Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

LITHIUM-ION BATTERIES 35.9
100 150 200 250 300 350 400
Temperature (°C)
-40
0
40
80
120
Heat Flow (W/g)
200 250
0
10
20
pure LiNiO
2
LiNi
0.7
Co
0.2
Ti
0.05
Mg
0.05
O
2
DSC 10°C/min
LiNi
0.8
Co
0.2
O
2
LiCoO
2
LiNiO
2
LiNi
0.8
Co
0.2
O
2
LiCoO
2
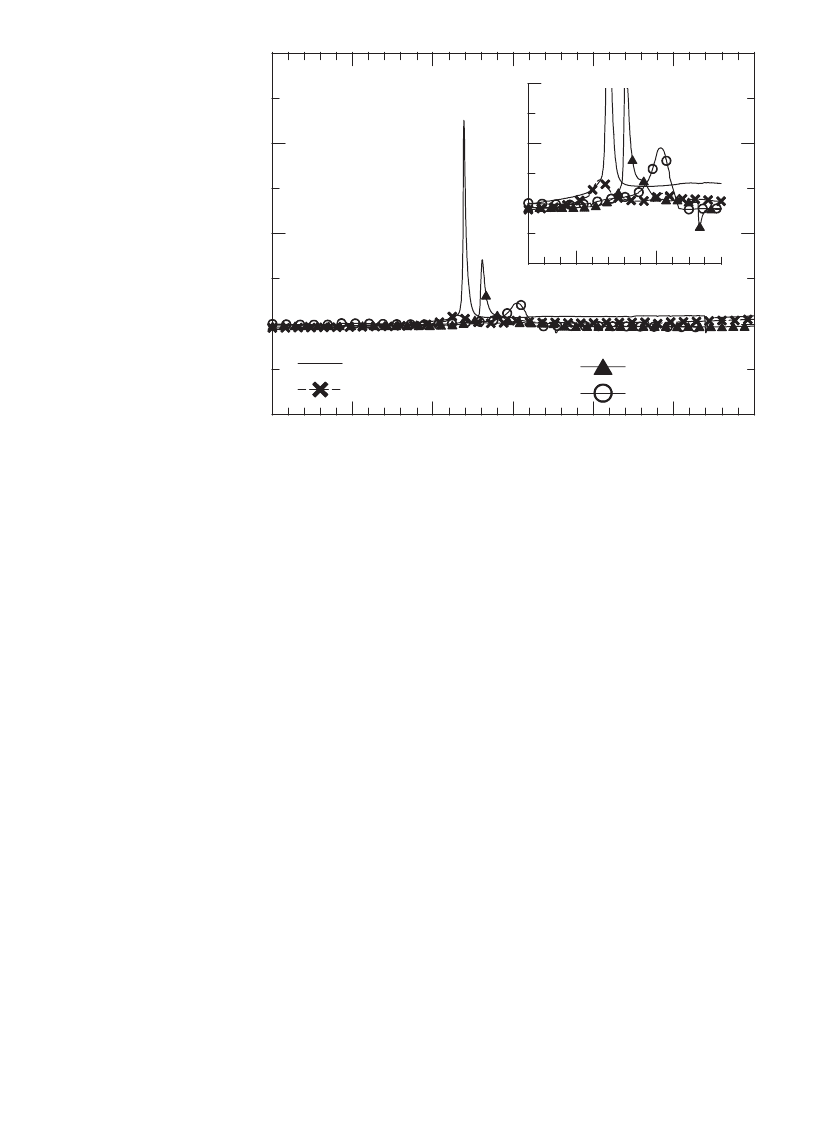
FIGURE 35.7 DSC data showing the onset temperature and energy released from
charged cathode materials upon heating. Electrode float charged to 4.5 V for 40 hours.
(Courtesy of FMC.)
To reduce the energy evolved during positive material decomposition, new materials have
been developed that offer either less evolved energy or higher onset temperatures. Exam-
ples include Al or Ti and Mg doped lithium nickel cobalt oxides. DSC data for
LiNi
0.7
Co
0.2
Ti
0.005
Mg
0.005
O
2
is included in Fig. 35.7. As shown, it evolves much less energy
than even LiCoO
2
, however its reversible capacity is 25 mAh/g less than LiNi
0.8
Co
0.2
O
2
.
Others have developed magnesium oxide coated LiNi
1
⫺
x
Co
x
O
2
type materials that offer elec-
trochemical performance comparable to un-modified materials, but with a higher self-heating
temperature (219
⬚C versus 211⬚C) and a reduced exotherm when heated in a DSC.
16
Electrical Properties of Positive Electrode Materials. The voltage characteristics of
LiMn
2
O
4
, LiCoO
2
, and LiNi
1
⫺
x
Co
x
O
2
, when cycled versus a Li counter electrode are illus-
trated in Fig. 35.8 (Charge) and Fig. 35.9 (Discharge). As indicated, LiMn
2
O
4
offers the
highest voltage (4.0 V) but the lowest capacity (
⬃120 mAh/g), LiNi
1
⫺
x
Co
x
O
2
the lowest
average voltage (
⬃3.75), but the highest capacity (⬃205 mAh/g), LiCoO
2
is intermediate
(3.88 V,
⬃155 mAh/g). Spinel is unique in that it exhibits two distinct voltage plateaus on
charge and discharge, more evident in the differential capacity plot shown in Fig. 35.10.
Also shown in Fig. 35.10 is the differential capacity for LiNi
0.8
Co
0.2
O
2
. As shown,
LiNi
0.8
Co
0.2
O
2
reversibly incorporates lithium over the range of 3.7 V to 4.25 V on charge
(deintercalation), and 3.4 V to 4.25 V on discharge (intercalation). Shown in Fig. 35.11 is
the differential capacity for LiCoO
2
when cycled versus Li. It intercalates the majority of its
capacity at 3.93 V with small peaks at 4.07 V and 4.20 V that correspond to small peaks
observed in the case of the LiNi
0.8
Co
0.2
O
2
material.
A comparison of three different LiNi
1
⫺
x
Co
x
O
2
materials, LiNi
0.7
Co
0.3
O
2
, LiNi
0.8
Co
0.2
O
2
,
and LiNi
0.9
Co
0.1
O
2
, is illustrated in Fig. 35.12, which shows first cycle charge curves, and
Fig. 35.13, which shows first cycle discharge curves. On charge, these LiNi
1
⫺
x
Co
x
O
2
mate-
rials offer similar voltage, except that as the cobalt content is reduced, higher capacity results,
up to 222 mAh/ g. This trend in capacity is also observed on discharge. The synthesis,
properties and electrochemical performance of LiNi
1
⫺
x
Co
x
O
2
(x ⫽ 0, 0.1, 0.2, 0.3) materials,
including charge, discharge, and irreversible capacities, and their thermal stability have been
reported.
13
35.10 CHAPTER THIRTY-FIVE
050100150200
3.2
3.4
3.6
3.8
4.0
4.2
4.4
Li
1+x
Mn
2
O
4
(118mAh/g)
LiCoO
2
(158mAh/g)
LiNi
0.8
Co
0.2
O
2
(205mAh/g)
Voltage (V vs. Li)
Specific Capacity (mAh/g)
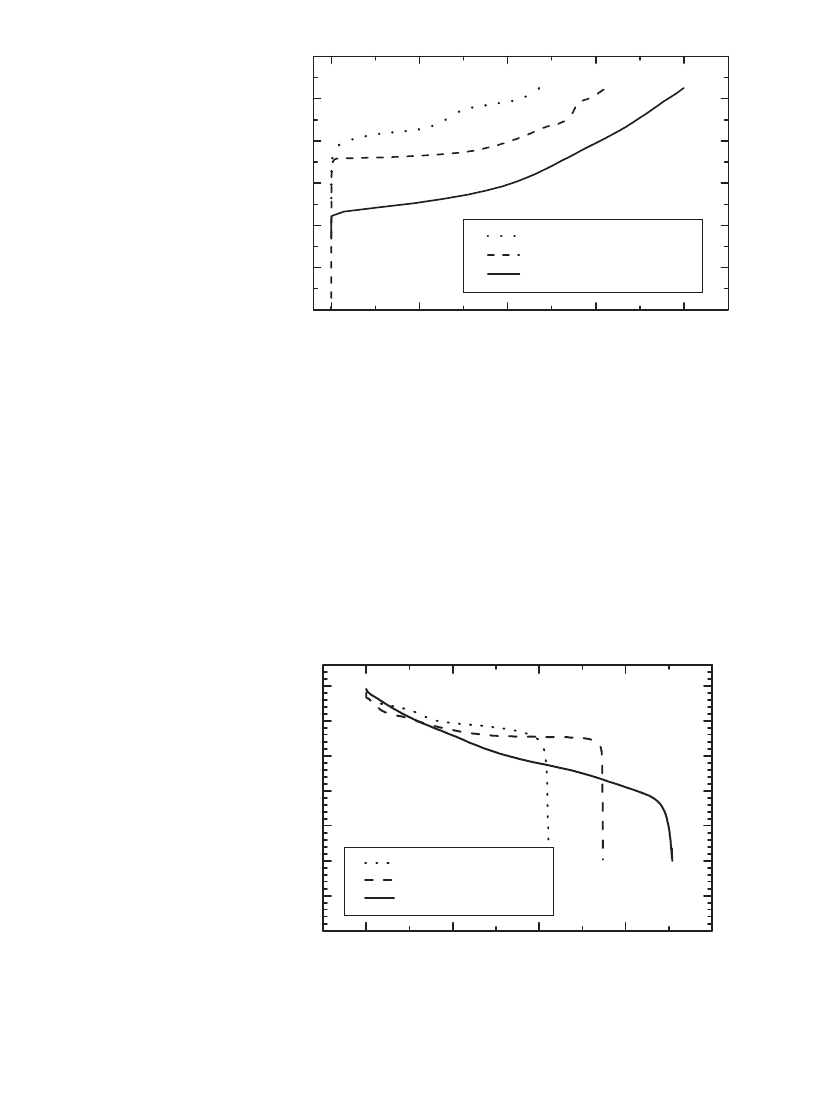
FIGURE 35.8 Voltage and specific capacity of common positive electrode
materials on the first charge at 25⬚C. All materials were charged at approx-
imately C / 20, the LiCoO
2
at 7.2 mA/ g, the LiNi
1
⫺
x
Co
x
O
2
materials at 0.16
mA/cm
2
, and the LiMn
2
O
4
at 0.09 mA / cm
2
.(Courtesy of FMC and Yardney
Technical Products, Inc.)
050100150200
2.50
2.75
3.00
3.25
3.50
3.75
4.00
4.25
LiMn
2
O
4
(106 mAh/g)
LiCoO
2
(136 mAh/g)
LiNi
0.8
Co
0.2
O
2
(190 mAh/g)
Voltage (V vs. Li)
Specific Capacity (mAh/g)
FIGURE 35.9 Voltage and specific capacity of common positive electrode
materials on the first discharge at the C / 20 rate. The LiNi
1
⫺
x
Co
x
O
2
materials
were discharged at 0.16 mA / cm
2
, the LiCoO
2
at 7.5 mA / g, and the LiMn
2
O
4
at 0.09 mA/ cm
2
.(Courtesy of FMC and Yardney Technical Products, Inc.)
LITHIUM-ION BATTERIES 35.11
3.00 3.25 3.50 3.75 4.00 4.25
-30
-20
-10
0
10
20
30
Discharge
Charge
Differential Capacity (dQ/dV)
Voltage (V vs. Li)
LiMn
2
O
4
LiNi
0.9
Co
0.1
O
2
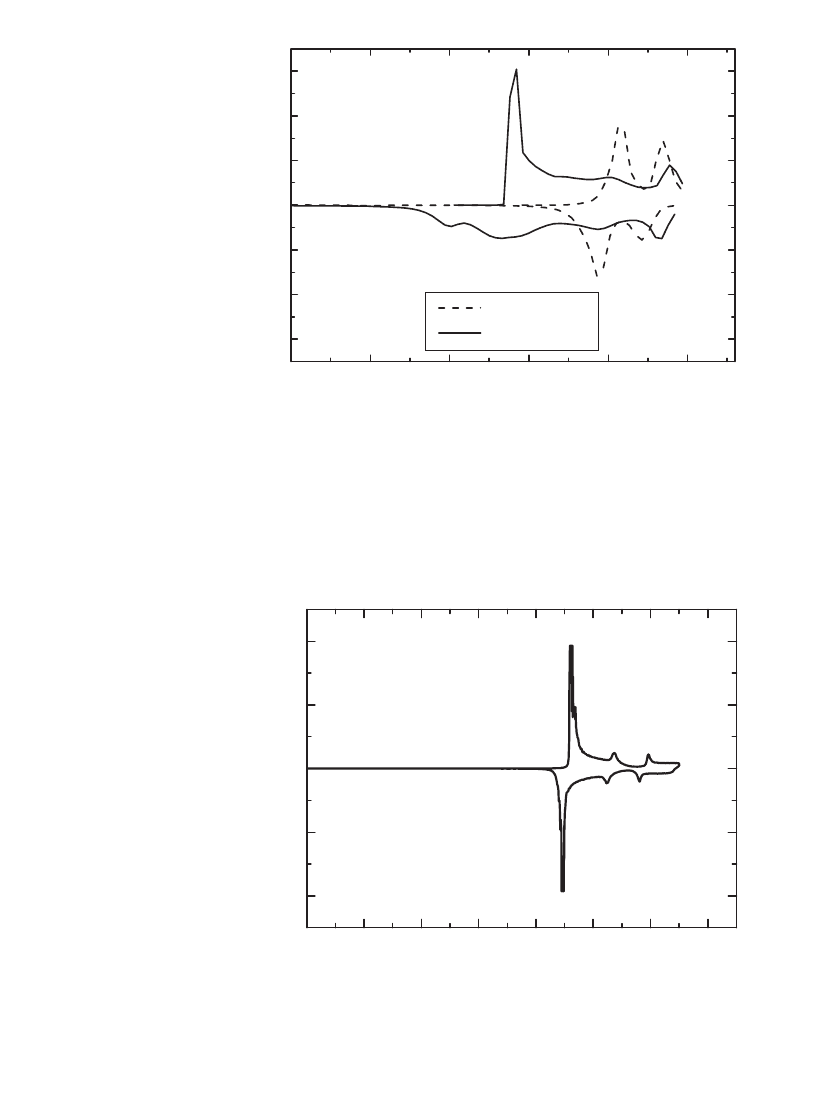
FIGURE 35.10 Differential capacity on the first cycle for LiMn
2
O
4
and
LiNi
0.9
Co
0.1
O
2
when cycled versus Li at the C / 15 rate. (Courtesy of Yardney
Technical Products, Inc.)
3.0 3.2 3.4 3.6 3
Voltage (V vs. Li)
.8 4.0 4.2 4.4
-40
-20
0
20
40
Differential Capacity (dQ/dV)
FIGURE 35.11 Differential capacity on the first cycle for LiCoO
2
when cy-
cled versus Li at the C/ 20 rate. (Courtesy of FMC and Yardney Technical
Products, Inc.)
35.12 CHAPTER THIRTY-FIVE
0 50 100 150 200
3.2
3.4
3.6
3.8
4.0
4.2
4.4
LiNi
0.9
Co
0.1
O
2
(222 mAh/g)
LiNi
0.8
Co
0.2
O
2
(205 mAh/g)
LiNi
0.7
Co
O.3
O
2
(190 mAh/g)
LiNi
0.7
Co
0.2
Ti
0.005
Mg
0.005
O
2
(183 mAh/g)
Voltage (V vs. Li)
Specific Capacity (mAh/g)
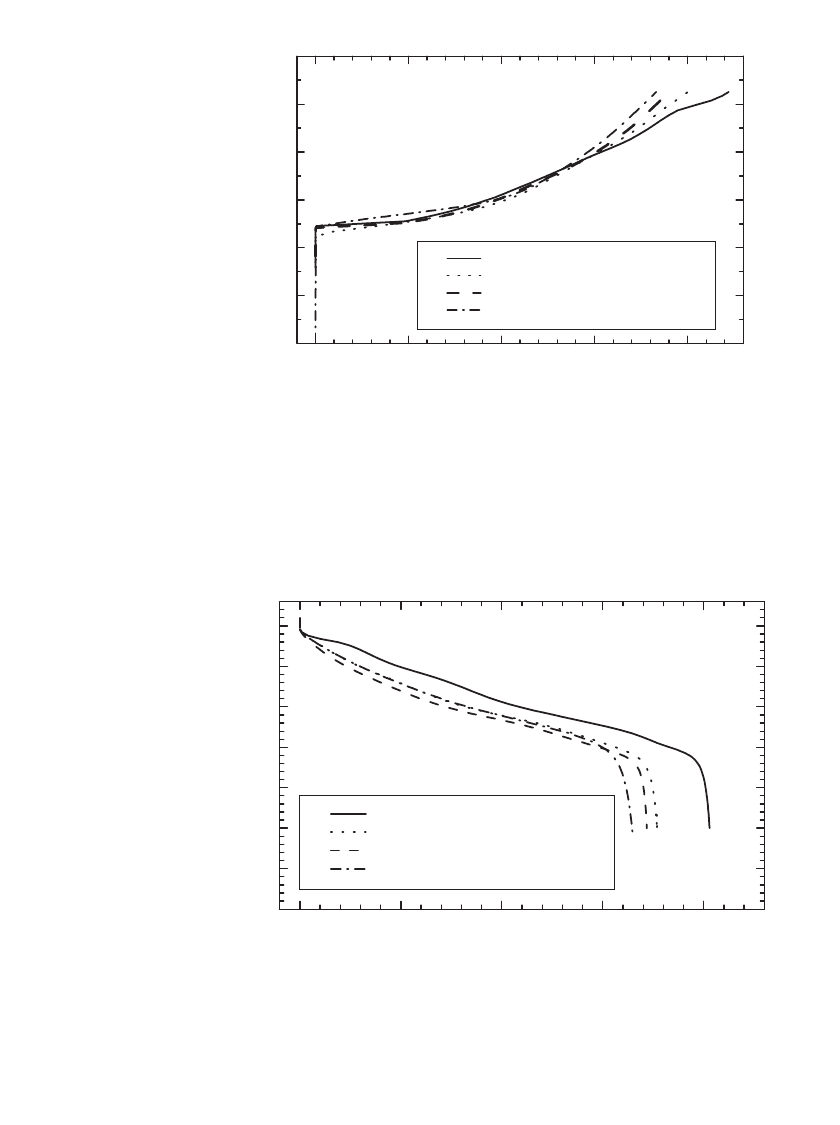
FIGURE 35.12 Charge curves for various LiNi
1
⫺
x
Co
x
O
2
materials charged at
the C / 20 rate. (Courtesy of Yardney Technical Products, Inc. and FMC.)
0 50 100 150 200
2.50
2.75
3.00
3.25
3.50
3.75
4.00
4.25
LiNi
0.9
Co
0.1
O
2
(203 mAh/g)
LiNi
0.8
Co
0.2
O
2
(190 mAh/g)
LiNi
0.7
Co
0.3
O
2
(172 mAh/g)
LiNi
0.7
Co
0.2
Ti
0.005
Mg
0.005
O
2
(165 mAh/g)
Voltage (V vs. Li)
Specific Capacity (mAh/g)
FIGURE 35.13 Discharge curves for various LiNi
1
⫺
x
Co
x
O
2
materials at the C / 20 rate
except LiNi
0.7
Co
0.2
Ti
0.005
Mg
0.005
O
2
which was discharged at the C / 7 rate. (Courtesy of
Yardney Technical Products, Inc. and FMC.)

LITHIUM-ION BATTERIES 35.13
The LiNi
0.7
Co
0.2
Ti
0.005
Mg
0.005
O
2
material, engineered to offer improved safety through
reduced exotherm on decomposition, offers slightly lower capacity, 183 mAh/g on the first
deintercalation, and 165 mAh/ g reversibly, but with voltage similar to the other LiNi
1
⫺
x
Co
x
O
2
materials described.
Synthesis of Lithiated Metal Oxides. The synthesis of lithiated metal oxides, including
LiCoO
2
and LiMn
2
O
4
, has be achieved through a wide variety of routes, although those
practiced commercially use inexpensive starting materials, such as lithium carbonate, lithium
hydroxide and the metal oxide. The physical and electrochemical properties of the materials
may be controlled by the choice of starting materials and the preparation conditions.
18
The easiest to prepare is LiCoO
2
. Its thermodynamic stability results in the desired phase
after treatment of a range of lithium and cobalt reagents, including carbonates,
19
oxides,
20
hydroxides, nitrates,
21
and organic acid complexes,
22
in the appropriate ratio at high tem-
perature, 600
⬚C to 1100⬚C, in air. LiCoO
2
can be prepared in bulk at lower temperatures,
400
⬚C, from either cobalt nitrate
23
or the acetates.
24
Preparation of LiCoO
2
at 300⬚C has
been achieved from hydroxide mixtures.
25
LiCoO
2
has also been prepared via nonaqueous
routes from nitrates,
26
under hydrothemal conditions,
27
and as thin films by laser ablation
28
or spray pyrolysis.
29
An overview of the preparation and properties of lithium cobalt oxides
is available.
30
Lithium nickel cobalt oxides can be prepared using routes similar to those used in the
preparation of LiCoO
2
, although the properties of the material are more sensitive to the
preparative method. Preparations of lithium nickel cobalt oxides are designed to achieve
molecular mixing of the cobalt and nickel materials prior to their reaction. Lithium cobalt
nickel oxides have been prepared from lithium, nickel and cobalt hydroxide co-precipitates
from nitrate solutions, treated between 400
⬚C and 800⬚C after removal of excess water.
31
Another preparation includes treatment of Li
2
CO
3
, CoCO
3
and Ni(NO
3
)
2
6H
2
O at 400⬚C.
32
The electrochemical properties of LiMn
2
O
4
materials are sensitive to the preparative
method, motivating the development of preparations that yield single phase material with
controlled Li, Mn, and O stoichiometry with the desired structure. The effect of preparation
conditions on the properties of spinels prepared from Li
2
CO
3
and MnO
2
, such as electrolytic
MnO
2
(EMD), at 600⬚Cto900⬚C,
33
has been the subject of numerous investigations.
34
Ma-
terials have also been prepared from LiOH and
␥
-MnO
2
(CMD),
35
or Mn
2
O
3
,
36
towards
spinels with improved capacity retention.
35.2.3 Capacity Fade in C/ LiMn
2
O
4
Cells
Manganese-based materials continue to receive industrial and commercial interest because
of their low cost, benign environmental qualities,
37
good electrochemical properties, and
excellent safety properties, despite the higher capacity and improved high temperature sta-
bility possible when cobalt- or nickel-based materials are used. The complex chemistry of
the lithium manganese oxides has been the focus of many academic
38
and industrial studies.
The most significant differences in performance between typical manganese- and cobalt-
based cells are their lower capacity, their higher rate of capacity fade when cycled or stored
in the charged or discharged state, and the ability of spinel cells to sustain abuse, attributed
to the stability of
-MnO
2
to deoxygenation, relative to NiO
2
or CoO
2
.
Capacity fade in spinel cells is the result of multiple processes,
39
including those related
solely to the spinel material and others involving the interaction of spinel with the electrolyte
and negative electrode materials. Most fade mechanisms can be attributed to three factors:
40
1. Dissolution of Mn
2
⫹
into the electrolyte after disproportionation of Li
x
Mn
2
O
4
:
3
⫹
4
⫹
2
⫹
2Mn → Mn ⫹ Mn
(solid) (solid) (solution)

35.14 CHAPTER THIRTY-FIVE
Dissolution of spinel into the electrolyte,
41,42
is promoted by acid-induced delithiation,
resulting in disproportionation and formation of
-MnO
2
. Li-ion electrolytes that employ
LiPF
6
are acidic, as the salt reacts with adventitious water to form HF. This reaction has
a secondary effect of reducing electrolyte conductivity:
4H O
⫹ LiPF → LiF ⫹ 5HF ⫹ HPO
26 34
However, studies of the material losses from spinel electrodes, and the resulting Mn
2
⫹
concentration in the electrolyte, found dissolution can account for only a fraction of the
capacity fade observed in spinel cells.
42
For example, when spinel cells were cycled at
50
⬚C, analysis of the electrolyte for manganese found spinel dissolution could account
for only 34% of the capacity fade observed.
43
2. Instability of the electrolyte
Decomposition of the electrolyte includes reaction of the solvent at electrode sur-
faces
42,44,45
to yield a passivation layer (Solid-Electrolyte Interphase or SEI), resulting in
an increase in electrode resistance, cell polarization, and apparent capacity loss.
43
The presence of lithium alkoxides within the SEI formed on the surface of graphite
negative electrodes, as well as LiOH, Li
2
CO
3
, and lithium alkyl carbonates, has been
established.
45,46
One proposed spinel degredation mechanism
47
starts with the reaction of
lithium alkoxides at the graphite negative with adventitious water to yield alcohol:
LiOMe
⫹ HO→ MeOH ⫹ LiOH
2
The alcohol can then react at the positive electrode where it is oxidized to H
2
O and CO
2
,
the spinel electrode serving as a source of oxygen:
MeOH
⫹ 1/
␦
Mn O → CO ⫹ 2H O ⫹ 1/
␦
Mn O
24 2 2 24
⫺
3
␦
This water and CO
2
may then return to the negative electrode and react with Li
x
C
6
to
yield immobilized Li in the form of LiOH or Li
2
CO
3
:
2H O
⫹ 1/
␦
LiC → 2LiOH ⫹ 1/
␦
Li C ⫹ H
26 1
⫺
2
␦
62
HCO ⫹ 1/
␦
LiC → Li CO ⫹ 1/
␦
Li C ⫹ H
23 6 23 1
⫺
2
␦
62
Any H
2
generated may be oxidized at the positive electrode to form more water:
H
⫹ 1/
␦
Mn O → HO⫹ 1/
␦
Mn O
2242 24
⫺
␦
This mechanism does not require positive electrode weight loss, Jahn-Teller distortion,
48
or lattice contraction. Consistent with this mechanism, analysis of C/LiMn
2
O
4
cells cycled
at 45
⬚C found the majority (75%) of the immobilized lithium within the graphite negative
electrode, the remainder (25%) was found within the positive electrode, implicating in-
volvement of the negative electrode in spinel cell capacity fade.
3. Jahn-Teller distortion in discharged cells (Li
1
Mn
2
O
4
)
Jahn-Teller distortion
49
occurs in LiMn
2
O
4
at 7⬚C (280 K).
50
This phase transition results
in a transformation from the cubic space group Fd3m to the tetragonal group I4
1
/amd.
The structural distortion results from interaction of the Jahn-Teller active species Mn
3
⫹
(t
2g
3
-e
g
1
), in contrast, Mn
4
⫹
(t
2g
3
-e
g
0
) and Mn
2
⫹
(t
2g
3
-e
g
2
) are not Jahn-Teller active. Because
of the low temperature of this transition, and that modified spinels have in general lower
transition temperatures, this mechanism may not be as relevant as others for currently
used spinel materials. Related mechanisms can cause strain and structural failure,
51
re-
sulting in electrically disconnected particles.
LITHIUM-ION BATTERIES 35.15
01020304050
60
70
80
90
100
110
120
130
140
Specific Capacity (mAh/g)
Cycle
25°C
55°C
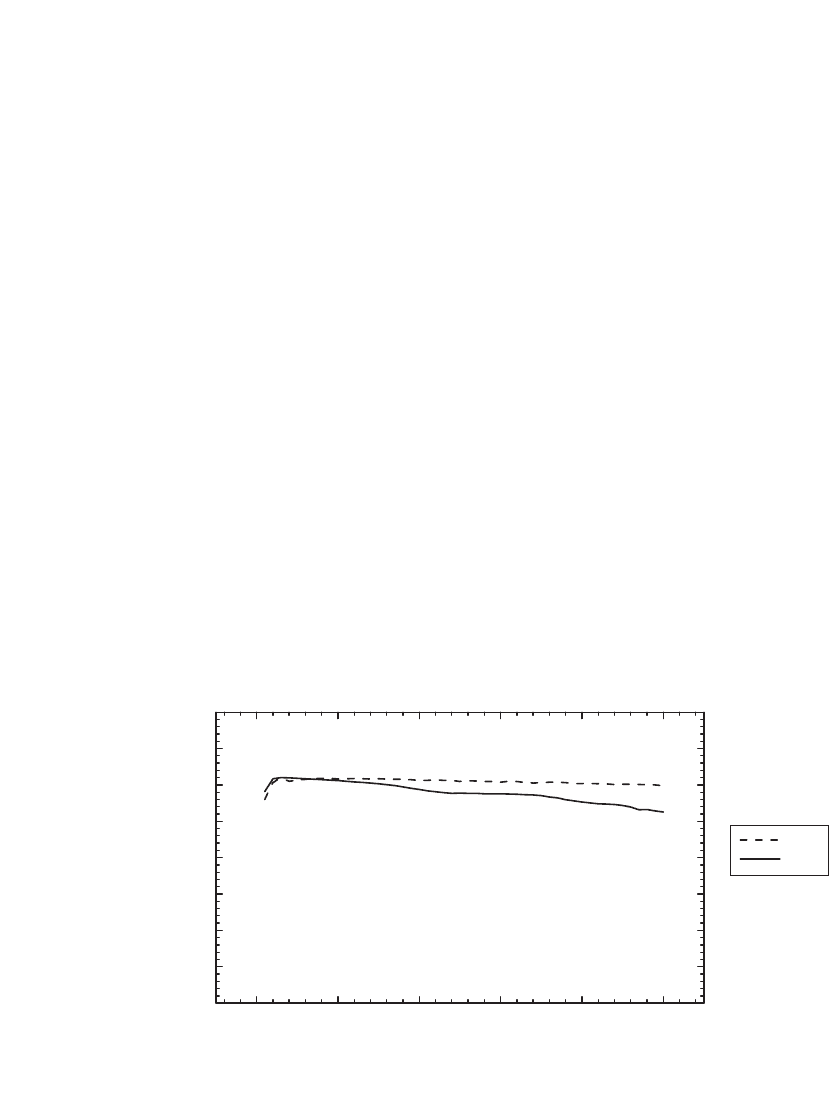
FIGURE 35.14 The specific capacity of a manganese spinel cycled at 55⬚C and 23⬚C at the C / 2 rate
between 4.3 and 3.5 V. (Courtesy of Carus Chemical.)
Capacity fade in spinel cells is prominent when cells are stored in either the charged or
discharged state.
43
An unmodified spinel will lose over 20% of its capacity when stored for
three weeks at 50
⬚C. Coated spinels, such as those protected by an inorganic coating of
Li
2
CO
3
or LiCoO
2
, offer improved stability, loss of 1% to 3% per week at 50⬚C is typical.
The coatings inhibit electrolyte decomposition and acid formation, thus are most effective
in mitigating capacity loss in the charged state.
Modified spinels that contain excess lithium, and preferably an admetal
(Li
1
⫹
x
M
y
Mn
2
⫺
x
⫺
y
O
4
,M⫽Al
3
⫹
,Cr
3
⫹
,Ga
3
⫹
), offer improved storage stability in the discharged
state as manganese disproportionation is inhibited when the Mn
3
⫹
:Mn
4
⫹
ratio is reduced.
Materials with admetal content offer fade rates at 55
⬚C of 0.05%/cycle (0.05 mAh/g per
cycle),
52
whereas current coated materials offer irreversible capacity loss on storage of less
than 1% per week at 55
⬚C in the discharged state, or 20% capacity loss after 500 cycles at
55
⬚C.
53
Alternatively, improved electrolytes, in particular those with additives to reduce water and
HF impurity levels, such as hexamethyldisilazane,
54
permit spinel cells to offer improved
performance. To illustrate the ability of the improved spinel materials to cycle at elevated
temperature (55
⬚C), Fig. 35.14 shows the specific capacity of a spinel material when cycled
versus lithium at 23
⬚C and 55⬚C. As shown, at 23⬚C the material demonstrated a fade rate
of 0.04% /cycle and at 55
⬚C, 0.15% /cycle. While these fade rates are higher than those
demonstrated for LiCoO
2
, they are viable for applications that value the low cost and benign
safety properties of the manganese oxides.
Since reaction with the electrolyte or dissolution processes occur at the particle surface,
materials with lower surface area and specially coated surfaces have been developed, such
as LiCoO
2
or Li
2
CO
3
coated LiMn
2
O
4
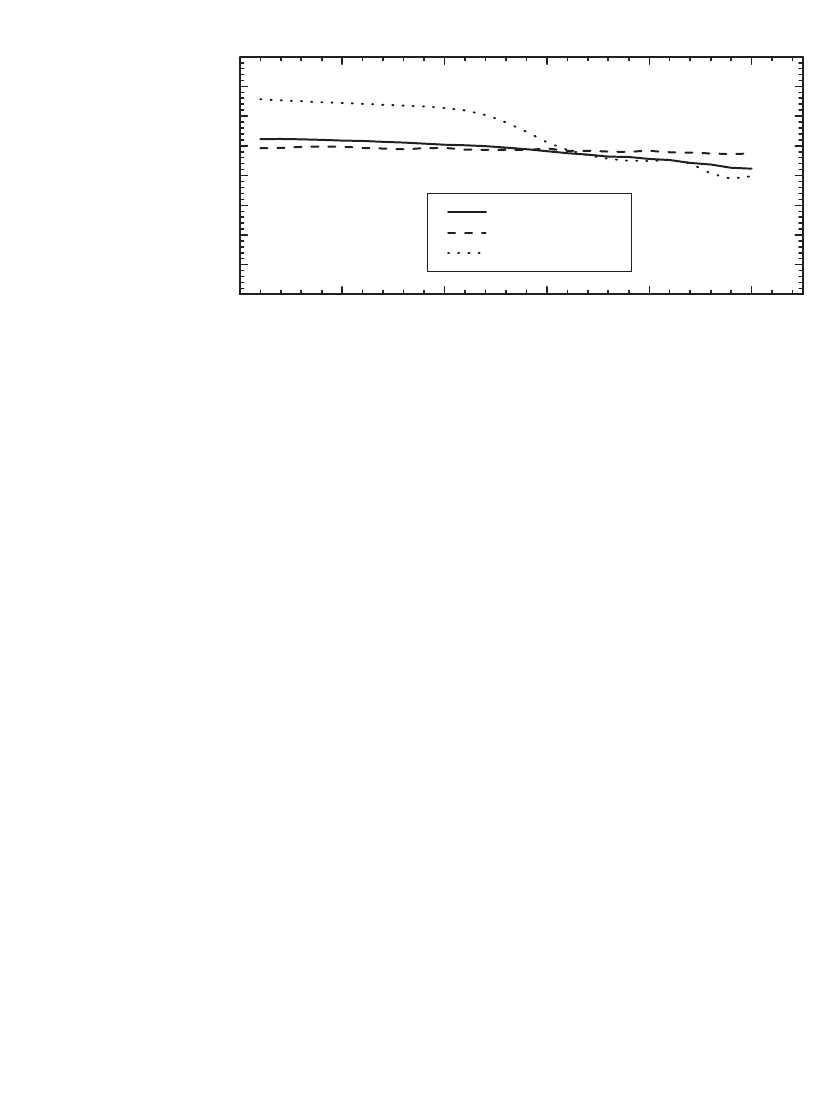
. To illustrate the ability of these materials to sustain
cycling at 55
⬚C, Fig. 35.15 shows the specific capacity of un-coated and LiCoO
2
coated
LiMn
2
O
4
materials cycled at C/ 2 at 23⬚Cor55⬚C. As illustrated, at 55⬚C the un-coated
LiMn
2
O
4
material initially provides 126 mAh/ g but the fade rate increased after 10 cycles
to 1.3% /cycle. In contrast, the LiCoO
2
coated material demonstrated lower capacity fade at
55⬚C, 0.6% /cycle.
35.16 CHAPTER THIRTY-FIVE
0 5 10 15 20 25
60
70
80
90
100
110
120
130
140
Specific Capacity (mAh/g)
Cycle
55°C, coated
23°C, coated
55°C, un-coated
FIGURE 35.15 Specific capacity of LiCoO
2
coated and un-coated spinels cycled at the C/ 2 rate
between 4.2 and 3.5 V at 23⬚Cor55⬚C. (Courtesy of Carus Chemical.)
35.2.4 Negative Electrode Materials
Historical Overview. Since the early 1970s, intercalation compounds have been considered
as electrode materials for secondary lithium batteries. However, secondary lithium battery
development effort throughout the 1970s and early 1980s focused on the use of lithium metal
as the negative electrode because of the high specific capacity of the metal. Cells with
impressive performance were developed and some were commercialized, however safety
issues with lithium metal batteries
55
caused the industry to concentrate on using lithium
intercalation into carbon at the negative electrode instead of lithium metal.
56
The safety issues
with lithium metal have been attributed to the changing morphology of lithium as a cell is
cycled. As described in Chapter 34, the safety properties of negative electrodes may be
correlated to their surface area, thus while the properties of lithium metal negative electrodes
change with use, carbon electrodes offer stable morphology resulting in consistent safety
properties over their useful life.
56
By utilizing low surface area carbons, electrodes with
acceptable self-heating rates may be fabricated.
The first Li-ion batteries marketed by Sony utilized petroleum coke at the negative elec-
trode. Coke-based materials offer good capacity, 180 mAh/g, and are stable in the presence
of propylene carbonate (PC)-based electrolytes, in contrast to graphitic materials. The dis-
order in coke materials is thought to pin the layers inhibiting reaction or exfoliation in the
presence of propylene carbonate.
56
In the mid-1990s most Li-ion cells utilized electrodes
employing graphitic spheres, in particular a Mesocarbon Microbead (MCMB) carbon.
MCMB carbon offers higher specific capacity, 300 mAh/g, and low surface area, thus pro-
viding low irreversible capacity and good safety properties. Recently, a wider variety of
carbon types has been used in negative electrodes. Some cells utilize natural graphite, avail-
able at very low cost, while others utilize hard carbons that offer capacities higher than
possible with graphitic materials.
Types of Carbon. Many types of carbon materials are industrially available and the struc-
ture of the carbon greatly influences its electrochemical properties, including lithium inter-
calation capacity and potential. The basic building block for carbon materials is a planar
sheet of carbon atoms arranged in a hexagonal array, as shown in Fig. 35.16. These sheets
are stacked in a registered fashion in graphite. In Bernal graphite, the most common type,
ABABAB stacking occurs, resulting in hexagonal or 2H graphite. In a less common poly-
morph, ABCABC stacking occurs, termed rhombohedral or 3R graphite.
3
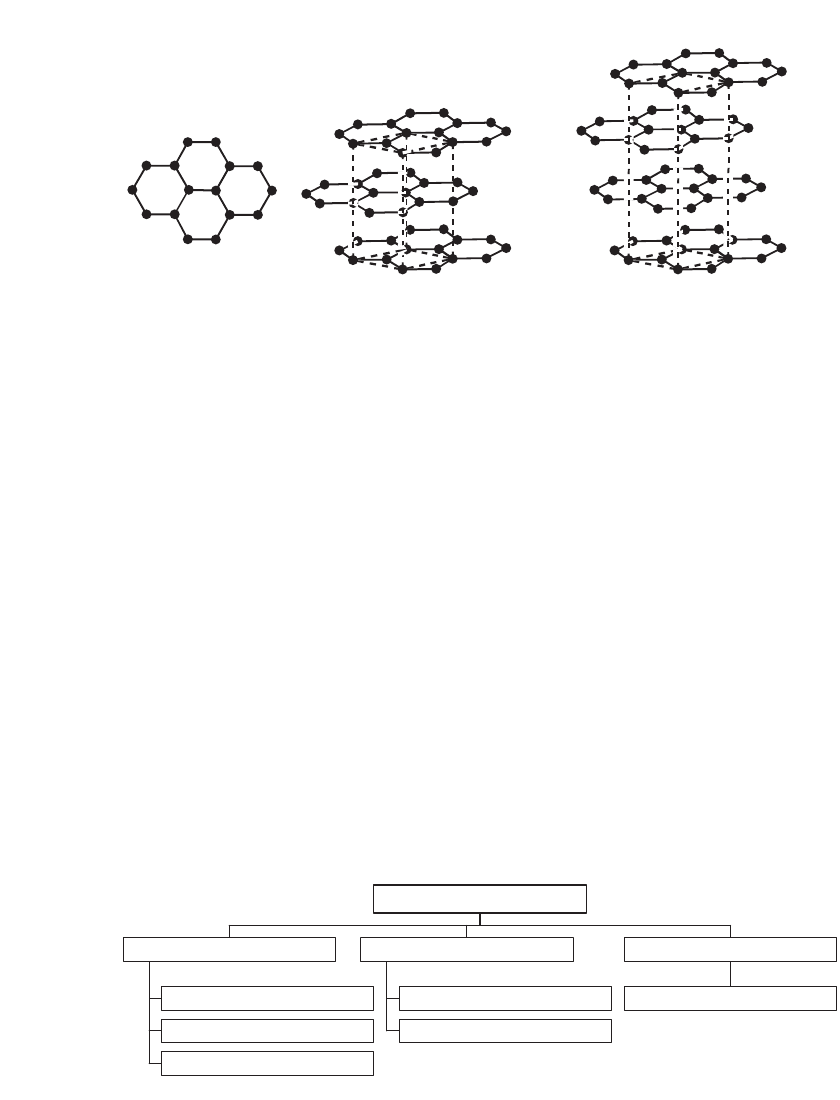
LITHIUM-ION BATTERIES 35.17
C
B
A
A
B
A
A
FIGURE 35.16 The hexagonal structure of a carbon layer and the structures of hexagonal (2H) and rhom-
bohedral (3R) graphite.
Artificial Graphite
Petroleum Coke
Coal Tar Coke
Liquid Phase Precursor
Vapor Grown Carbon Fiber
Acetylene Black
Vapor Phase Precursor
Resin Pyrolytic Graphite
Solid Phase Precursor
Carbon
FIGURE 35.17 Carbons classified by the precursor phase.
Most real materials contain disorder, including the 2H and 3R stacking orders as well as
random stacking, thus a more precise way to identify a graphite is to specify the relative
fractions of 2H, 3R and random stacking. Forms of carbon have been developed with a range
of stacking disorders and different morphologies. Stacking disorders include those where the
graphitic planes are parallel but shifted or rotated, termed turbostratic disorder,
57
or those in
which the planes are not parallel, termed unorganized carbon.
56
Particle morphologies range
from the flat plates of natural graphites, to carbon fibers, to spheres.
Carbon materials can be considered as different aggregations of a basic structural unit
(BSU) consisting of two or three parallel planes with a diameter of 2 nm.
58
The BSU’s may
be oriented randomly, resulting in carbon black, or oriented to a plane, axis or point, resulting
in a planar graphite, a whisker or a spherule.
The types of carbon may alternatively be organized based on the type of precursor ma-
terial, as illustrated in Fig. 35.17 as the precursor material, and the processing parameters
determine the nature of carbon produced. Materials that can be graphitized by treatment at
high temperature (2000
⬚C to 3000⬚C) are termed soft carbons. Upon graphitization, the
turbostratic disorder is removed and strain in the material relieved.
59
Hard carbons, such as
those prepared from phenolic resin, cannot be readily graphitized, even when treated at
3000
⬚C. Coke type materials are prepared at ⬃1000⬚C, typically from aromatic petroleum
precursors.
35.18 CHAPTER THIRTY-FIVE
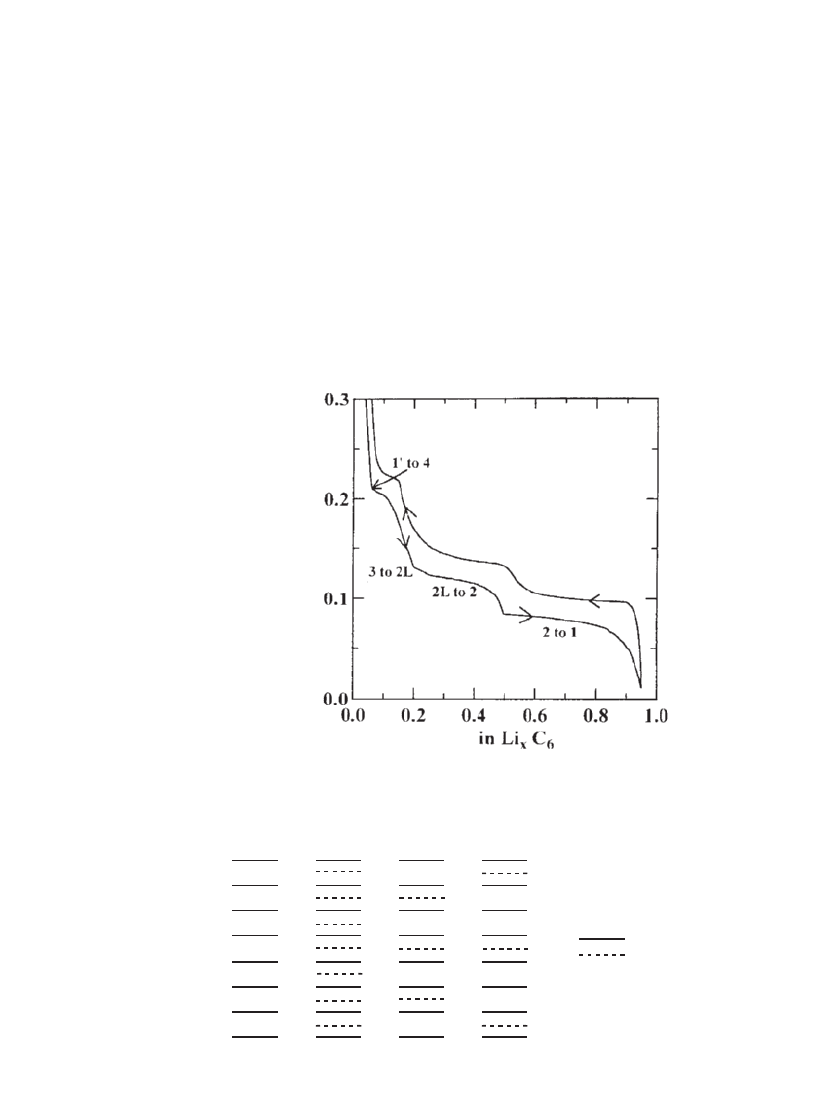
x
Voltage (V)
FIGURE 35.18 The voltage of a Li/ graphite cell illustrating
Li staging upon intercalation of graphite. (Reproduced with
permission from Physical Review B. From Ref. 59,)
Graphite
123
Carbon laye
r
Lithum laye
r
Stage
FIGURE 35.19 Schematic diagram of lithium staging in graphite.
Staging and Electrochemical Intercalation into Carbon. When lithium is intercalated into
graphite, the ABAB structure transforms to an AAAA structure and distinct voltage plateaus
are observed. This is illustrated in Fig. 35.18, which shows the voltage of a Li /graphite cell
over one cycle at low rate for a highly ordered graphite. Voltage plateaus are observed upon
lithium intercalation as distinct phases (stages) are formed.
60
A classical model of lithium
staging is illustrated in Fig. 35.19. As shown, lithium forms ‘‘islands’’ within graphite instead
of distributing homogeneously. The most lithium rich stage, LiC
6
, is termed stage 1 and is
formed at the lowest voltage, as indicated in Fig. 35.18. As lithium is removed from the
graphite, higher stages are formed, as indicated in the figure.
In graphites used in Li-ion cells, less distinct stages are observed and a flat discharge
profile results. In contrast, when petroleum coke or another disordered material is used, a
continuous, sloping voltage profile is observed. This is illustrated in Fig. 35.20, which shows
the first intercalation (charge) and deintercalation (discharge) for coke and artificial graphite.
As shown, the coke material does not exhibit distinct stages and has a higher average voltage,
0.3 V versus lithium.
