Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

PORTABLE FUEL CELLS—INTRODUCTION 42.15
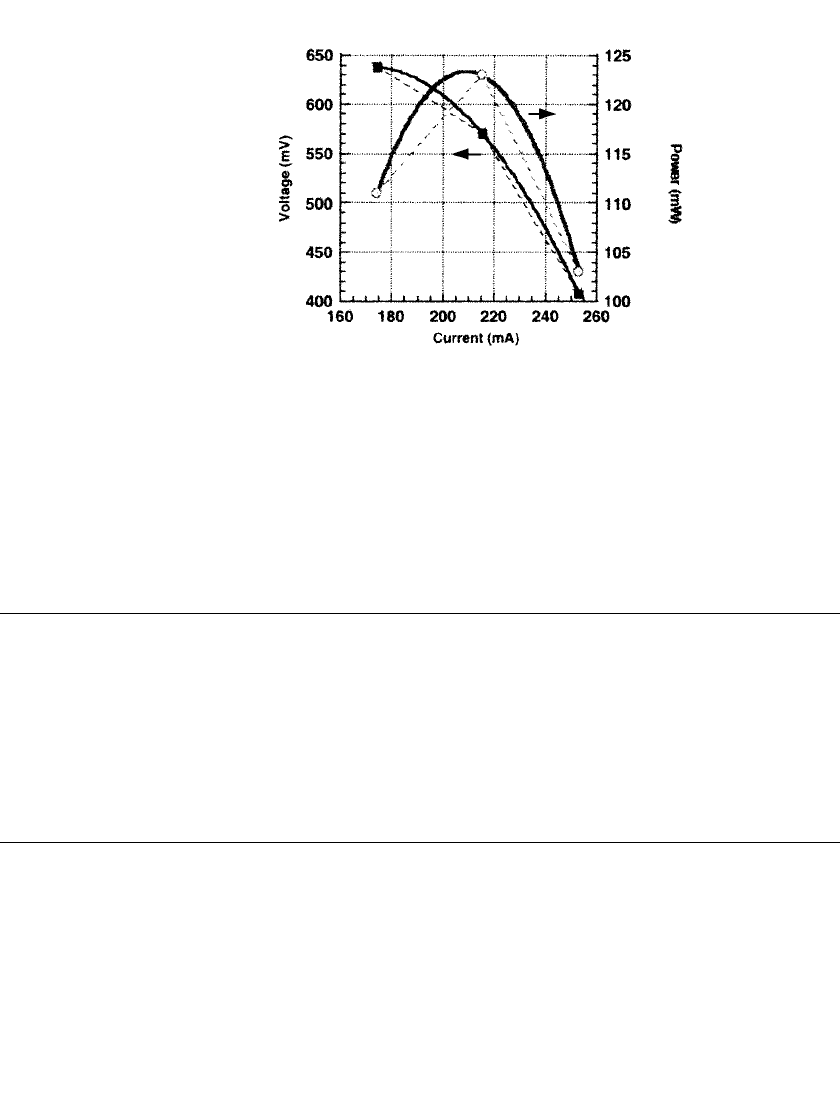
FIGURE 42.6 Polarization curve of the thin film fuel cell
with Pt electrodes (from Ref 4).
These various miniature fuel cells illustrate some of the work that is ongoing, but which
is still in the very early development stages. Although initial prototypes have been built,
none has yet been definitely demonstrated as a viable, compact device that would be com-
petitive in performance, cost and convenience with a battery. The objective remains a chal-
lenging, but potentially fruitful goal.
REFERENCES
1. Christopher F. Dyer, Replacing the Battery Portable Electronics, Scientific American, July 1999, p.
88.
2. Manhattan Scientifics, New York, N.Y.
3. Sandia National Laboratories.
4. Joseph Bostaph et al., ‘‘Thin Film Fuel Cells for Low Power, Portable Applications,’’ Proc. of the
39th Power Sources Conf., p. 152, June 2000.
BIBLIOGRAPHY
See Appendix F.
43.1
TABLE 43.1 Small Fuel Cell Applications
Application Power range Status Reference
Backup power supply 250 W, 500 W Limited production 9, 10, 11
Military field battery charger 50 W, 100 W Limited production 2, 12
General purpose portable power supply 10–100 W Limited production 1, 2, 8, 10
Briefcase power supply 35 W Prototype 13
Professional video camera power supply 35 W Prototype 13
Backup for solar photovoltaic power in telecommunications 20–100 W Prototype 9, 10
Backup for solar photovoltaic traffic sign 50–100 W Commercial 8, 9
CHAPTER 43
SMALL FUEL CELLS (LESS THAN
1000 WATTS)
Arthur Kaufman and H. Frank Gibbard
43.1 GENERAL
Small fuel cells that provide power at ratings below 1000 watts, are being introduced into
and groomed for application areas such as portable power sources, mobile power sources,
remote or unattended power sources, and propulsion power for small, off-the-road vehicles.
These existing and candidate applications may include power systems that are fuel-cell-only,
fuel-cell/ battery hybrids, and fuel-cell/solar/battery hybrids, depending on the nature of the
system’s requirements. Representative examples of such applications are illustrated in Table
43.1.
The interest in small fuel cells stems from their potential to replace engine-generators
with more efficient and environmentally friendly conversion systems and, as replacements
or supplements to batteries, because of their potentially higher specific energy.
The energy-storage and power-generating elements of a fuel cell system are separate
entities—the fuel storage and the fuel cell stack plus its auxiliaries, respectively. In a battery,
on the other hand, the energy-storage and power-generating elements are the same. Hence,
the fuel cell system can be designed to relate optimally to its operating mode—the fuel cell
stack to satisfy the power requirements, and the fuel storage to satisfy the energy require-

43.2 CHAPTER FORTY-THREE
ments. This can be particularly advantageous in applications where the energy requirement
is great and the power requirement is minimal, that is, in applications of long duration (see
Sec. 43.4.1). In such applications the fuel cell stack, with its auxiliaries, becomes relatively
insignificant within the overall system; and the system’s energy density and specific energy
approach that of the fuel storage subsystem alone (see Fig. 42.3). The mission duration
beyond which fuel cells would tend to be favored over batteries, by providing a smaller and/
or lighter system, depends on the specific application requirements.
Certain applications are well suited to a fuel-cell/ battery hybrid system by nature of their
duty-cycle. Those that have high peak-to-average load ratios and relatively short-duration
peaks are generally attractive candidates. Such a system allows the fuel cell to be rated near
its average power while a relatively small battery provides excess power on demand and is
recharged by the fuel cell during normal-load operation. Hybrid systems thus exploit the
strengths of both batteries and fuel cells—the wide dynamic power range of the former, and
the high energy content per unit weight or volume of the latter.
Solar/ battery power systems can also be combined advantageously with fuel cells in
various applications. Use of the fuel cell can often allow solar power to be exploited without
the need for excessive battery size and weight associated with prolonged or unpredictable
lack of availability of solar energy.
Small fuel cells are also expected to become an alternative to small engine-generator sets
in some applications. In this sector, the fuel cell is unlikely to offer any advantage from an
energy density or initial-cost point of view. However, in applications where system life,
reliability, efficiency (fuel consumption), noise, and emissions are important, the fuel cell
system could become competitive.
43.2 APPLICABLE FUEL CELL TECHNOLOGIES
Various types of fuel cell systems are either in use or under development, and these are
generally distinguished on the basis of their electrolyte. These systems exhibit viable oper-
ation in different temperature regimes:
1. Phosphoric acid fuel cells (PAFC)—use highly concentrated aqueous phosphoric acid
electrolyte and generally operate in the 160 to 200
⬚C range
2. Molten carbonate fuel cells (MCFC)—use mixed alkali-carbonate molten salt electrolyte,
operate typically at about 600
⬚C
3. Solid oxide fuel cells (SOFC )—use solid oxygen-ion-conducting metal oxide electrolyte
at about 1000
⬚C, although some development activity is focusing on somewhat lower
temperatures
4. Alkaline fuel cells (AFC )—typically use liquid solutions of potassium hydroxide electro-
lyte at temperatures ranging from ambient to about 80
⬚C
5. Proton-exchange membrane fuel cells (PEMFC)—use solid-polymer proton-conducting
membrane electrolyte at temperatures generally ranging from ambient to 90
⬚C. Today’s
technology primarily uses the trifluoromethanesulfonic-acid-based electrolyte membrane,
such as DuPont’s Nafion
䉸.
Small fuel cells can be exploited most effectively if they can stand by and operate at
ambient temperatures (and can therefore start rapidly), can operate on ambient air, can re-
spond rapidly to load changes, have a non-migrating (solid) electrolyte, and have a reason-
ably high power density and specific-power. The fuel cell type that best suits these criteria
is clearly the PEMFC, despite a drawback related to the fact that liquid water embodied in
the solid polymer tends to freeze, and thereby impedes proton conduction, when its temper-
ature drops below the freezing point. The PEMFC can stand by under freezing conditions,
SMALL FUEL CELLS 43.3
however, and can generally operate under these conditions as well, taking advantage of self-
generated heat; external means, such as power from a battery or an electric grid, are some-
times required to execute a sub-freezing start-up or to prevent freezing.
Proton-exchange membrane fuel cells are indeed the type that has received the predom-
inant share of development and implementation in the small fuel cell arena.
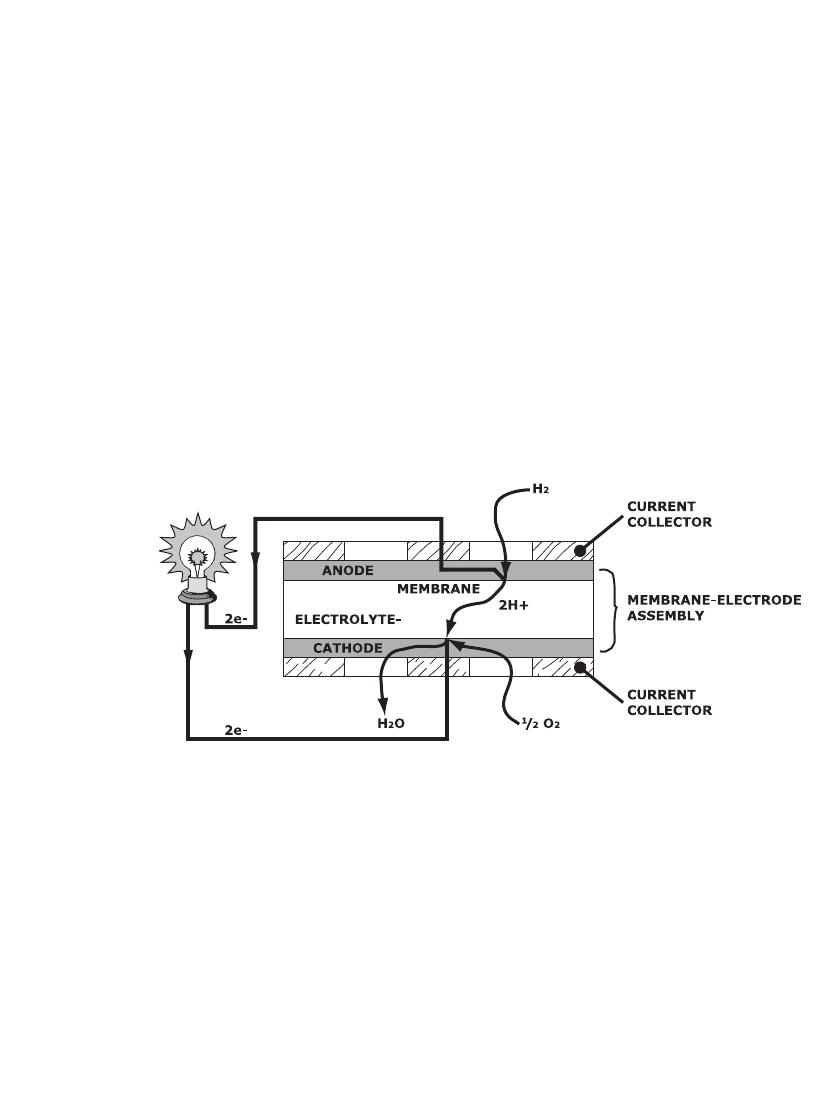
As the electrolyte, these PEM electrochemical cells use ion-exchange membranes in the
acidic form; i.e., with protons as the exchangeable ions. The electrolyte membrane supports
cell current generation via proton transport from the anode at one membrane surface to the
cathode at the opposite surface, as illustrated in Fig. 43.1. Hydrogen ions are produced at
the anode-electrolyte interface from hydrogen gas that diffuses through the anode structure.
Electrons generated in this process migrate through the electronically conductive phase of
the anode to an adjacent current collector. Hydrogen ions, reaching the cathode-electrolyte
interface, react with electrons returning from the external load and with oxygen gas that
diffuses through the cathode structure, producing water.
FIGURE 43.1 Schematic representation of an individual proton-exchange membrane fuel cell.
Since cell voltages generated in this process are typically in the 0.6 to 0.8 V range,
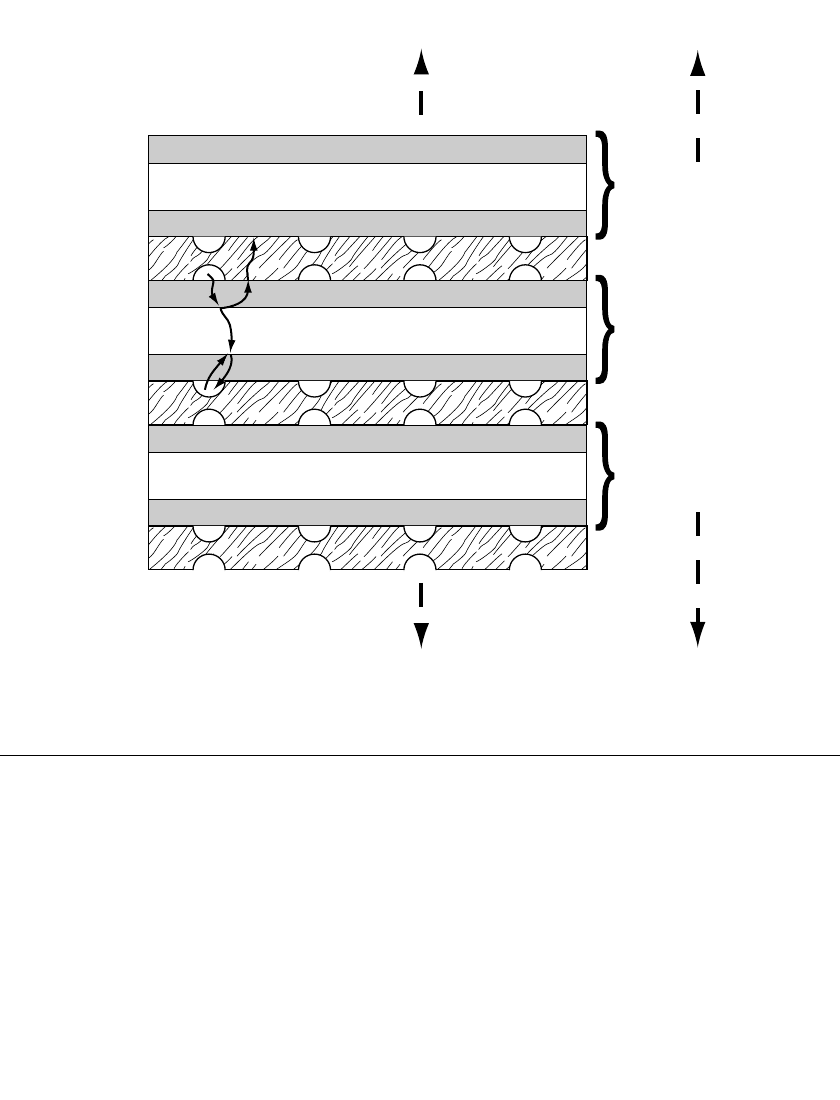
multiple cells are generally stacked in a series-bipolar array to obtain practical voltage levels;
this is illustrated in Fig. 43.2. The electrochemically active cell elements, shown schemati-
cally in Figs. 43.1 and 43.2, are commonly referred to as membrane-electrode assemblies,
or MEAs. The MEAs are interleaved with bipolar plates that have multiple functions: con-
duction of electronic current from cell to cell, dispersion of the active hydrogen and oxygen
gases through flow channels on the two opposing surfaces, prevention of mixing of these
gases, and in many cases providing a means for removing the heat that is generated during
the operation of the fuel cell. The voltage or current of the stack shown in Fig. 43.2 can be
raised by increasing the number of cells or their active area, respectively.
43.4 CHAPTER FORTY-THREE
ANODE
CATHODE
BIPOLAR PLATE
ELECTROLYTE-MEMBRANE
MEMBRANE-ELECTRODE
ASSEMBLY
ANODE
CATHODE
BIPOLAR PLATE
ELECTROLYTE-MEMBRANE
MEMBRANE-ELECTRODE
ASSEMBLY
ANODE
CATHODE
BIPOLAR PLATE
ELECTROLYTE-MEMBRANE
MEMBRANE-ELECTRODE
ASSEMBLY
2H+
H
2
H2O
1
/2 O2
2e-
2e-
FIGURE 43.2 Schematic representation of proton-exchange membrane fuel cell stack.
43.3 SYSTEM REQUIREMENTS
The requirements for small fuel cell systems vary from application to application. The more
important requirements for a given rated-power output include size, weight, cost, energy
storage, voltage output, ambient-temperature operating range, and start-up characteristics.
The approach followed for small fuel cell system design stresses simplicity of operation and
maintenance as well as compactness.
43.3.1 Fuel Supply
To date small fuel cell systems have, for the most part, utilized some form of hydrogen fuel
storage. For simplicity, fuel conservation, and safety, hydrogen is provided to the anodes at
a modest pressure (typically at a gauge pressure from 10 to 50 kPa) and dead-ended, allowing
the anodes to consume the amount of hydrogen required to sustain the electrochemical
reaction at any given rate. A momentary ‘‘purge’’ of the exit port is implemented to allow
accumulated impurities and water to be discharged from the anode compartment at selected
time intervals. (See Sec. 43.4 on Fuel Processing and Storage.)

SMALL FUEL CELLS 43.5
43.3.2 Air Supply
Small fuel cell stacks can operate on either diffused or forced reactant air. Diffused-air stacks
are generally limited in their applicability because of air supply rate issues and their impact
on geometry; the requisite openness of the air compartments in such devices also tends to
render them vulnerable to atmospheric conditions. Hence, diffused-air (static) fuel cells are
usually practical only in certain particularly low-power applications, up to perhaps 25 Watts.
1
Forced-air fuel cell stacks are practical over the entire power range of small fuel cells.
The reactant air is delivered to the fuel cell stack at whatever pressure is necessary to
overcome the pressure drop through the stack and associated plumbing; this is typically in
the range of 1 to 20 kPa (a small fraction of atmospheric pressure), depending on stack
design characteristics. The air-moving devices are usually small air pumps, such as rotary-
vane or diaphragm types. The stack’s utilization rate of the oxygen in the reactant air will
vary in accordance with operating conditions, but a typical rate is about 50 percent. The exit
air is generally discharged to the atmosphere.
43.3.3 Water Management
A key design issue for small PEM fuel cells (and other PEM fuel cells as well) is the
management of water with respect to the fuel cell stack. The trifluoromethanesulfonic-acid-
based electrolyte membrane requires a certain level of water content in order to conduct
protons efficiently, with water molecules effectively serving as carriers for the protons in
their migration across the membrane. Accordingly, the system design must provide for a
reasonably high relative humidity in the reactant passages that are in communication with
the membrane.
The moisture requirements for the reactant streams place significant limitations on op-
erating regimes. Ambient (non-humidified) reactant air is highly preferred in small fuel cells
in order to achieve the simplicity and compactness that are generally sought in small power
sources. The use of ambient air, however, requires design measures to prevent the membrane
from drying out. The flow rate of air must be limited to reduce the drying effect, and the
cell design must be tailored to take advantage of the product water. The threat of drying
clearly becomes far more acute as the ambient temperature increases and as the current
density of the stack is increased, thereby increasing the stack’s temperature in relation to
ambient.
The water management burden is not limited to preventing membrane dryout. The need
to operate at relatively high oxygen utilization rates (relatively low air flow rates) increases
the tendency to form water droplets within the cell from the formation of product water at
the cathode. This can lead to accumulation of water on the surface of or within the electrode
substrate or in the air distribution channels of the cathode flow-field. Such events could result
in serious performance losses from the ensuing restriction of air access to impacted regions
of the electrocatalyst. Consequently, the cell design approach must also serve to prevent such
accumulation of water droplets.
43.3.4 Thermal Management
The thermal management requirements for small PEM fuel cells (and other PEM fuel cells
as well) are intimately associated with water management. As discussed above, the level of
hydration of the electrolyte membrane must be maintained in order to prevent dryout and
thus loss of proton conduction in the membrane. The temperatures within the stack must
accordingly be restricted. Cooling of the individual cells of the stack is carried out to assure
that temperatures are moderate and rather uniform throughout the stack.
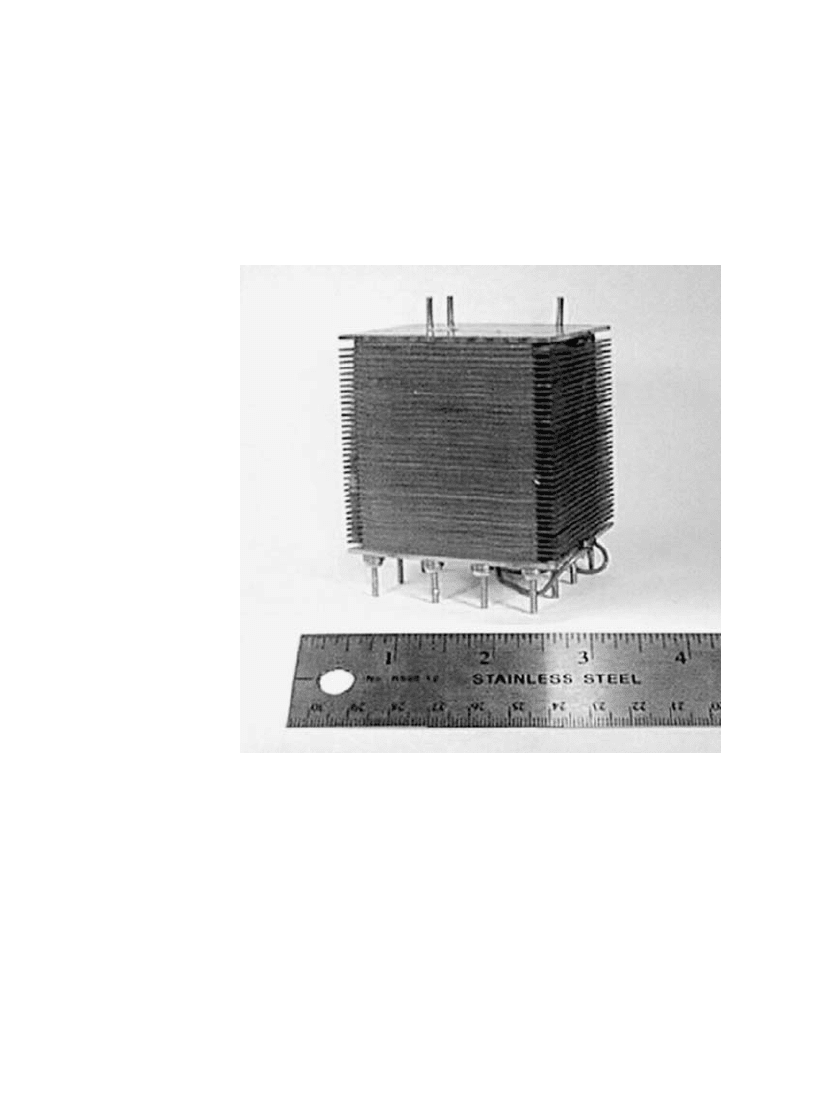
43.6 CHAPTER FORTY-THREE
Stack cooling in larger PEM fuel cells is generally carried out using a liquid coolant,
often water. This methodology is very effective because of the relatively high thermal con-
ductivity and high volumetric heat capacity of liquids. However, as discussed above, small
fuel applications predominantly require simple and compact systems, and a circulating liquid
loop with external heat rejection is not compatible with these requirements. The preferred
approach for small fuel cell stack cooling is based on the use of ambient air delivered across
the external surface of each cell via a fan. The effectiveness of this method is typically
enhanced by expanding the cells’ external surface-area (as by providing finned extensions of
the bipolar plates; see Fig. 43.3 and Sec. 43.5 on Stack Design).
FIGURE 43.3 Air-cooled fuel cell stack.
43.3.5 Operational Control
Small fuel cell systems, especially those operating on hydrogen fuel, are characteristically
simpler than larger systems, as discussed earlier. Nevertheless, certain control elements must
be imposed to foster stable operation. Representative methodology for these is as follows:
1. The reactant-air flow rate must be controlled as a function of load to assure that neither
excessive water build-up (low flow rate) nor cell dry-out (high flow rate) is encountered.
This requires measurement of stack current and corresponding speed adjustment in the
air-moving device.
2. Stack temperature must be controlled to prevent operation in a dry-out condition (too
hot). This requires that the stack cooling fan (or fans) be either turned on or ramped to
a higher speed in response to a high-temperature signal from the stack’s temperature
sensor.

SMALL FUEL CELLS 43.7
3. Since hydrogen-fueled systems run dead-ended, a timer (or coulometer) is utilized to
impose a brief open-close cycle to a solenoid valve in the exit line to purge the anode
compartment of accumulated impurities and water on a regular basis.
4. Other control means are provided on an application-specific basis as appropriate.
43.4 FUEL PROCESSING AND STORAGE TECHNOLOGIES
The advantages of small fuel cells often depend on the method used for the supply of fuel.
Currently small fuel cell systems predominantly use some form of hydrogen storage. It is
anticipated, however, that in the future, small systems will also utilize processed forms of
common fuels (e.g., methanol, natural gas, and liquefied petroleum gas) as well as unpro-
cessed methanol.
43.4.1 Hydrogen Storage
The hydrogen storage means that are presently being implemented in small fuel cell systems
include compressed hydrogen gas canisters and metal hydride canisters. The challenge is to
develop lightweight and small ‘‘containers’’ with high specific energy to make the overall
system more attractive.
Compressed Hydrogen Gas Storage. The simplest form of fuel storage and utilization for
fuel cell systems is compressed hydrogen. Such storage is impractical for larger fuel cells,
except in cases where operation is limited to brief periods in a back-up mode, because of
the higher cost and inferior logistics of transport for hydrogen in comparison with common
fuels. With respect to small fuel cells, however, compressed hydrogen is sometimes an ac-
ceptable storage option, especially at very low power levels and in back-up service.
Compressed hydrogen is generally selected for missions in which compactness is not
particularly critical and in which storage pressures of 10 to 30 MPa are not objectionable.
Since the weight of the active material is negligible, compressed hydrogen storage provisions
can be exploited where light weight is a priority. This would require the use of lightweight
canisters and pressure regulators, and the associated cost factors must be taken into account.
Metal Hydrides. The storage of hydrogen in the form of hydrides of metal-alloy powders
is often an attractive and convenient energy storage mode for small fuel cell systems. This
is attributable to their simplicity of operation and compactness; the benefits are most real-
izable in particularly small systems. The energy densities of these materials can range up to
500 to 1000 Watt-hours (elec.) per liter, substantially higher than that of compressed hydro-
gen. This reflects a hydrogen loading approaching 2 percent by weight, which is character-
istic of the maximum obtainable in alloys (typically AB
2
type, where, for example, A is Zr
or a mixture of Zr and Ti, and B is a mixture of transition metals) that have useful hydrogen
pressures at ambient temperatures. (Magnesium-based alloys have been formulated to obtain
hydrogen loadings on the order of 5 percent by weight; but these require discharge temper-
atures in the neighborhood of 300
⬚C, which necessitates combustion means with a percentage
of the hydrogen being sacrificed to generate heat.)
The equilibrium hydrogen pressure of the hydride at the desired operating temperature
can be appropriately tailored by adjusting the composition of the alloy mix. Pressure is
generally selected in the range of 400 to 2000 kPa, a small fraction of that used in the case
of compressed hydrogen. Nevertheless, the containment for the alloy usually constitutes a
significant fraction of the overall weight of the energy-storage provisions; and depending on

43.8 CHAPTER FORTY-THREE
TABLE 43.2 Representative Chemical Hydride Reactions
and Theoretical Hydrogen Yields and Specific Energy
Reaction
Theoretical
hydrogen yield
Theoretical
specific energy
LiH ⫹ H
2
O → LiOH ⫹ H
2
7.8 percent 2540 Wh /kg
CaH
2
⫹ 2H
2
O → Ca(OH)
2
⫹ 2H
2
5.2 percent 1700 Wh /kg
NaBH
4
⫹ 2H
2
O → NaBO
2
⫹ 4H
2
10.9 percent 3590 Wh /kg
Theoretical, including weight of hydride and water, in H
2
/ air fuel cell based on 1.23
V per cell.
the size and discharge rates required, additional means might be needed for internal hydrogen
manifolding and for internal heat transfer. (The latter relates to the endothermic reaction
associated with the discharge of hydrogen from the metal hydride lattice. For most small
systems, the heat necessary to sustain the reaction is simply supplied through the walls of
the canister from ambient air; but, even here, the size and discharge rate might be high
enough to require internal heat-conduction enhancement to prevent excessive temperature
gradients along with unacceptable diminution of hydrogen pressure.) The AB
2
type metal
hydrides (and AB
5
type, e.g., LaNi
5
as well) are quite dense (powder bulk densities on the
order of 3 g/ cm
3
), and their specific-energies are not particularly high (perhaps 200 to 300
Watt-hours per kilogram). As indicated above, the containment and other design provisions
will significantly reduce the specific energy for the energy-storage system as a whole.
The cost of the active metal hydride materials is usually quite acceptable in the context
of small fuel cell systems ($5 to 25 per kilogram). Clearly, however, as in the case of
compressed hydrogen, provisions must be made for recharging or reserve storage when the
fuel cell is used in continual operation. The charging pressure is quite low, but the associated
exothermic reaction limits the charging rate. Depending on size, canister design character-
istics, and degree of charging completeness, this process typically requires 5 to 30 minutes
using a conventional compressed hydrogen supply. Future methodology for very small sys-
tems (e.g., consumer electronic applications) could employ pressurized water electrolysis for
canister recharging.
Chemical Hydrides. Primary hydride systems of various types are being developed for use
in small fuel cell systems. These are irreversible (throw-away) chemical systems that generate
hydrogen on demand. The active reactant is generally an alkali or alkaline earth metal hydride
(sometimes in complex form). This typically is caused to react with water to form hydrogen
and a metal oxide (or mixed metal oxide). Analogous chemical hydride systems are also
being explored.
The potential advantages of chemical hydride systems include high specific energy, since
the hydrogen yield by weight can be a far higher percentage of the reactant weight in
comparison with reversible metal hydrides. The hydrogen-generating reaction, theoretical
hydrogen yield and the theoretical value for the specific energy of the fuel are shown in
Table 43.2 for representative chemical hydrides. (Note that the mass of water required to
generate the hydrogen is included in the calculations of percent hydrogen and specific energy
for each chemical hydride.) This advantage can be enhanced if product water from the fuel
cell can be recovered for use in the chemical hydride reaction, in which case the system
could be refueled simply via a stored reserve of reactant powder or granules. On the other
hand, energy densities (Wh /L) for these systems are not as attractive because of the relatively
low densities of the reactants.

SMALL FUEL CELLS 43.9
The challenges associated with chemical hydrides include the requirement that the re-
spective reactants be brought into contact so that the rate of reaction just meets the fuel
cell’s hydrogen needs. Also, since the reaction products are disposed of, as opposed to being
regenerated, the cost of the replenishing reactant chemicals must be taken into account in
evaluating the economics of system operation.
Carbon-Based Hydrogen Storage. Hydrogen-storage methodologies based on carbon ma-
terials are currently the subject of considerable technology development and could provide
opportunities for attractive energy-storage systems in the future. Some investigations have
focused on the sorption of hydrogen on activated carbons.
2
This phenomenon is greatly
enhanced by carrying out the sorption (and storage) at cold temperatures (typically below
⫺100⬚C). Under these conditions, the loading of hydrogen can be rather high (substantially
higher in weight percent than that of metal hydrides). Consequently, the specific energies
for such storage systems are potentially attractive, although, because of their relatively low
densities, the energy per unit volume parameters are not especially high. More important for
small fuel cells is the relative impracticality of cryogenic storage at this scale.
In recent years much attention has been devoted to hydrogen sorption on nanoscale carbon
or graphitic structures.
3
Hydrogen loadings at room temperature have been reported ranging
from a few percent by weight to far higher levels. Results appear to be irreproducible and
highly dependent on preparation methodologies. It is too early to predict the ultimate promise
of such approaches, but research is proceeding actively toward optimization of loading as
well as adsorption-desorption characteristics as a function of pressure and temperature. These
activities could play a significant role in determining future energy-storage concepts for small
fuel cells (and perhaps larger fuel cells as well).
43.4.2 Fuel Processing
The application of small fuel cells could clearly be greatly enhanced if compact systems
using conventional fuels are implemented. In most cases, this requires a fuel processor to
convert the fuel into a hydrogen-rich gas that would be delivered to the fuel cell. Much of
the challenge in such an approach relates to attaining a sufficiently compact and low-cost
fuel processor. In the case of methanol in particular, there is also the potential for systems
that use direct fuel feed to the fuel cell anodes.
A variety of common fuels and chemicals can be considered as candidate fuels for small
fuel cells. These include the following.
Ammonia. Ammonia is commonly used in industry and agriculture in the form of a liquid
stored at its own, modest vapor pressure. Liquid ammonia offers high specific energy and
energy density based on a relatively simple thermocatalytic hydrogen-generating dissociation
reaction
2NH 3H ⫹ N
322
Thus, ammonia can yield hydrogen at about 17 percent of its own weight. This corre-
sponds to about 3 kWh (elec.) per kilogram and almost 2 kWh (elec.) per liter, assuming a
practical cell operating voltage of 0.7 V. However, a fraction of the hydrogen formed must
be consumed in generating heat to sustain the endothermic dissociation reaction. LPG is
generally preferred as a fuel over ammonia because of its greater distribution infrastructure,
even higher energy density, and lower cost per unit of energy content, and because of am-
monia’s reputation as a toxic chemical. Nevertheless, ammonia could play a role in selected
small fuel cell applications as a result of its far simpler hydrogen-generating process.
