Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

LITHIUM-ION BATTERIES 35.19
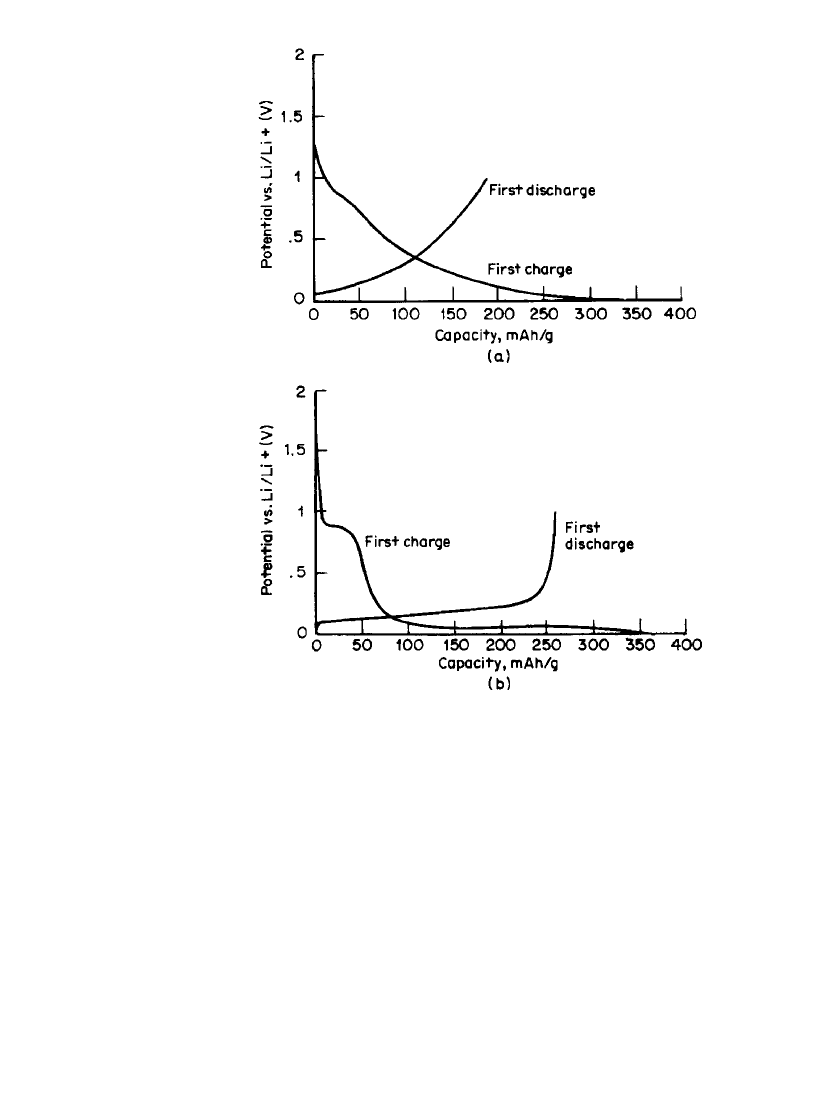
FIGURE 35.20 Potential of the carbon negative electrode in a Li-
ion cell on the first cycle illustrating the irreversible capacity asso-
ciated with (a) coke or (b) artificial graphite materials.
On the first cycle, passivation layers are formed on the surface of the electrodes. These
layers have been shown to result from the reaction of the electrolyte with the electrode
surface. The passivation layers contain lithium that is no longer electrochemically active,
thus their formation results in irreversible capacity, an undesirable property of all current
materials that occurs largely on the first cycle. The capacity difference between the charge
and discharge curves in Fig. 35.20 results from irreversible capacity.
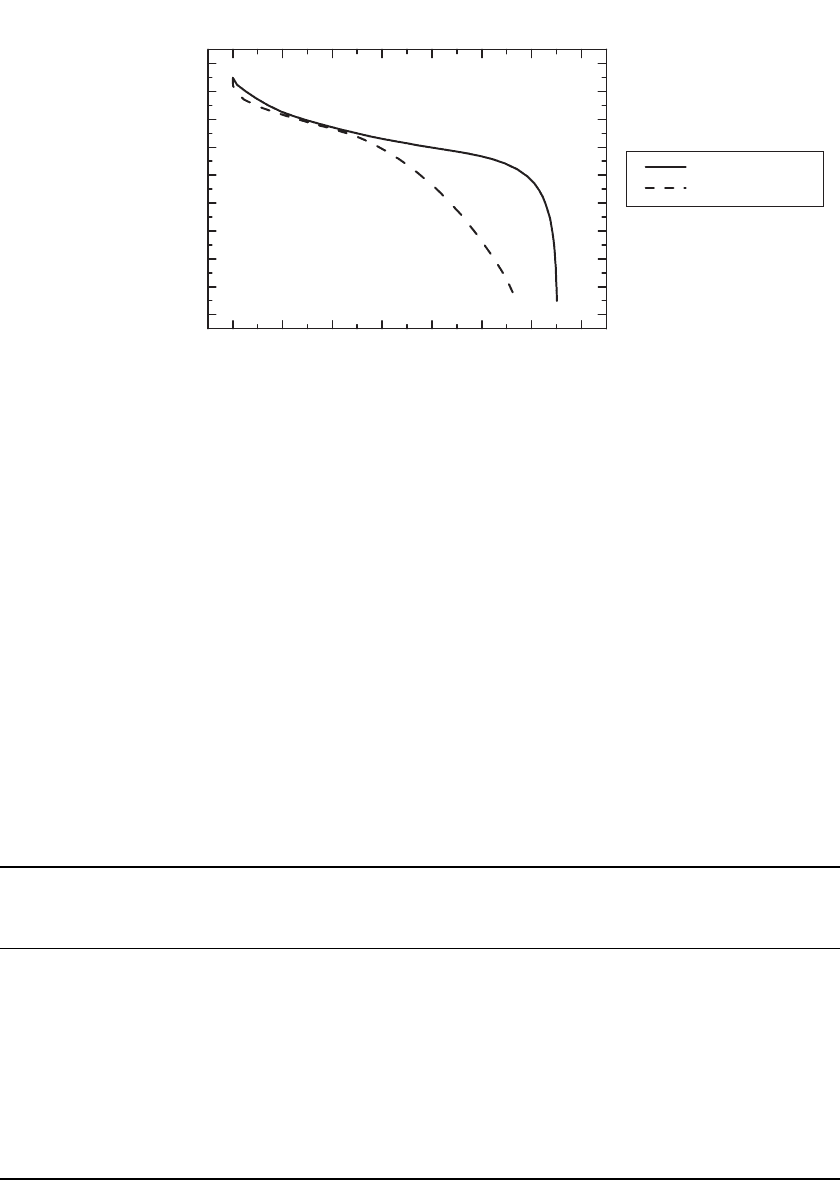
To emphasize the impact of the negative electrode material on cell voltage, Fig. 35.21
shows the discharge voltage of commercial 18650-type C/LiCoO
2
Li-ion cells with different
electrode materials. As shown, cells with graphite negatives have flatter discharge curves
than cells with coke negative electrodes. Since most commercial products now on the market
have a flat discharge curve and high average voltage, they apparently use a graphitic negative
electrode material.
35.20 CHAPTER THIRTY-FIVE
0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4
2.4
2.6
2.8
3.0
3.2
3.4
3.6
3.8
4.0
4.2
Cell Voltage (V)
Discharge Capacity (Ah)
Graphite/LiCoO
2
Coke/LiCoO
2
FIGURE 35.21 Effect of the carbon type on the discharge profile of Li-ion cells. (Courtesy of the
University of South Carolina.)
Properties of Carbons. Performance and physical characteristics of various carbons are
included in Table 35.4. An ideal material would offer high specific capacity without irre-
versible capacity. The carbon used in the cells commercialized by Sony in 1990 was a
petroleum coke. Cokes are compatible with a wide variety of electrolyte solvents, including
PC, but have lower capacity than graphitic materials. MCMB carbons offer good capacity,
⬃300 mAh /g, and low irreversible capacity, ⬃20 mAh/g. Lower cost graphites offer higher
capacity,
⬃350 mAh /g, but higher irreversible capacity, ⬃50 mAh/g, and have higher fade
rates than MCMB carbons, thus not necessarily higher energy density.
61
This is illustrated
in Fig. 35.22, which compares the reversible and irreversible capacity and energy density of
two MCMB materials and an artificial graphite. In this case, the graphite offers higher
capacity but also higher irreversible capacity than the MCMB’s, thus intermediate energy
density. In general, irreversible capacity may be correlated to the surface area of a material,
thus the interest in low surface area, spherical materials. The MCMB 25-28 has lower specific
surface area than MCMB 10-28, thus less irreversible capacity. In practice, a particle size
less than
⬃30
m is required for rate capability to the C rate. MCMB carbon can have a
variety of structures, depending on how the graphite planes are oriented within the sphere.
The performance of MCMB is related to its structure. The laboratory preparation and prop-
erties of a variety of MCMB carbons have been reported.
62
TABLE 35.4 Properties and Performance of Various Carbons (Experimental Values). (From Ref. 67.)
Carbon Type
Specific
capacity
(mAh/ g)
Irreversible
capacity
(mAh/ g)
Particle
size
D
50
(
m)
BET surface
area (m
2
/g)
KS6 Synthetic graphite 316 60 6 22
KS15 Synthetic graphite 350 190 15 14
KS44 Synthetic graphite 345 45 44 10
MCMB 25-28 Graphite sphere 305 19 26 0.86
MCMB 10-28 Graphite sphere 290 30 10 2.64
Sterling 2700 Graphitized
Carbon Black
200 152 .075 30
XP30 Petroleum coke 220 55 45 N /A
Repsol LQNC Needle coke 234 104 45 6.7
Grasker Carbon fiber 363 35 23 11
Sugar carbon Hard carbon 575 215 N /A 40
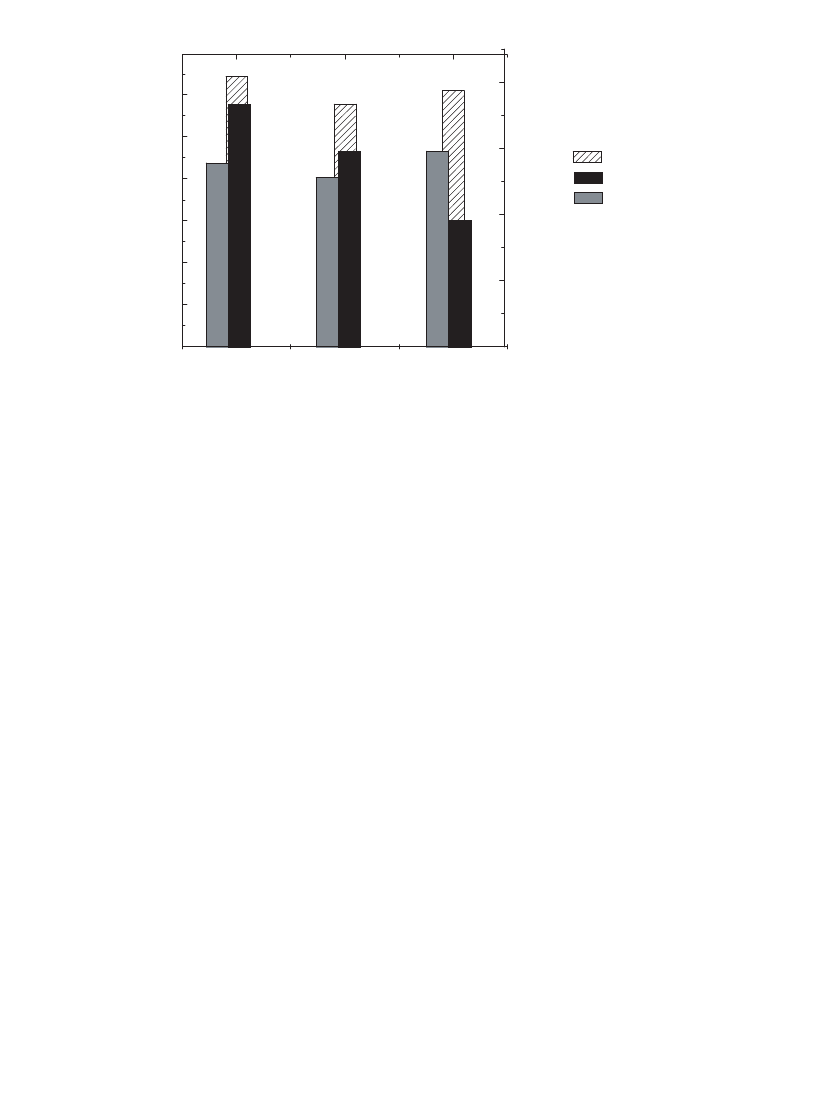
LITHIUM-ION BATTERIES 35.21
LK702 MCMB 10-28 MCMB 25-28
0
50
100
150
200
250
300
Energy Density (mWh/cm
3
) and Q
IRREV
(mAh/g)
Q
REV
(mAh/g)
Anode Material
Q
REVERSIBLE
(mAhg) - Cycle 8
AVE
0
10
20
30
40
Q
IRREVERSIBLE
(mAh/g) - Cycle 1
AVE
Energy Density (mWh/cm
3
) - Cycle 8
AVE
FIGURE 35.22 The energy density, reversible and irreversible capacity of carbons commonly used as
negative electrode materials.
The theoretical specific capacity of carbon (LiC
6
) is 372 mAh/ g. Hard carbon materials
offer higher capacity, over 1000 mAh /g, but have not achieved broad acceptance because
they have greater irreversible capacity and higher voltage,
⬃1 V vs. lithium,
63
than graphitic
materials. Hard carbons are highly disorganized. Mechanisms have been proposed to explain
incorporation of Li above the theoretical capacity of graphite. That proposed by Sato suggests
lithium occupies nearest neighbor sites between pairs of graphene sheets.
64
A mechanism
proposed by Dahn et al. suggests additional lithium may bind hydrogen containing regions
of carbon,
65
as supported by theoretical studies which illustrate the importance of hydrogen-
terminated edge regions.
66
35.2.5 Electrolytes
Four types of electrolytes have been used in Li-ion batteries: liquid electrolytes, gel electro-
lytes, polymer electrolytes and ceramic electrolytes. Liquid electrolytes are solutions of a
lithium salt in organic solvents, typically carbonates. As described in a monograph,
68
a
polymer electrolyte is a liquid- and solvent-free material, where an ionically conducting
phase is formed by dissolving a salt in a high molecular weight polymer, whereas a gel
electrolyte is an ionically conductive material wherein a salt and a solvent are dissolved or
mixed with a high molecular weight polymer. Gel electrolytes developed for Li-ion batteries
are typically films of PVDF-HFP, LiPF
6
or LiBF
4
salt, and carbonate solvent. Fumed silica
may be added to the PVDF-HFP film for additional structural integrity. Potential advantages
of polymer electrolytes include improved safety properties resulting from their low volatility
and high viscosity, as they do not contain a volatile, flammable solvent component. A pos-
sible advantage of gel electrolytes is that the liquid phase is absorbed within the polymer,
thus less likely to leak from a battery, however, in a typical Li-ion battery employing a liquid
electrolyte, the electrolyte is almost completely absorbed into the electrode and separator
materials. In the marketplace and the literature, gel electrolytes are often termed gel-polymer
electrolytes, and cells that employ gel (or gel-polymer) electrolytes are termed gel-polymer
or simply polymer cells. Ceramic electrolytes refer to inorganic, solid-state materials that are
ionically conductive.
35.22 CHAPTER THIRTY-FIVE
Most Li-ion electrolytes in current use utilize LiPF
6
as the salt as its solutions offer high
ionic conductivity,
⬎10
⫺
3
S/ cm, high lithium ion transference number (⬃0.35), and accept-
able safety properties. As reviewed below, many other salts have attracted industrial interest,
notably LiBF
4
. Electrolytes in current use are formulated with carbonate solvents. Carbonates
are aprotic, polar, and have a high dielectric, thus can solvate lithium salts to high concen-
tration (
⬎1 M). They also provide compatibility with cell electrode materials over a broad
range of potential. While the industry focused initially on propylene carbonate (PC)-based
solutions, current formulations utilize other carbonates, notably ethylene carbonate (EC),
dimethyl carbonate (DMC), ethyl methyl carbonate (EMC) and diethyl carbonate (DEC), as
PC causes degradation in graphite electrodes as it co-intercalates with lithium, resulting in
exfoliation. The choice of solvents for a Li-ion electrolyte is also influenced by any low
temperature requirements of the application. Low temperature electrolytes utilize low vis-
cosity solutions with low freezing points.
Salts. Salts commonly used in Li-ion cells are listed in Table 35.5. Most cells currently
marketed use LiPF
6
as its solutions have high conductivity and good safety properties. How-
ever, the salt is costly, hygroscopic, and LiPF
6
yields hydrofluoric acid (HF) upon reaction
with water, thus must be handled in a dry environment. Organic salts have also been devel-
oped. They are more stable to water, thus easier to handle. In particular, BETI (lithium
bisperfluoroethanesulfonimide) has received significant attention as its solutions offer high
conductivity, it is stable to water, it can be easily dried, and it does not cause aluminum
corrosion—an issue with other organic salts such as lithium triflate.
TABLE 35.5 Salts Used in Electrolytes for Li-ion Cells
Common name Formula
Mol. wt.
(g/ mol)
Typical
impurities Comments
Lithium
hexafluorophosphate
LiPF
6
151.9 H
2
O (15ppm)
HF (100ppm)
Most commonly used
Lithium
tetrafluoroborate
LiBF
4
93.74 H
2
O (15ppm)
HF (75ppm)
Less hygroscopic than
LiPF
6
Lithium perchlorate LiClO
4
106.39 H
2
O (15ppm)
HF (75ppm)
When dry, less stable than
alternatives
Lithium
hexafluoroarsenate
LiAsF
6
195.85 H
2
O (75ppm)
HF (15ppm)
Contains arsenic
Lithium triflate LiSO
3
CF
3
156.01 H
2
O
(100ppm)
Al corrosion above 2.8 V,
stable to water
Lithium
bisperfluoroethane-
sulfonimide (BETI)
LiN(SO
2
C
2
F
5
)
2
387 N / A No Al corrosion below
4.4 V, stable to water
Solvents. A wide variety of solvents, including carbonates, ethers and acetates, has been
evaluated for non-aqueous electrolytes. The industry has now focused on the carbonates as
they offer excellent stability, good safety properties and compatibility with electrode mate-
rials. Neat carbonate solvents typically have intrinsic solution conductivity less than 10
⫺
7
S/ cm, dielectric constant ⬎3, and solvate lithium salts to high concentration. Table 35.6
presents the properties of some commonly used solvents. The properties of these and other
materials are included in a recent review.
69
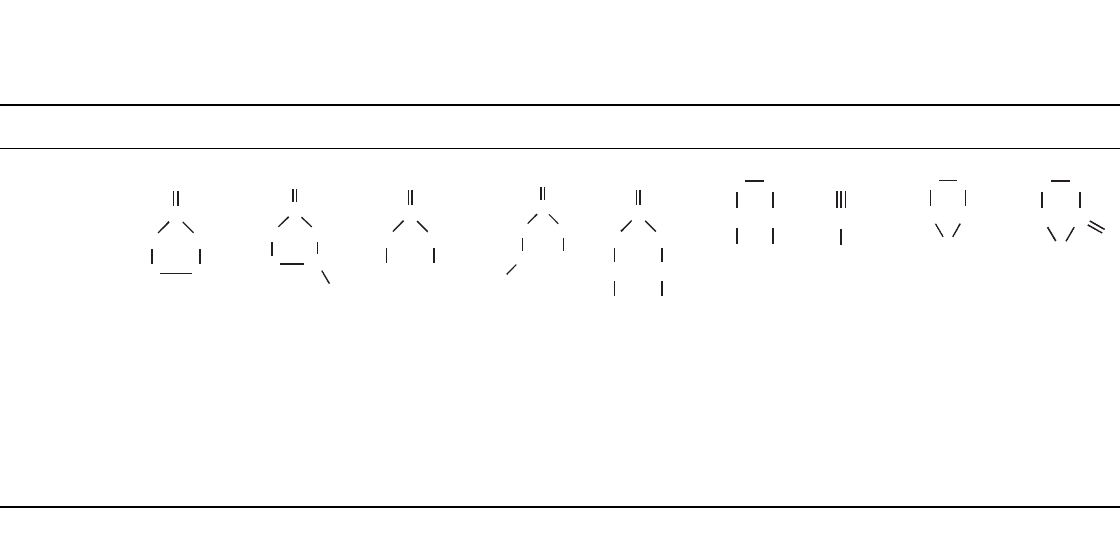
35.23
TABLE 35.6 Characteristics of Organic Solvents
Characteristic EC PC DMC EMC DEC 1,2-DME AN THF
␥
-BL
Structure
O
C
OO
CH
2
H
2
C
O
C
OO
CH
H
2
C
CH
3
O
C
OO
CH
3
CH
3
O
C
OO
CH
3
CH
2
H
3
C
O
C
OO
CH
2
CH
2
CH
3
CH
3
H
2
CCH
2
OO
CH
3
CH
3
CH
3
C
N
H
2
CCH
2
CH
2
H
2
C
O
H
2
CCH
2
CH
2
C
O
O
BP (⬚C) 248 242 90 109 126 84 81 66 206
MP (
⬚C) 39 ⫺48 4 ⫺55 ⫺43 ⫺58 ⫺46 ⫺108 ⫺43
Density (g / ml) 1.41 1.21 1.07 1.0 0.97 0.87 0.78 0.89 1.13
Viscosity (cP) 1.86 (40
⬚C) 2.5 0.59 0.65 0.75 0.455 0.34 0.48 1.75
Dielectric
constant
89.6 (40
⬚C) 64.4 3.12 2.9 2.82 7.2 38.8 7.75 39
Donor
number
16.4 15 8.7
70
6.5
70
8
70
— 14 –—
Mol. wt. 88.1 102.1 90.1 104.1 118.1 90.1 41.0 72.1 86.1
*EC ⫽ ethylene carbonate, PC ⫽ propylene carbonate, DMC ⫽ dimethyl carbonate, EMC ⫽ ethyl methyl carbonate,
DEC
⫽ diethyl carbonate, DME ⫽ dimethylether, AN⫽acetonitrile, THF⫽tetrahydrofuran,
␥
BL ⫽
␥
-butyrolactone.
(From Refs. 69, 70, 71 and 72.)
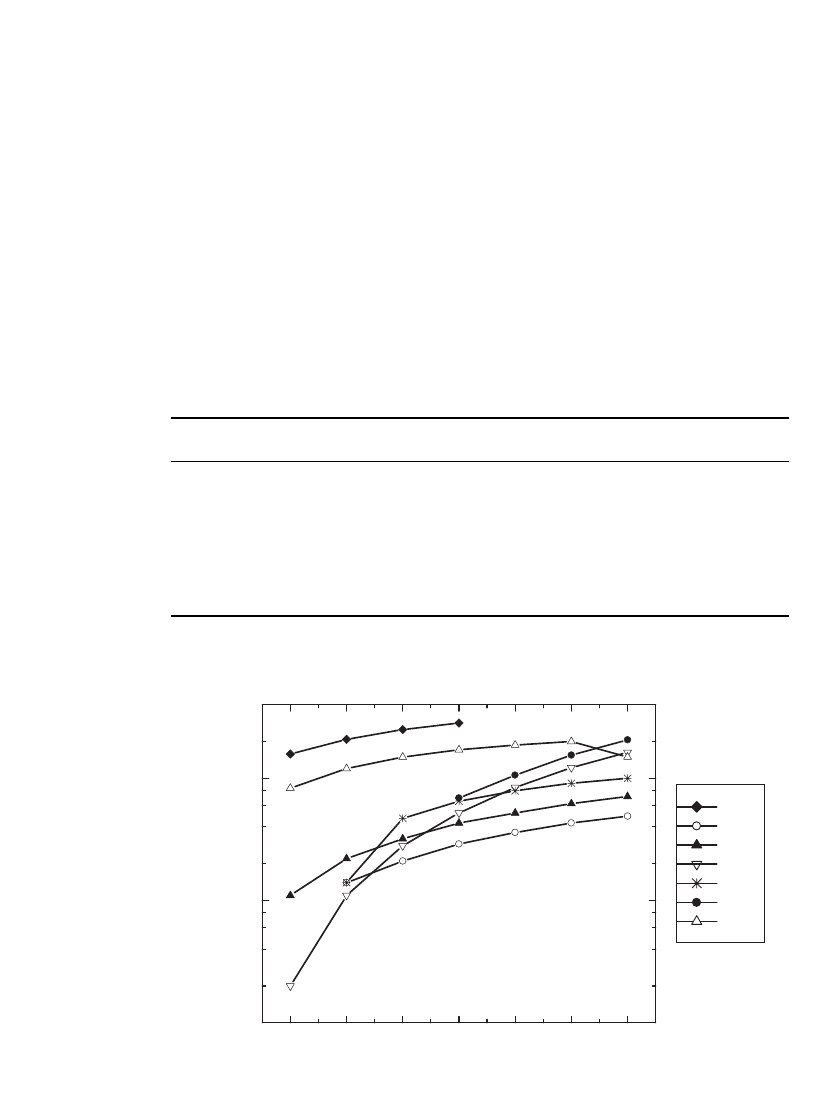
35.24 CHAPTER THIRTY-FIVE
TABLE 35.7 Conductivity, in mS/ cm, of 1M LiPF
6
Solutions in Various Solvents.
(Courtesy of Merck KGaA, Darmstadt, Germany.)
Solvent ⫺40.0⬚C ⫺20.0⬚C 0.0⬚C 20.0⬚C 40.0⬚C 60.0⬚C 80.0⬚C
DEC — 1.4 2.1 2.9 3.6 4.3 4.9
EMC 1.1 2.2 3.2 4.3 5.2 6.2 7.1
PC 0.2 1.1 2.8 5.2 8.4 12.2 16.3
DMC — 1.4 4.7 6.5 7.9 9.1 10.0
EC ———6.9 10.6 15.5 20.6
MA 8.3 12.0 14.9 17.1 18.7 20.0 —
MF 15.8 20.8 25.0 28.3 ———
* DEC ⫽ diethyl carbonate, EMC ⫽ ethyl methyl carbonate, PC ⫽ propylene carbonate, DMC ⫽
dimethyl carbonate, EC⫽ethylene carbonate, MA⫽methyl acetate, MF ⫽ methyl formate.
-40-200 20406080
0.1
0.2
0.4
0.6
0.8
1
2
4
6
8
10
20
40
Conductivity (mS/cm)
Temperature (°C)
MF
DEC
EMC
PC
DMC
EC
MA
FIGURE 35.23 Conductivity, in mS /cm, of 1M LiPF
6
solutions in various solvents.
(Courtesy of Merck KGaA, Darmstadt, Germany.)
Conductivity of Electrolytes. Electrolyte formulations in current Li-ion cells typically util-
ize two to four solvents. Formulations with multiple solvents can provide better cell per-
formance, higher conductivity, and a broader temperature range than possible with a single
solvent electrolyte. For example, ethylene carbonate (EC) is associated with low irreversible
capacity and low capacity fade when used in conjunction with graphitic negative electrodes.
Ethylene carbonate is found in many commercial electrolyte formulations, but is a solid at
room temperature. Multiple solvent formulations often include EC, thereby incorporating its
desirable properties, while using other solvents to lower the freezing point and viscosity of
the mixture.
The conductivity of 1 M LiPF
6
solutions in solvents commonly used in Li-ion electrolytes
is given in Table 35.7 and plotted in Fig. 35.23 for temperatures between
⫺40⬚C and 80⬚C.
In general these solutions offer high conductivity, 10
⫺
2
S/ cm, and a few solvents, such as
PC and EMC, offer good conductivity at low temperature and a high boiling point. MA and
MF offer high conductivity, but cell performance is poor if either is used at levels greater
than 25% by weight.
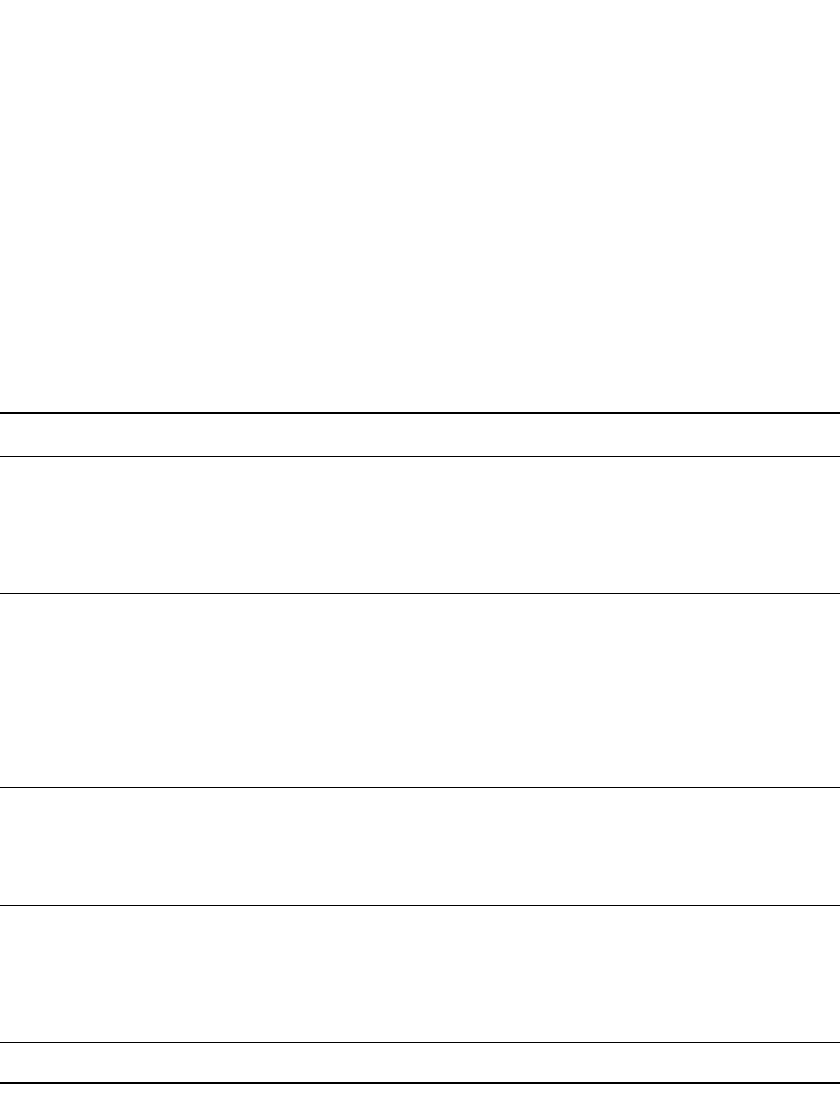
LITHIUM-ION BATTERIES 35.25
TABLE 35.8 Conductivity, in mS/ cm, of LiPF
6
Solutions in Binary Mixtures, 1:1 by Weight. C ⫽ partially
crystallized, S
⫽ saturated. (Courtesy of Merck KGaA, Darmstadt, Germany.)
Solvents Concentration ⫺40⬚C ⫺20⬚C0⬚C20⬚C40⬚C60⬚C80⬚C
EC:DEC
0.25 M
0.50 M
1.00 M
1.25 M
1.50 M
1.75 M
—
—
0.7
0.4
—
—
—
2.5 (C)
2.2
1.7
—
—
1.7 (C)
3.0
4.2
3.6
—
—
4.2
6.4
7.0
6.4
5.6
4.8 (S)
5.8
8.7
10.3
9.7
—
—
7.3
11.1
13.9
13.5
—
—
8.8
13.6
17.5
17.4
—
—
0.25 M ——4.2 5.8 7.8 9.7 11.5
0.50 M ——6.5 9.3 12.8 16.0 19.1
0.75 M — 3.8 6.9 10.3 14.0 17.9 21.6
1.00 M — 3.7 7.0 15.0 19.5 24.0
EC:DMC 1.25 M 0.7 2.7 5.6 9.3 13.7 18.4 23.3
1.50 M — 2.2 5.4 9.3 14.1 19.2 24.7
1.75 M —— —7.5 ———
2.00 M —— —6.7 ———
2.25 M —— —0.9 (S) ———
EC:EMC
0.25 M
0.50 M
1.00 M
1.25 M
3.50 M
—
—
0.9
0.6
—
—
3.0
2.7
2.3
—
3.7
5.1
5.3
4.7
—
5.3
7.5
8.5
8.0
0.9 (S)
7.2
10.2
12.2
12.0
—
9.1
12.8
16.3
16.2
—
10.9
15.4
20.3
20.6
—
EC:MA
0.25 M
0.50 M
1.00 M
1.25 M
3.0 M
3.5 M
2.4 (C)
3.1 (C)
3.8
—
—
—
4.6
6.7
7.8
7.1
0.5
—
6.3
9.8
12.2
11.8
2.1
—
8.3
13.1
17.1
17.2
5.2
3.4 (S)
10.4
16.0
22.3
22.7
—
—
12.4
19.3
27.3
28.4
15.4
—
—
—
—
—
21.8
—
EC:MPC 1.00 M C 1.5 3.6 6.3 9.5 12.9 16.8
* DEC ⫽ diethyl carbonate, EMC ⫽ ethyl methyl carbonate, DMC ⫽ dimethyl carbonate, EC ⫽ ethylene carbonate, MA ⫽ methyl
acetate.
The conductivity of binary 1:1 mixtures of EC with common Li-ion electrolyte solvents
over a range of salt concentrations and temperatures is given in Table 35.8. As indicated,
for many solvent pairs, conductivity is highest with 1 M LiPF
6
, and these formulations are
liquid from
⫺40⬚Cto80⬚ C. The conductivity of 1 M LiPF
6
binary solutions with EC are
plotted in Fig. 35.24. As shown, the EC:MA solution offers the highest conductivity although
such levels of MA are associated with high capacity fade.
74
Other mixtures, including EC:
DEC, EC:DMC, and EC:EMC offer good conductivity and low capacity fade. In particular,
EC:EMC mixtures offer 0.9 mS/cm conductivity at
⫺40⬚C, and low capacity fade.
The conductivity of PC:DME 1:1 solutions of LiPF
6
, and a variety of organic salts, is
illustrated in Fig. 35.25, which shows the conductivity of the solutions from 0.25 M to 2 M.
As shown, LiPF
6
offers the highest conductivity, 13 mS/ cm at 1.2 M, although solutions
with the organic salts offer comparable conductivity, up to 11 mS/ cm.
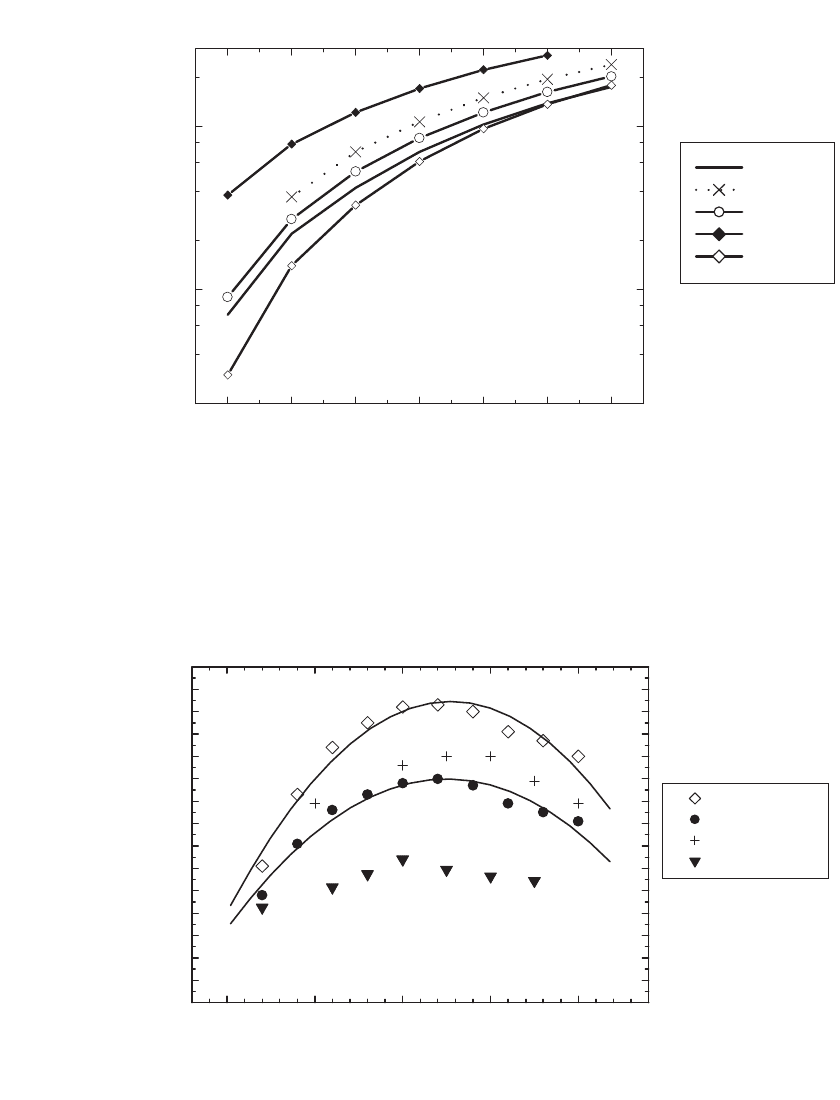
35.26 CHAPTER THIRTY-FIVE
-40-20 0 20406080
0.2
0.4
0.6
0.8
1
2
4
6
8
10
20
Conductivity (mS/cm)
Temperature (°C)
EC:DEC
EC:DMC
EC:EMC
EC:MA
EC:PC
FIGURE 35.24 Conductivity of 1M LiPF
6
solutions in various binary mixtures, 1:1 by weight. (Courtesy
of Merck KGaA, Darmstadt, Germany.)
0.0 0.5 1.0 1.5 2.0
0
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
Conductivity (mS/cm)
Salt Concentration (M)
LiPF
6
LiN(SO
2
CF
3
)
2
LiN(SO
2
C
2
F
5
)
2
LiSO
3
CF
3
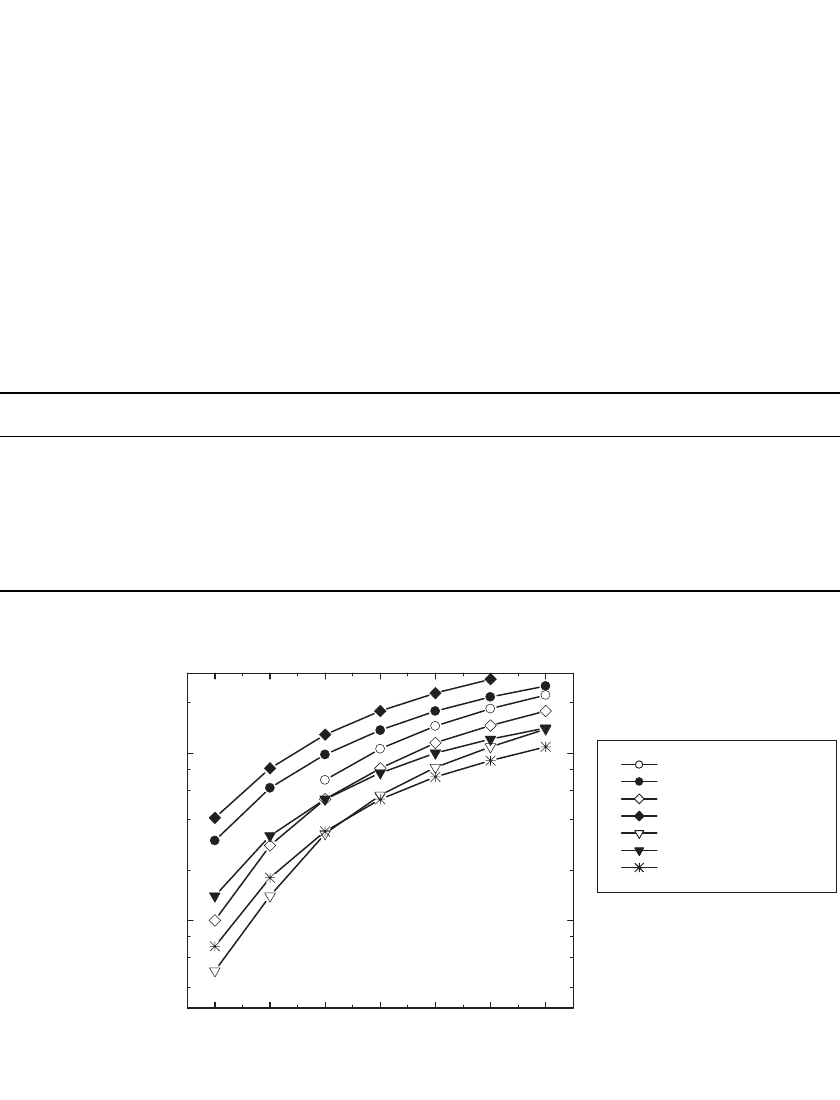
FIGURE 35.25 Conductivity of electrolytes at 20⬚C prepared with PC:DME (1:1, V /V) and various salts.
(Courtesy of 3M, St. Paul, MN.)
LITHIUM-ION BATTERIES 35.27
TABLE 35.9 Conductivity, in mS/ cm, of M LiPF
6
Solutions in Various Ternary Solvent Mixtures. (Courtesy of Merck
KGaA, Darmstadt, Germany.)
Solvent Wt. ratio ⫺40⬚C ⫺20⬚C0⬚C20⬚C40⬚C60⬚C80⬚C
EC:PC:DMC 20:20:60 ——6.9 10.6 14.5 18.4 22.2
EC:PC:EA 15:25:60 3 6.2 9.8 13.7 17.8 21.6 25.1
EC:PC:EMC 15:25:60 1 2.8 5.3 8.1 11.5 14.6 17.8
EC:PC:MA 15:25:60 4.1 8.1 12.9 17.8 22.8 27.6 boils
EC:PC:MPC 15:25:60 0.5 1.4 3.3 5.6 8.2 10.9 13.9
EC:DMC:EMC 15:25:60 1.4 3.2 5.3 7.6 10 12.1 14.1
EC:DMC:MPC 15:25:60 0.7 1.8 3.4 5.3 7.2 9 10.9
* DEC ⫽ diethyl carbonate, EMC ⫽ ethyl methyl carbonate, PC ⫽ propylene carbonate, DMC ⫽ dimethyl carbonate, EC ⫽ ethylene
carbonate, MA
⫽ methyl acetate, MPC ⫽ methyl propyl carbonate, EA ⫽ ethyl acetate.
-40°C -20°C0°C20°C40°C60°C80°C
0.4
0.6
0.8
1
2
4
6
8
10
20
Conductivity (mS/cm)
Temperature (°C)
EC:PC:DMC, 20:20:60
EC:PC:EA, 15:25:60
EC:PC:EMC, 15:25:60
EC:PC:MA, 15:25:60
EC:PC:MPC, 15:25:60
EC:DMC:EMC, 15:25:60
EC:DMC:MPC, 15:25:60
FIGURE 35.26 Conductivity of 1 M LiPF
6
solutions in ternary solvent mixtures. (Courtesy of Merck KGaA,
Darmstadt, Germany.)
The conductivity of selected ternary 1 M LiPF
6
solutions is given in Table 35.9 and
plotted in Fig. 35.26. These mixtures contain 33% EC, as is typical in current Li-ion elec-
trolytes, but still offer high conductivity and a broad temperature range, illustrating the utility
of multiple component mixtures. For example, four of these mixtures offer at least 1 mS /
cm at
⫺40⬚C, of which three are liquid at 80⬚C. Quaternary mixtures have also been devel-
oped to provide electrolytes with better low temperature performance. The conductivity of
1.0 M LiPF
6
solutions in various quaternary solvent mixtures is shown in Fig. 35.27. As
illustrated, these solutions offer over 1 mS/ cm at
⫺40⬚C and up to 0.6 mS/cm at ⫺60⬚C.
A survey of the conductivity and solvent properties of electrolytes utilizing either LiAsF
6
,
LiPF
6
, LiSO
3
CF
3
, or LiN(SO
2
CF
3
)
2
and a wide variety of solvents, including EC, DME,
ethyldiglyme, triglyme, tetraglyme, sulfolane, Freon, and methylene chloride has been pub-
lished.
73
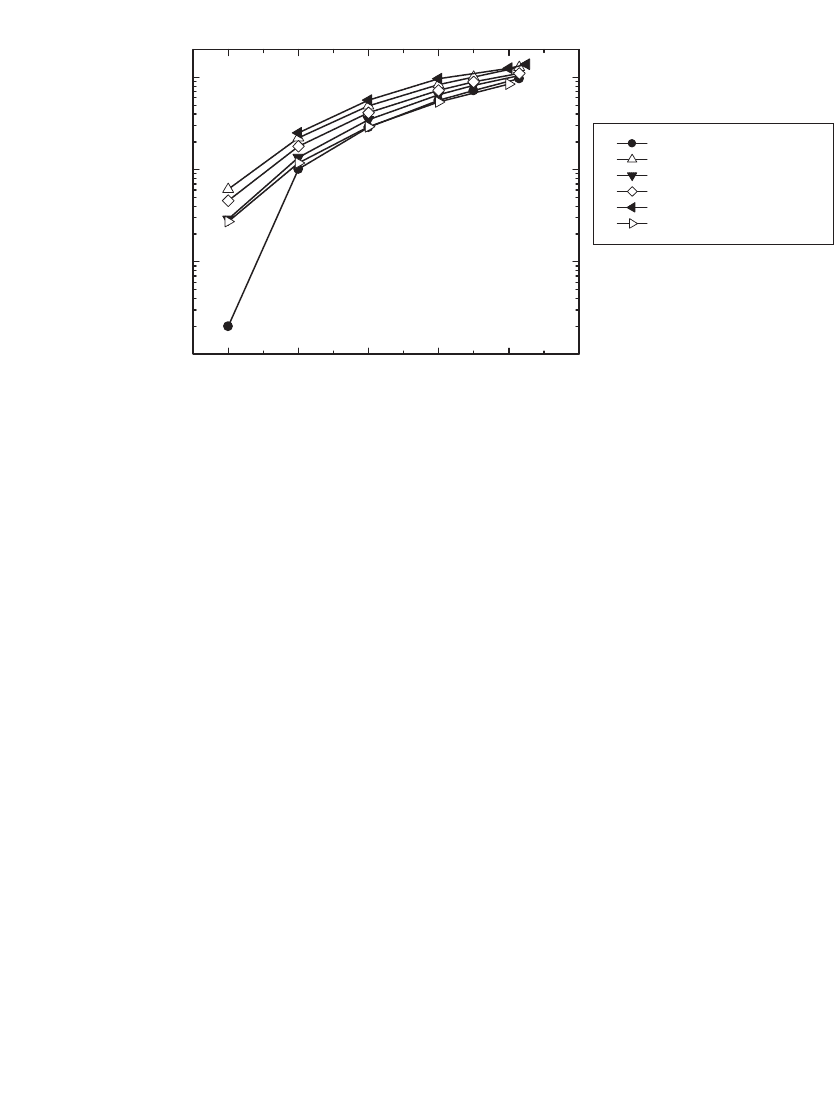
35.28 CHAPTER THIRTY-FIVE
-60 -40 -20 0 20 40
0.01
0.1
1
10
Conductivity (mS/cm)
Temperature (°C)
EC:DEC:DMC (1:1:1)
EC:DEC:DMC:MA (1:1:1:1)
EC:DEC:DMC:GBL (1:1:1:1)
EC:DEC:DMC:EA (1:1:1:1)
EC:DEC:DMC:MF (1:1:1:1)
EC:DEC:DMC:EB (1:1:1:1)
FIGURE 35.27 The conductivity of 1.0 M LiPF
6
solutions in quaternary solvent mixtures. (Courtesy of
JPL.)
Electrolyte Formulation, Irreversible Capacity and the SEI. Various electrolytes have
been used in Li-ion batteries. The solvents used must be stable at both the anodic and
cathodic potentials found in Li-ion cells, 0 V to 4.2 V vs. lithium. No practical solvents are
thermodynamically stable with lithium or Li
x
C
6
near 0 V vs. Li, but many solvents undergo
a limited reaction to form a passivation film on the electrode surface. This film spatially
separates the solvent from the electrode, yet is ionically conductive, and thus allows passage
of lithium ions. The passivation film, termed the solid electrolyte interphase (SEI), therefore
imparts extrinsic stability to the system allowing the fabrication of cells that are stable for
years without significant degradation.
74
When the SEI is formed, lithium is incorporated into the passivation film. This process
is irreversible and is thus observed as a loss of capacity, primarily on a cell’s first cycle. The
amount of irreversible capacity is dependent on the electrolyte formulation and the electrode
materials, in particular the type of carbon used in the negative electrode. As the reaction
occurs at the particle surface, materials with low specific surface area typically offer lower
irreversible capacity.
Cells with electrolyte formulations that contain alkyl carbonates, in particular EC, have
been shown to offer low capacity fade, low irreversible capacity and high capacity.
75
In EC
containing electrolytes, the passivation film formed on the surface of Li-ion electrodes is
formed with a minimum amount of lithium. This SEI has been shown to consist primarily
of Li
2
(OCO
2
(CH
2
)
2
OCO
2
)
2
,
76
and related reaction products, including Li
2
CO
3
and LiOCH
3
,
77
of the electrolyte solvent with either lithium or a lithiated species such as Li
x
C
6
. While
solvents other than EC, typically esters or alkyl carbonates such as EMC or MPC,
78
also
form stable passivation films, most solvents do not. If an ester or alkyl carbonate is not used,
graphite can be cycled in a solvent that does not form a stable passivation film if an additive,
such as a crown ether
79
or CO
2
,
76,80
is added to the electrolyte.
