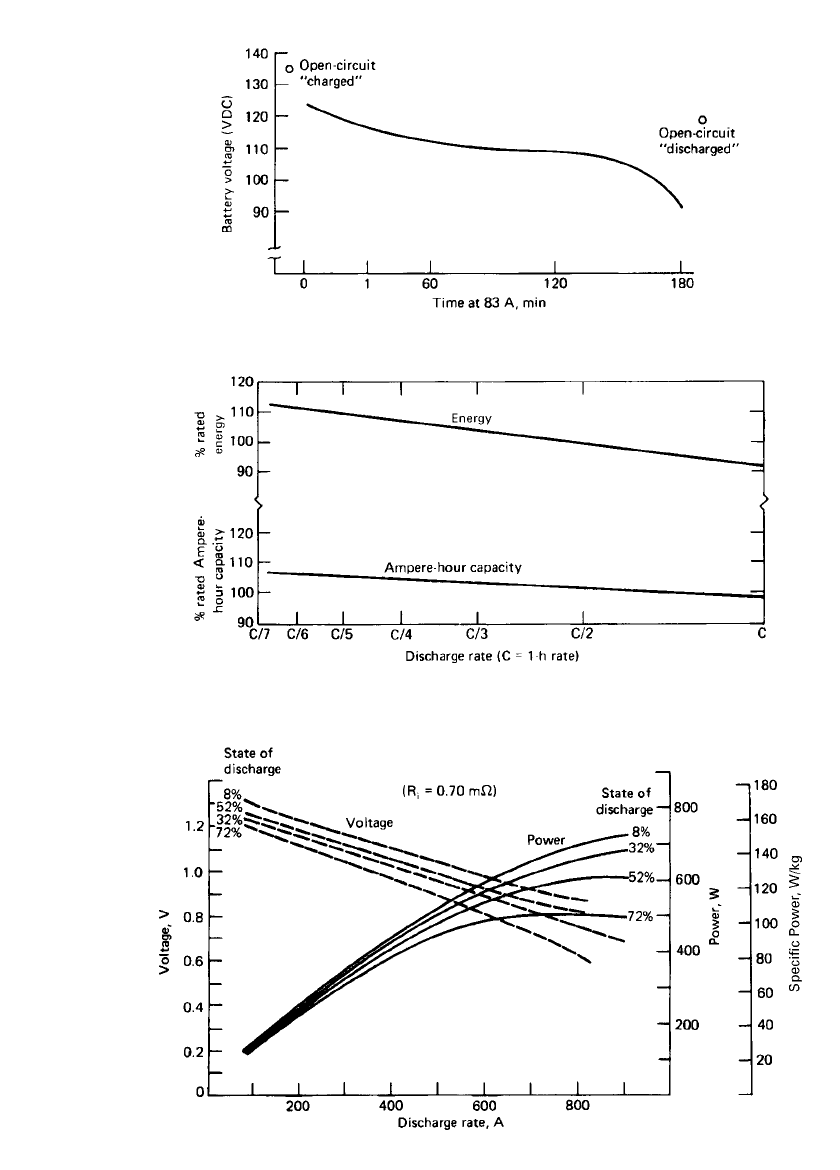
Westinghouse system, are summarized in Table 25.4. Figure 25.13 shows a typical discharge
curve for a 90-cell electric-vehicle battery at the C/ 3 rate. The battery capacity and energy
as a function of discharge rate are shown in Fig. 25.14. Cell power and voltage character-
istics, as a function of discharge rate and state of discharge, are presented in Fig. 25.15. The
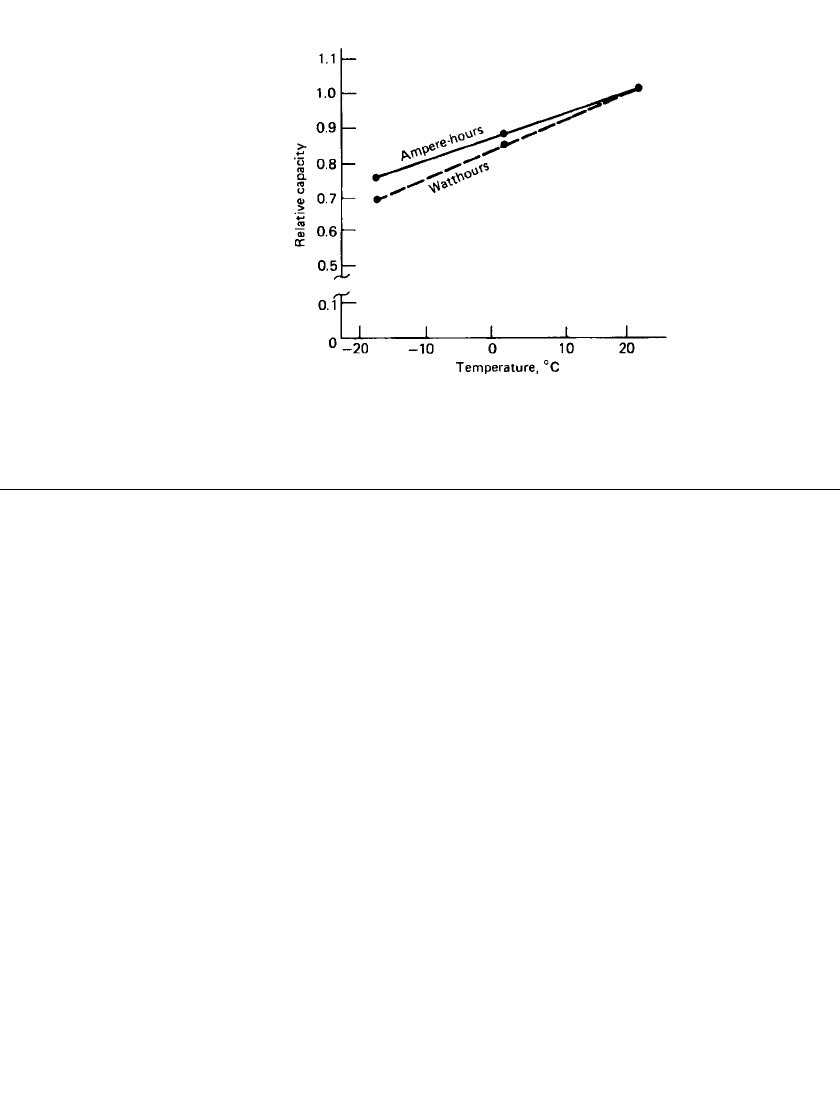
variation in battery capability with temperature, based on tests on five-cell modules, is shown
in Fig. 25.16.
10–12
The Eagle-Picher Company also developed a nickel-iron battery using
sintered-nickel electrodes similar to those described in Chap. 27. The iron electrode is similar
to the Swedish National Development Corporation iron electrode discussed in Sec. 25.5. The
performance of this battery is similar to that given in Figs. 25.13 to 25.16.
13
TABLE 25.4 Advanced Nickel-Iron Battery Performance Characteristics*
Demonstrated as of December 1991
Capacity, † Ah 210
Specific energy, † Wh/ kg 55
Energy density, † Wh/ L 110
Specific power, ‡ W / kg 100
Cycle life§
⬎900
Urban range, km
with regenerative braking 154
Without regenerative braking 125
Projected production cost, $/ kWh (1990 $) 200–250
* Based on the Westinghouse nickel-iron battery.
† At the C / 3 rate.
‡ 30-s average at 50% state of charge.
§ Cycle to 100% depth of discharge; life to 75% of rated energy.
IRON ELECTRODE BAT TERIES 25.15
FIGURE 25.13 Battery voltage at C/ 3 (83-A) discharge rate.
FIGURE 25.14 Capacity as a function of discharge rate. (Courtesy of Westinghouse
Electric Corp.)
FIGURE 25.15 Power characteristics of 210-Ah nickel-iron battery. (Courtesy of Westinghouse
Electric Corp.)
25.16 CHAPTER TWENTY-FIVE
FIGURE 25.16 Effect of temperature on capacity and
energy of nickel-iron battery (C / 3 rate).
25.5 IRON/AIR BATTERIES
Rechargeable metal/air batteries have an advantage over conventional systems as only one
reactant (the anode material) need be contained within the battery. The electrically recharge-
able iron/ air cell has a lower specific energy than the mechanically rechargeable cells (see
Chap. 38) but has the advantage of potentially lower life-cycle costs. Unlike zinc, the iron
electrodes do not suffer a severe redistribution of active materials or gross shape change
upon prolonged electrical cycling. The iron /air cell is another candidate as a motive power
source, especially for electric vehicles. The cell reactions are
discharge
—— —
O ⫹ 2Fe ⫹ 2H O 2Fe(OH) (first plateau)
———
22
2
charge
discharge
1
—— —
–
3Fe(OH) ⫹ OFeO⫹ 3H O (second plateau)
———
222
34 2
charge
The iron electrode kinetics are covered in Sec. 25.2. The oxygen electrode reactions
follow the kinetic path with peroxide as an intermediate. The oxygen electrode reactions in
simple form are
⫺
O ⫹ 2H O ⫹ 2e → HO ⫹ 2OH
22 22
⫺
HO ⫹ 2e → 2OH
22
The single most important life-limiting factor in this battery system is the stability of the air
electrode, which loses its ability to function reversibly as it undergoes repeated charges and
discharges. The oxygen and peroxide evolved on charge and discharge may attack the sub-
strate, alter the activity of the catalyst, and delaminate the wetproofing film. Separate air
(oxygen) electrodes and circuits can be employed in the charge and discharge modes; how-
ever, considerations of system weight and volume favor the use of a bifunctional electrode,
that is, a single electrode capable of sustaining either oxygen reduction or evolution. These
electrodes must be stable over the potential range of both reactions, a fact which poses
constraints on material stability and electrode design.
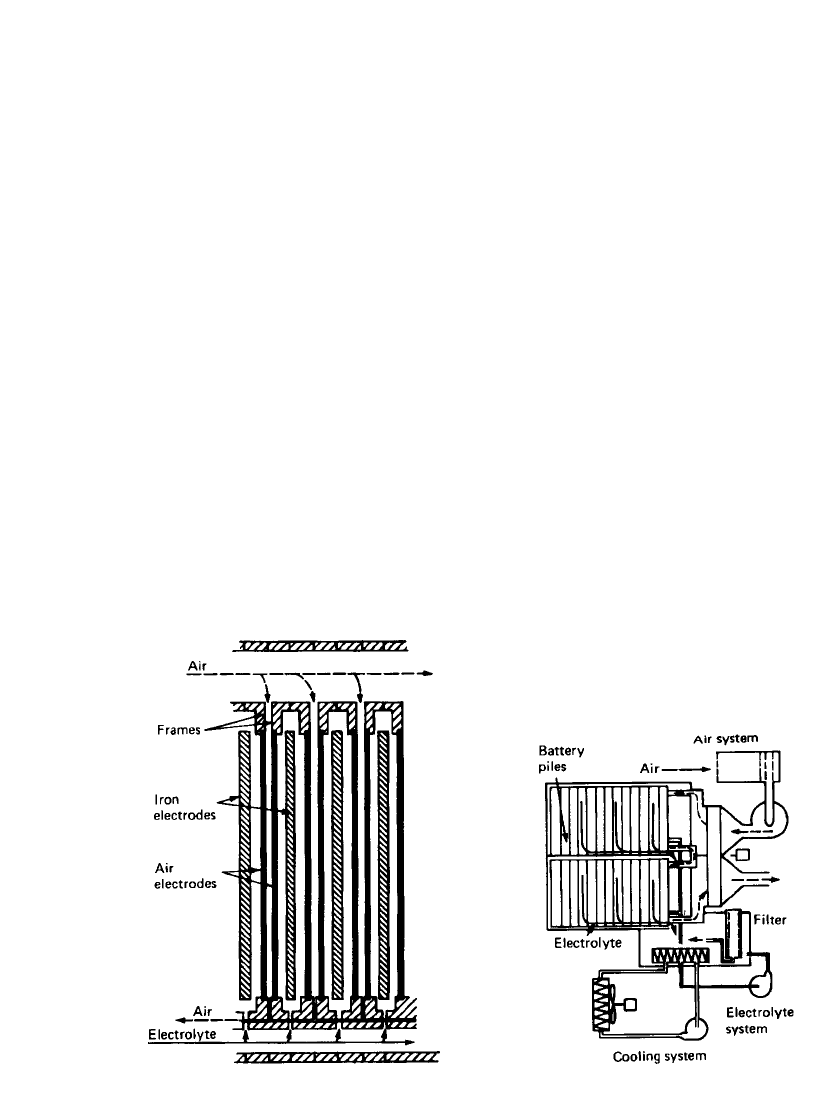
IRON ELECTRODE BAT TERIES 25.17
FIGURE 25.17 Cross section of Swedish National
Development Corporation’s iron /air battery pile.
(From Ref. 5.)
FIGURE 25.18 Schematic cross section of Swedish
National Development Corporation’s iron /air battery
including auxiliary system. (From Ref. 5.)
Several designs were developed, but most work on iron/ air has been discontinued in favor
of zinc /air.
The Swedish National Development Corporation’s iron/air cell used the sintered-iron-
mesh anode construction.
5,14,15
A pore-forming material could be included to control the
development of the optimum electrode structure. The resulting pressed matrix was treated
in H
2
at 650⬚C. The pore-forming material could be leached out after treatment. The active
material utilization approached 65%. The air electrode was a porous-nickel double-layer
structure (0.6 mm thick) composed of sintered nickel of coarse and fine porosities. The coarse
layer on the electrolyte side was catalyzed with silver and impregnated with hydrophobic
agents. The electrodes were welded into a polymer frame and formed into cells, as shown
in Fig. 25.17. There were two air electrodes for each iron electrode. Air was forced past the
electrode at about 2 times the stoichiometric requirement during operation. A schematic and
a photo of a 30-kWh battery are shown in Fig. 25.18 and Fig. 25.19, respectively. Electrolyte
circulation was used to control heat balance and remove gases generated during operation.
Carbon dioxide was scrubbed from the incoming air using NaOH. The air was then humid-
ified to minimize electrolyte loss. Overall the auxiliary systems require less than 10% of the
system output.
Typical charge-discharge curves for an average battery in the iron /air battery are shown
in Fig. 25.20. The marked difference in charge and discharge voltages accounts largely for
the low overall system efficiency. Figure 25.21 shows the power-producing characteristics.
The system is capable of over 1000 cycles, limited by the gradual deterioration of the air
electrode.
The Westinghouse iron /air battery used a construction similar to that described for the
Swedish National Development Corporation’s system.
16
The sintered-iron electrode was
somewhat similar to that described before. The iron electrode for this iron /air battery had a
high active iron content and lower cycle life compared with the electrode described previ-
ously. Particles of iron powder were sintered to form a structure without the steel fiber
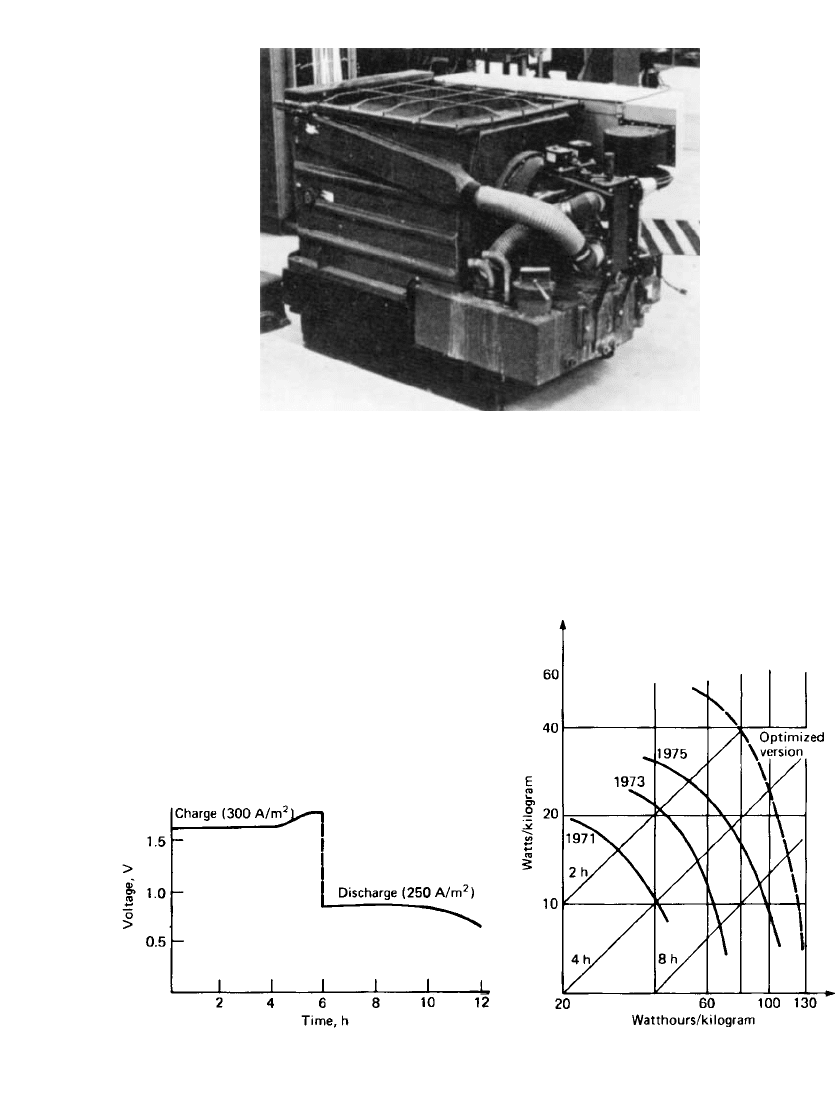
25.18 CHAPTER TWENTY-FIVE
FIGURE 25.19 Swedish National Development Corporation’s 30-
kWh iron /air battery system. (Courtesy of Swedish National De-
velopment Corp.)
FIGURE 25.20 Charge-discharge voltages for battery
in Swedish National Development Corporation’s iron / air
battery. (From Ref. 5.)
FIGURE 25.21 Performance of Swedish Na-
tional Development Corporation’s iron/ air batter-
ies. (From Ref. 5.)
IRON ELECTRODE BAT TERIES 25.19
substrate. Electrodes with this construction demonstrated up to 0.44 Ah/g. The air electrode
was bifunctional and used a Teflon-bonded carbon-based structure with complex silver cat-
alysts (silver content was less than 2 mg/ cm
2
) supported on a silver-plated nickel screen.
The Westinghouse system used a horizontal flow concept to improve performance and control
gas and heat. Good life was demonstrated for over 300 cycles with an air electrode of
potentially very low cost. A summary of the projected characteristics of the 40-kWh battery
is given in Table 25.5.
The Siemens cell was similar except that the air electrode was fabricated with two layers:
a hydrophilic layer of porous nickel on the electrolyte side for oxygen evolution and a
hydrophobic layer [carbon black bonded with Teflon
威 (PTFE) and catalyzed with silver] on
the air side for oxygen reduction. The dual porosity helped to shield the silver catalyst from
oxidation. As many as 200 cycles were achieved.
17
TABLE 25.5 Characteristics of Westinghouse Iron/ Air Electric-Vehicle Battery
Electric vehicle:
Weight 900 kg curb weight
Range 240 km
Battery:
Capacity 40 kWh
Power 10 kW continuous power
Weight 530 kg
Volume 0.04 m
3
Cost $150/ kWh
25.6 SILVER-IRON BATTERY
The silver-iron battery has been limited in use because of its high cost. Its theoretical energy
density is essentially equal to that of the more popular silver-zinc system. The silver-iron
battery has good cycle life compared with the silver-zinc and provides a battery of high
reliability, long life, and better durability where high specific energy content is essential.
18–23
Figure 25.22 shows a 3.5-kWh battery designed for telecommunication use, whereas Fig.
25.23 shows a 9.5-kWh battery designed for use in a submersible.
The cell reactions are
discharge
—— —
Fe ⫹ 2AgO ⫹ H O Fe(OH) ⫹ Ag O (first plateau)
———
2
22
charge
discharge
—— —
Fe ⫹ Ag O ⫹ H O Fe(OH ) ⫹ 2Ag (second plateau)
———
22
2
charge
discharge
—— —
3Fe(OH) ⫹ Ag O Fe O ⫹ 3H O ⫹ 2Ag (third plateau)
———
22
34 2
charge
In practice, only the first and second discharge plateaus are used. The overall reaction is
discharge
0
—— —
2Fe ⫹ 2AgO ⫹ 2H O 2Fe(OH) ⫹ 2Ag E ⫽ 1.34 V
———
2
2
charge
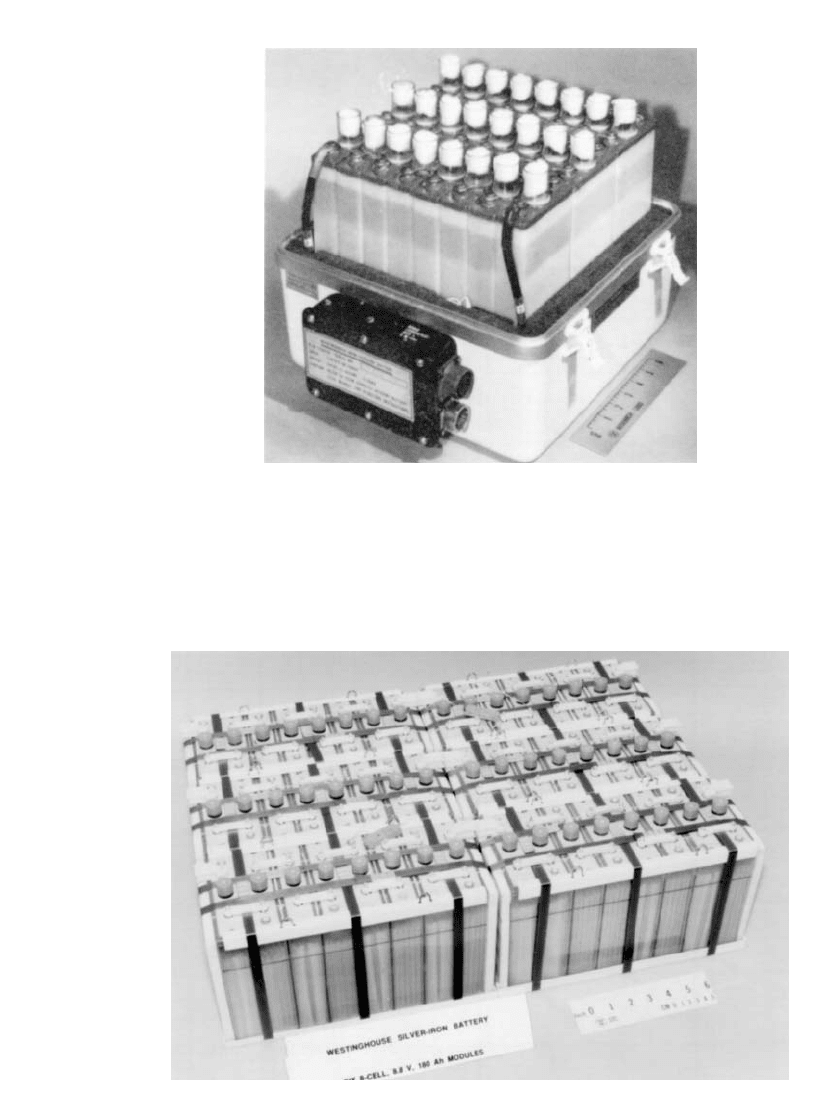
25.20 CHAPTER TWENTY-FIVE
FIGURE 25.22 3.5-kWh telecommunications iron /silver oxide
battery. (Courtesy of Westinghouse Electric Corp.)
FIGURE 25.23 9.5-kWh iron / silver oxide battery for submersible vehicle. (Courtesy of West-
inghouse Electric Corp.)
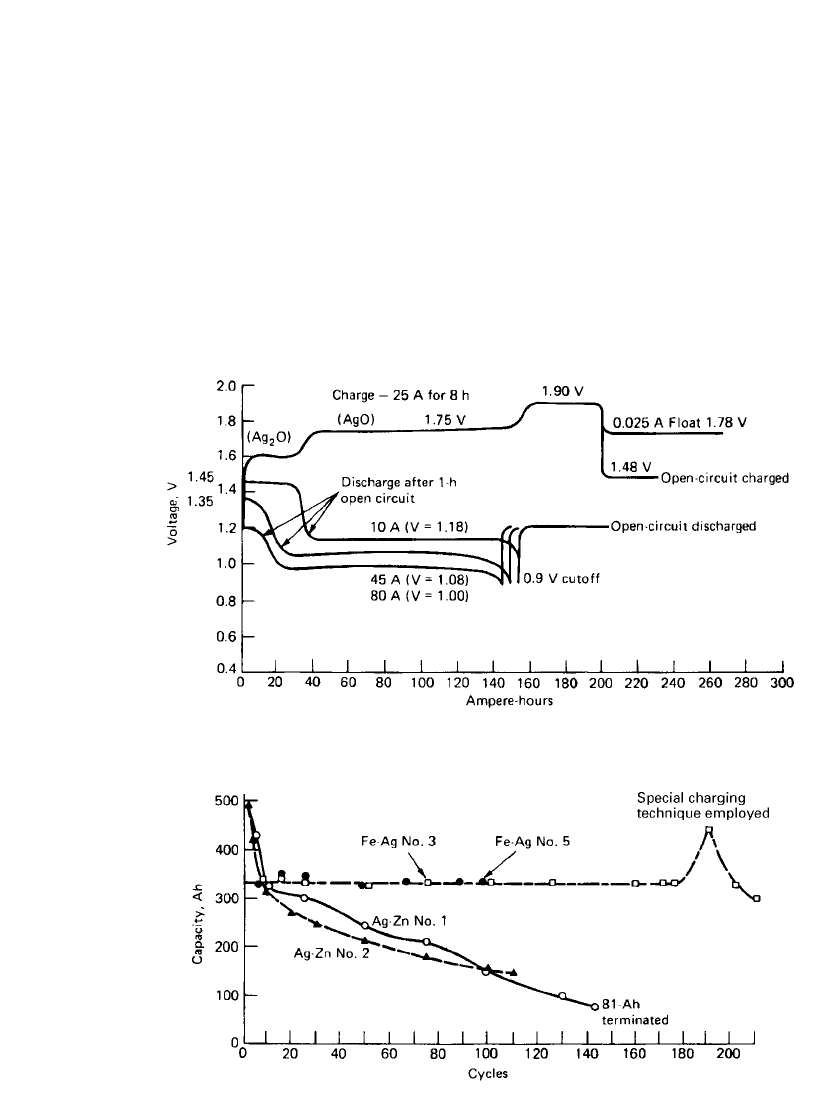
IRON ELECTRODE BAT TERIES 25.21
FIGURE 25.24 Charge-discharge characteristics of nominal 140-Ah iron / silver oxide battery.
(From Ref. 20.)
FIGURE 25.25 Cyclic life performance of zinc/ silver oxide and iron/ silver oxide
prototype batteries. (From Ref. 20.)
Charge/ Discharge Characteristics. Typical charge-discharge curves for a silver-iron bat-
tery of the type shown in Fig. 25.22 are given in Fig. 25.24. The electrolyte is KOH of 1.31
specific gravity with 15 g/L LiOH added. The batteries can withstand several complete
reversals without appreciable adverse effect on capacity.
Separator System and Cycle Life. Multilayer microporous polyethylene, nonwoven felt
polypropylene, and cellophanes are some of the materials typically used as separators in this
system. It is important to note that the choice of separator has very little to do with the iron
electrode, which is extremely stable in KOH and does not react with the separator system.
Rather, the separator system must be selected to retard the migration of silver to the iron
electrode and to withstand the oxidative effects of the silver electrode itself. The particular
separator system chosen will therefore determine the cycle life, the shelf life, and the power
capabilities. Consequently the separator system is usually application-specific. The typical
cycle life at 100% depth of discharge and 10% overcharge is shown in Fig. 25.25.
