Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.


43.10 CHAPTER FORTY-THREE
Methanol. Methanol is a widely available chemical that is relatively easy to handle and
store as a liquid at atmospheric pressure. It can also be converted to a hydrogen-rich gas by
a process that is simplest among those for carbon-containing fuels. The endothermic reaction
CH OH ⫹ HO CO ⫹ 3H
3222
is carried out at modest temperature (about 250⬚C) since methane (favored by thermodynamic
equilibrium) cannot be formed when conventional methanol steam-reforming catalysts are
used. Accordingly, little downstream processing is needed because very little carbon mon-
oxide is formed at these temperatures. Here again, whereas LPG is favored from the points
of view of energy content and cost, methanol is an attractive fuel for small fuel cells because
its processing burden is greatly eased.
Ethanol. Ethanol is similar to methanol in its handling and storage. Its reaction with steam
can be expressed as
CHOH⫹ H O 2CO ⫹ 4H
25 2 2
Unlike methanol, it is considered non-toxic. Its availability and cost as a fuel are irregular;
more important, ethanol cannot be processed at the low temperatures characteristic of meth-
anol steam reforming. Therefore, its potential attractiveness as a fuel for compact fuel cell
systems is substantially diminished.
Liquefied Petroleum Gases (LPG). As indicated above, LPG (principally propane in the
U.S., but sometimes principally butane, as in Japan) is often the preferred fuel for dispersed
fuel cell systems. It has substantially higher specific energy density than both ammonia and
methanol along with lower price per unit energy. Indeed, in the absence of pipeline natural
gas, it is the fuel of choice for stationary fuel cells. However, small fuel cells generally have
a different set of criteria. In a relative sense, fuel cost is perhaps less important; and com-
pactness, simplicity, and hardware cost more important (although this cannot be over-
generalized; e.g., missions with very long durations could be an exception).
Natural Gas. Small fuel cell systems that are stationary and have ready access to a natural
gas pipeline will predominantly take advantage of the natural gas availability (just as in the
case of a larger stationary fuel cell). The principal constituent of natural gas is generally
methane, CH
4
. The cost per unit energy for natural gas is the most attractive, and compact-
ness is presumably not a major issue. Since its storage characteristics are not attractive and
its processing system is no more favorable than that of LPG, these are likely to be the only
circumstances under which natural gas would be utilized in small fuel cells.
Aviation Fuel or Diesel Fuel. Aviation and diesel fuels are preferred for military appli-
cations since they are the most available and safest (very low vapor pressure). Their avail-
ability is also a key factor in certain under-developed regions. On the other hand, these are
the most difficult to process (leaving out heavier fuels, like heating oil). This is attributable
to the difficulty of breaking down their larger molecules and to their relatively high sulfur
content. Therefore, such fuels would generally be avoided for small fuel cell systems.
43.4.3 Methodologies For Fuel Processing
The generation of hydrogen from carbon-containing fuels (with the exception of methanol)
requires a high-temperature (usually catalytic) process. The fuel is reacted with steam cat-
alytically (steam reforming, or SR), with sub-stoichiometric oxygen from air (partial oxi-
dation, homogeneous or catalytic, POX), or with steam and oxygen catalytically (autother-
mal, ATR). SR is an endothermic reaction carried out typically at 650
⬚C, or higher; POX is

SMALL FUEL CELLS 43.11
an exothermic process, usually carried out at higher temperatures (perhaps 1000⬚C), while
the ATR process is almost thermally neutral and typically operates at or somewhat above
SR temperatures. Representative reactions for these processes using methane as the fuel can
be expressed as follows:
SR: CH ⫹ HO CO⫹ 3H
42 2
POX: CH ⫹ 0.5O CO ⫹ 2H
42 2
ATR: CH ⫹ 0.25 O ⫹ 0.5H O CO ⫹ 2.5H
422 2
In simplistic terms, the SR process generally provides the highest hydrogen yield and
consequently the highest efficiency. Because of the endothermic reaction, its thermal man-
agement tends to be the most complex and bulky. Conversely, POX is typically the least
efficient but has the simplest configurations. The ATR process tends to be intermediate to
the other two in both respects.
The selection of a preferred fuel processor type depends heavily on the application re-
quirements. For example, a conventional stationary fuel cell system operating continuously
on natural gas might be best suited to the SR processor to minimize fuel cost, while a small,
mobile system requiring rapid start-up might be best served via a POX, or ATR, system.
Process Gas Upgrading. The high-temperature processes described above yield a reformate
gas that is high in carbon monoxide (CO) content (usually greater than 10 percent). In order
to maximize the hydrogen yield (and, in the case of low-temperature fuel cells like PEM,
minimize the fuel cell anode-catalyst inhibiting effects of CO), the reformate gas is then
passed through a catalytic water-gas shift-converter at lower temperature (perhaps in two
stages) where the following reaction takes place
CO ⫹ HO CO ⫹ H
222
Here again, as in the case of methanol steam reforming, the formation of methane under
these conditions is prevented via the specificity of the shift-converter catalyst. For PEM fuel
cells, further reduction in CO concentration is necessary (from about 0.5 percent down to
less than 100 ppm). This is often carried out by way of catalytic preferential oxidation of
CO in the presence of hydrogen with the addition of air at a flow rate that is a small multiple
of the stoichiometric rate required for complete oxidation of the CO.
It is evident that steam or water vapor is an essential player in the fuel processor, whether
it be in the reforming reaction itself or in the subsequent shift-conversion stage. The source
of this water must come from storage, make-up, or condensation and recovery from the fuel
cell system. In any event, the design and logistics for water management must be provided
for the system, and the selected mode must best reflect the requirements for the specific
application.
The complexity of the overall fuel processing system for carbonaceous fuels indicates the
challenge associated with adapting conventional fuels for use in small fuel cell systems.
Considerable effort is required in designing and optimizing the system to achieve the req-
uisite miniaturization and low cost in these applications.
43.4.4 Direct-Methanol Fuel Cell (DMFC) Technology
It is clear that a system that can utilize a liquid fuel directly at the fuel cell anodes would
be particularly appealing in small fuel cell applications. As mentioned earlier, at this time
methanol is the only carbonaceous species that can both serve as a practical fuel and provide
reasonable electrochemical performance at the fuel cell anode. Substantial research and de-
velopment efforts have been directed at direct-methanol fuel cells using PEM electrolyte for
more than a decade, in the context of small, field-deployable systems.

43.12 CHAPTER FORTY-THREE
While the direct-methanol approach is inherently appealing for small fuel cells, this tech-
nology is challenging and complex. Specifically, (a) the electrochemical activity at the anode
is poor, producing low cell voltages and requiring relatively high precious metal loadings to
attain desirable current densities; (b) methanol is soluble in conventional PEM electrolytes,
and its ‘‘cross-over’’ to the cathode results in wasted fuel consumption along with inhibition
of normal oxygen transport and electrochemical reduction at this electrode; and (c) main-
tenance of water balance within the cells requires that water vapor discharged at the cathode
flowfield exit be condensed and returned to the circulating methanol-water anode feed so-
lution which, in order to retard methanol migration across the electrolyte membrane to the
cathode, is maintained in a very dilute state, typically about 3 percent by weight.
Despite the issues cited above, the DMFC is considered to have much potential for im-
plementation in small fuel cell systems. Research and development work that specifically
focuses on these challenges is proceeding
4
and meaningful advances are anticipated in the
period ahead. Also, for applications requiring extremely low power, approaches involving
the feed of methanol, or methanol and water, without recirculation can be considered since
heat removal (generally via a heat-exchanger in the recirculation loop) could be accomplished
via natural convection.
4,5,6,7
In any event, when DMFCs are ready for commercial service,
they will offer attractive incentives, especially for extended-duration missions, because of
their simplicity of storage and relatively high energy densities and specific energies (ap-
proximately 2000 Wh/ kg at a practical cell voltage of 0.4 V).
43.5 FUEL CELL STACK TECHNOLOGY
The technology of the PEM fuel cell stack must be in keeping with the small fuel cell system
design approach described in Sec. 43.3.
43.5.1 Design
The requirements of the small fuel cell system translate into a fuel cell stack that is compact,
externally air-cooled and utilizes unconditioned ambient air as a reactant. For hydrogen-
fueled systems (which are the majority to date) the anode compartments of the stack are
virtually dead-ended. The need for compactness calls for internal reactant manifolding and
bolting as well as thin end-plates. A representative stack for a small fuel cell system is shown
in Fig. 43.3. The cell components, of course, should also be as thin as possible.
43.5.2 Electrolyte
The most suitable electrolyte for small fuel cell systems is the proton-exchange membrane
(PEM). For the most part, these are based on trifluoromethanesulfonic acid in a tetrafluo-
roethylene-based polymer backbone. The material’s equivalent weight is typically 1100, al-
though versions with an equivalent weight near 1000 are also in use. Membranes with lower
equivalent weight tend to have superior proton-exchange and water-transport properties but
they also have greater vulnerability to dry-out conditions. The thickness of membranes in
use in fuel cells of all types today is roughly in the 20 to 200
m range. Thinner membranes,
of course, exhibit higher proton-conductance as well as rate of water transport, while posing
a somewhat higher risk of failure, either in processing or in operation. Membranes used in
small fuel cells are generally on the thinner side, under 100
m and sometimes far thinner.

SMALL FUEL CELLS 43.13
43.5.3 Electrodes
Electrodes used in PEM fuel cells typically employ an electrocatalyst layer with a porous,
carbonaceous, electronically-conductive substrate that has been rendered hydrophobic. The
catalyst layer usually comprises platinum, or a platinum-containing alloy, on a carbonaceous
support (typically carbon black), dispersed ionomeric material similar in constituency to the
electrolyte-membrane, and dispersed hydrophobic polymer such as polytetrafluoroethylene.
The substrate, which serves as a reactant-gas diffusion layer, may be a carbon paper or a
woven or non-woven cloth. The catalyst layer may be deposited directly onto the electrolyte-
membrane or onto the substrate and later placed in contact with the membrane.
Anodes are usually very similar to, if not identical to those that serve as cathodes. Anodes
that operate on reformed-hydrocarbon fuels, which contain some carbon monoxide, generally
utilize a platinum-alloy catalyst to enhance co-tolerance. The catalyst-layer structure is some-
times altered between anodes and cathodes to adjust their respective hydrophobicity and
reactant-diffusion properties. The thickness of the catalyst layer typically ranges from 10 to
20
m, that of the substrate from 0.1 To 0.5 mm (uncompressed).
43.5.4 Bipolar Plates
The typical stack construction for PEM (and other) fuel cells is a series-connection of cells
with bipolar plates interposed between adjacent cells (or membrane-electrode assemblies).
The bipolar plate must provide for electronic conduction from one cell to the next; isolation
of fuel on one side from oxidant (air) on the other side; and distribution of fuel and air
reactants to the respective adjacent anode and cathode. In an edge-cooled stack of the type
described above, the bipolar plate also contributes to heat rejection by conducting heat lat-
erally to its finned edges, where forced-air convection is employed.
Graphite-based bipolar plates are often preferred for small fuel cell stacks because of their
relatively high thermal conductivity as well as their electrochemical stability. These plates
are generally comprised of graphite and a polymeric resin that have been compression-
molded from powders into a nominally pore-free structure. High resin content promotes
impermeability, but graphite content in the neighborhood of 80 percent by weight is usually
necessary in order to achieve acceptable electronic conductivity. Their thickness requirements
must take into account the depth of fuel and air reactant channels as well as structural
soundness and avoidance of reactant permeability. Nevertheless, thicknesses down to about
1 mm can be implemented in small stacks.
43.5.5 Seals
Sealing means must be provided at the edges of the cells to prevent reactants from escaping
to the atmosphere from porous elements of the electrodes. Also, since reactant manifolding
is usually internal to the stack in small fuel cells (for the sake of compactness), sealing
around manifold holes is required to prevent the mixing of reactants between manifolds and
electrodes; the considerations are the same for internal tie-bolt holes.
The sealing methodology generally employed involves a polymeric frame element (gas-
ket) on each side of the electrolyte-membrane, separating the respective, truncated electrodes
from the outside. These elements contain appropriate holes to allow for reactant manifolding
and tie-rods in these regions.
43.14 CHAPTER FORTY-THREE
43.6 HARDWARE AND PERFORMANCE
The general requirements for small fuel cells include simplicity and compactness, especially
for hydrogen-based systems. Accordingly, small fuel cells typically use reactant air, ambient
pressure and temperature with no external humidification, and are cooled via ambient air.
Operating current densities are, therefore, usually modest; e.g. in the range of 100 to 250
mA/cm
2
. Since fuel storage capacity is often a limiting factor, the design point tends to
provide relatively high cell voltage, generally higher than 0.7 V and sometimes as high as
0.8 V, depending on system requirements.
43.6.1 Electrical Characteristics
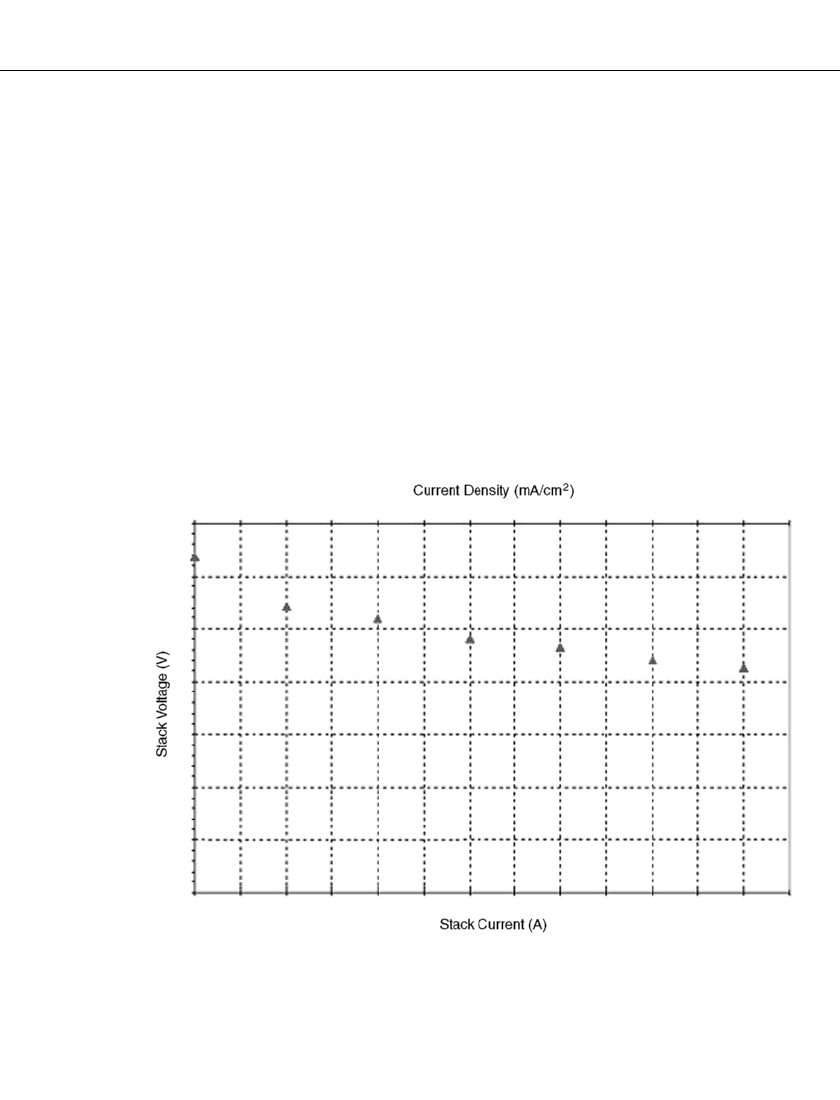
A representative voltage-current curve for a fuel cell stack in a nominal 50-Watt system is
shown in Fig. 43.4. This stack has an active area of 16.5 cm
2
per cell with 32 cells and a
cell pitch of six per centimeter (i.e. a stacking density of six cells per centimeter). The stack
uses hydrogen as a fuel (dead-ended) the stoichiometric ratio for the air is 2.0 to 2.5.
0 15 29 44 58 73 88 102 117 132 140 161 176 190
35
30
25
20
15
10
5
0
0.00 0.25 0.50 0.75 1.00 1.25 1.50 1.75 2.00 2.25 2.50 2.75 3.00 3.25
FIGURE 43.4 Voltage vs. current density for 32-cell stack.
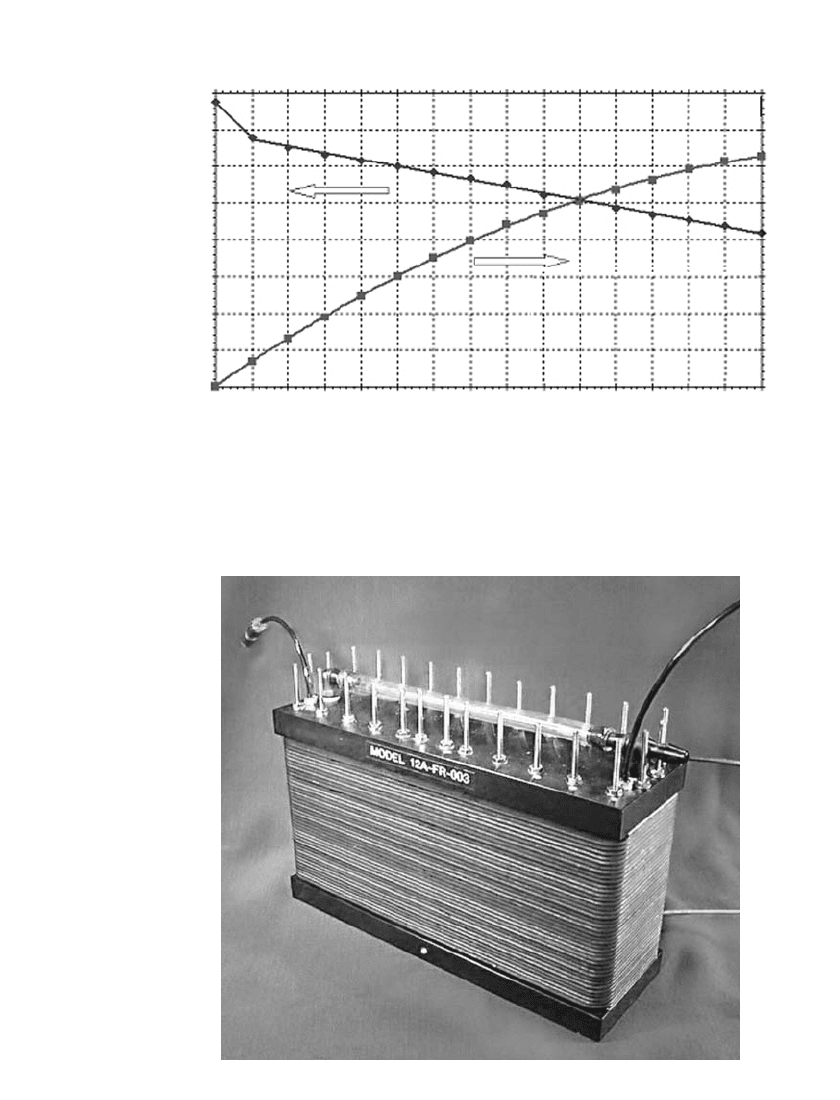
Figure 43.5 illustrates a typical performance curve for a stack in a nominal 250-Watt
system. This stack, shown in Fig. 43.6, has an active area of 77 cm
2
per cell with 40 cells
and a cell pitch of more than four per centimeter. Hydrogen is the fuel and the air is at
atmospheric pressure.
SMALL FUEL CELLS 43.15
0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30
1.000
0.875
0.750
0.625
0.500
0.375
0.250
0.125
0.000
0 26 51 77 103 129 154 180 206 231 257 283 308 334 360 386
20
18
15
13
10
8
6
3
0
Current (A)
Current Density (mA/cm_)
Cell Power (W)
Cell Voltage (V)
FIGURE 43.5 Performance of 40-cell fuel cell stack.
FIGURE 43.6 40-cell fuel cell stack. Dimensions (cm): 15.5(H), 8.6(W),
23.2(L). Weight: 4.5 kg.
43.16 CHAPTER FORTY-THREE
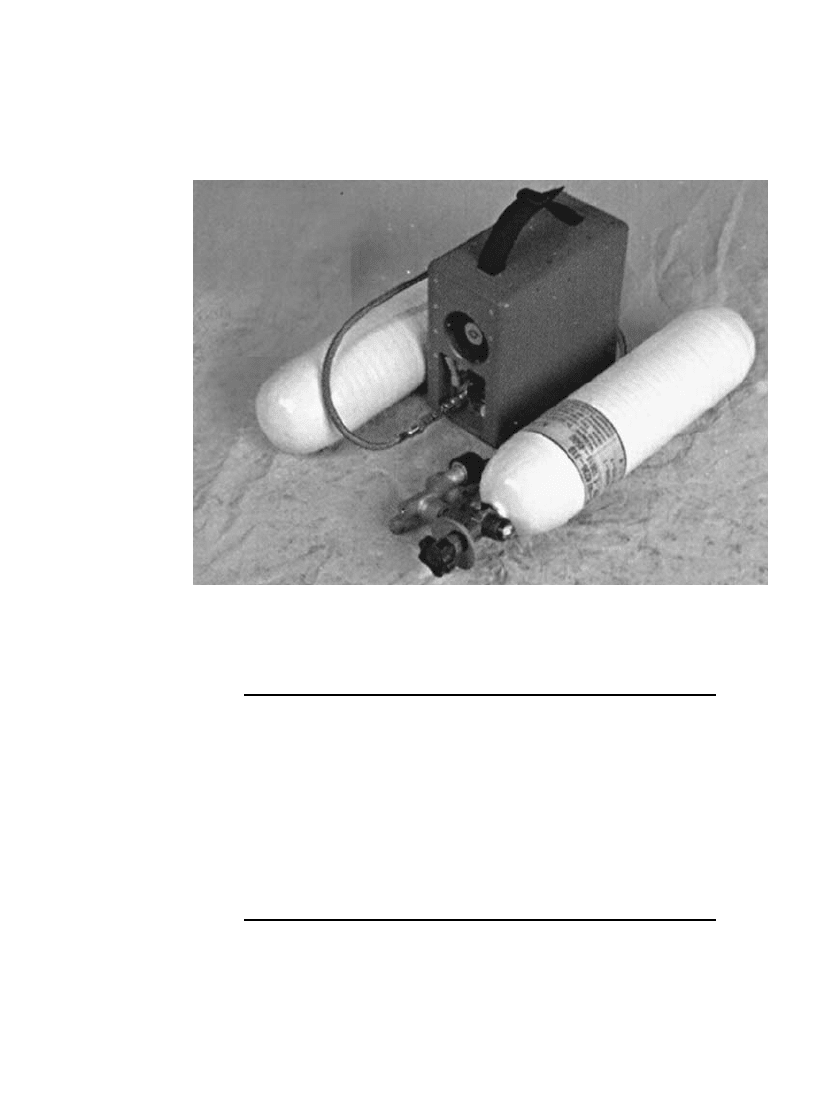
43.6.2 50-Watt Fuel Cell, Compressed Hydrogen Cylinder
Figure 43.7 is an illustration of a lightweight 50-Watt system developed for military field
use incorporating two compressed hydrogen cylinders for the fuel source. The characteristics
of this fuel cell system are summarized in Table 43.3.
FIGURE 43.7 50-Watt fuel cell system with compressed hydrogen gas. (Courtesy of
Ball Aerospace and Technologies Corp.)
TABLE 43.3 Characteristics of 50-Watt Fuel Cell
Size (cm)—fuel cell only 10.9(W), 19.6(H), 20.3(L)
Weight (kg)—fuel cell only 2.95
Weight (kg)—fuel source
(1) High pressure H
2
tanks: 2.3 kg / kWh
(2) Chemical hydride H
2
source: 2.5 kg / kWh
Total system weight (kg) with
high pressure H
2
tanks (for 1 kWh
of operation)
5.22
Power (Watts) at 12 V 50
Peak power (Watts), at
⬎10 V 65
Source: Ball Aerospace and Technologies Corp.
SMALL FUEL CELLS 43.17
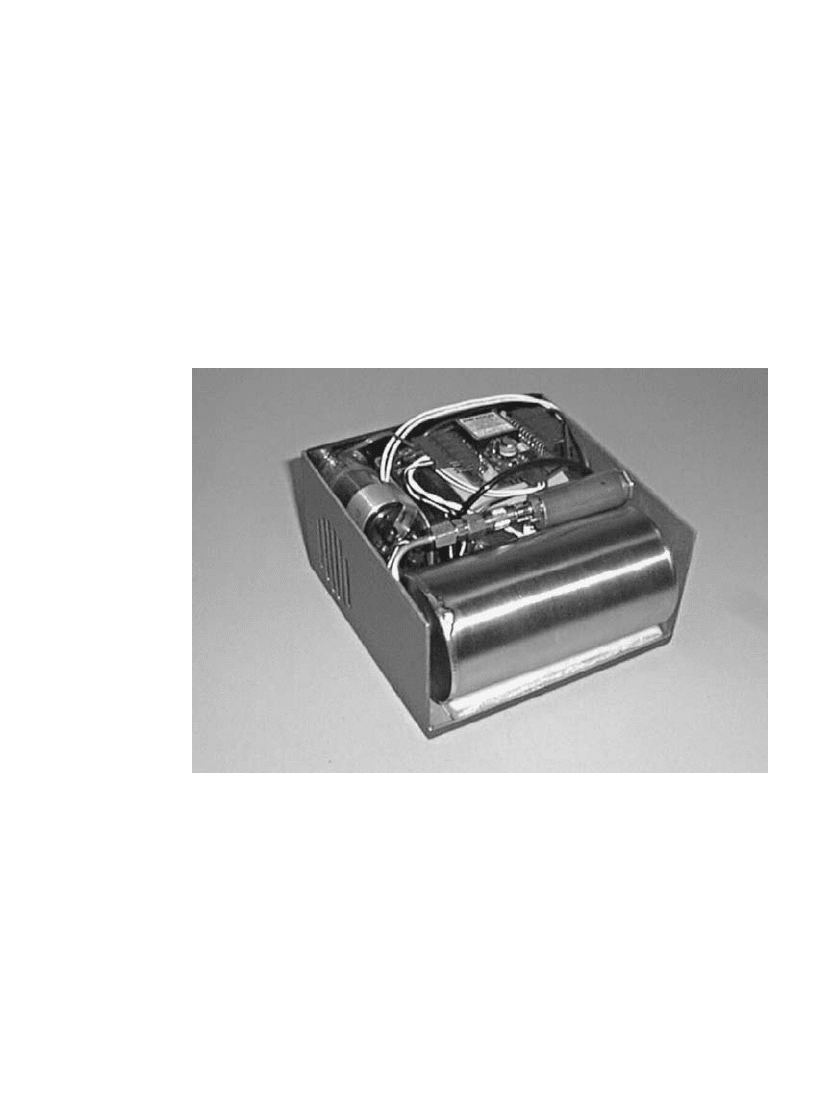
43.6.3 20-Watt Fuel Cell, Metal Hydride Canister
An example of a small fuel cell system fueled via a metal hydride canister is illustrated in
Fig. 43.8. This unit was assembled to fit within the dimensions of military Battery BA-5590
(see Sec. 6.4.2). The fuel cell is rated at approximately 20 Watts continuous and 40 Watts
peak. The canister delivers hydrogen for about 110 Watt-hours of operation at a specific
energy of about 160 Wh/kg. The specific energy of the overall system is about 75 Wh/kg.
On a volume basis, the energy density of the fuel cell system is 110 Wh /L and the replace-
ment canisters deliver about 420 Wh/L.
Although the weight of the fuel cell system is high compared to Battery BA-5590, which
has a specific energy of 160 Wh/kg, the fuel cell could be advantageous when used over
an extended period. This is illustrated in Fig. 42.3. The specific energy of the replacement
canisters is close to that of the battery and its cost, when commercialized, should be signif-
icantly lower than that of the battery. Improved devices for hydrogen storage could make
the fuel cell system more attractive.
FIGURE 43.8 Metal hydride fueled system using BA-5590 type case. (The figure is
of a system design having larger dimensions than the BA-5590.)
43.6.4 Commercial Fuel Cells
Manufacturing and testing of prototype small PEM forced-air fuel cells for commercial
application, mainly using hydrogen as a fuel, began in the mid-1990s. Some examples of
current developments include the following.
50-Watt to 500-Watt Fuel Cell Systems. Fuel cell systems with higher power ratings, up
to 500 Watts, are illustrated in Figs. 43.9 and 43.10. These units have either AC or DC
outputs and are manufactured in both portable and rack-mounted configurations. The weight
of the 250-Watt unit is 10 kg. The 500-Watt AC unit (120 VAC) is specifically suited for
back-up service in the home and operates on hydrogen fuel stored in metal hydride canisters.
The 50-Watt and 100-Watt portable fuel cell systems, illustrated in Fig. 43.11, are de-
signed for military field use. The figure shows the units operating and recharging batteries
of military radios in the field.

43.18 CHAPTER FORTY-THREE
FIGURE 43.9 250-Watt fuel cell power system. Dimensions (cm):
24.7(H), 15.8(W), 40.7(L). Weight: 10.2 kg. (Courtesy H-Power Corp.)
FIGURE 43.10 500-Watt fuel cell power system. Dimensions (cm):
29.2(H), 20.3(W), 40.6(L). Weight: 17.3 kg.

SMALL FUEL CELLS 43.19
FIGURE 43.11 50-Watt and 100-Watt systems charging batteries of military field radios.
Remote or Unattended Application. Another fuel cell application for remote telecommu-
nications is the use of fuel cells to back-up the solar /battery array at weather stations and
microwave-repeater stations. Fuel cells in the 20 to 100 Watt range are used with hydrogen
supplied in the form of compressed gas cylinders.
An example of an unattended application is illustrated in Fig. 43.12, a power source for
variable-message highway signs. This system consists of a 50 Watt hydrogen-air fuel cell
stack and its auxiliary components. A second system is provided for redundancy. The two
systems operate together under normal circumstances, sharing the load, but one unit could
provide full power in the case of failure of the other. These systems are designed to start
when the output of the main solar-battery power system is inadequate to maintain the bat-
teries at an acceptable state of charge. Thus, when solar unavailability is sustained long
enough to allow the voltage of the batteries of the hybrid system to drop below a cutoff
point, the fuel cell takes over and supplies power to the load and charges the batteries to the
extent possible.
The hydrogen fuel is supplied in the form of four small aluminum industrial cylinders
which are placed in a compartment positioned to replace a portion of the battery bank. The
fuel supply could sustain operation without any solar power for 12 days of continuous
operation. In actual practice, it tends to last for about six weeks during the winter, when
solar availability is most diminished. It is usually not needed during other seasons of the
year.
Prognosis. The introduction of small subkiloWatt fuel cells into commercial and industrial
applications has demonstrated the operational viability of the PEM technology and the po-
tential advantage that could be gained in energy density as a result of the use of hydrogen
and other high energy-rich fuels. In some cases, e.g., replacement of lead-acid batteries used
for wheelchair propulsion, prototype fuel cell power sources have shown clear superiority in
operating time for a given weight and volume. The actual depth of penetration into the
market traditionally served by batteries will be strongly influenced by a number of factors,
including cost reduction, fuel logistics, demonstration of reliability, and identification of
applications where fuel cells provide geater performance at acceptable costs.
