Lin S.D. Water and Wastewater Calculations Manual
Подождите немного. Документ загружается.

Wastewater Engineering 765
Step 2. Calculate the mass of calcium carbonate formed
(a) Calculate the mass of Ca added in the lime dosage
Mass of Ca in Ca(OH)
2
⫽ 280 mg/L ⫻ 40/(40 ⫹ 17 ⫻ 2)
⫽ 151.4 mg/L
(b) Determine the mass of Ca
2⫹
in Ca
5
(PO
4
)
3
(OH)
Mass of Ca in Ca
5
(PO
4
)
3
(OH) ⫽ 5 (40 mg/mM) ⫻ 0.08 mM/L
⫽ 16 mg/L
(c) Calculate the mass of total Ca present in CaCO
3
Ca in CaCO
3
⫽ Ca in Ca(OH)
2
⫹ Ca in influent
⫺ Ca in Ca
5
(PO
4
)
3
(OH) ⫺ Ca in effluent
⫽ (151.4 ⫹ 62 ⫺ 16 ⫺ 20) mg/L
⫽ 177.4 mg/L
(d) Convert the mass of Ca as expressed in CaCO
3
Mass of Ca as CaCO
3
⫽ 177.4 mg/L ⫻ (40 ⫹ 12 ⫹ 48)/40
⫽ 443.5 mg/L
Step 3. Determine the mass of Mg(OH)
2
formed
(a) Calculate the mass of Mg removed
Mole of Mg
2⫹
removed ⫽ 3 mg/L/(24.3 g/mol ⫻ 1000 mg/g)
⫽ 0.123 ⫻ 10
⫺3
mol/L
⫽ 0.123 mM/L
(b) Calculate the mass of Mg(OH)
2
formed
Mass of Mg(OH)
2
⫽ 0.123 mM/L ⫻ (24.3 ⫹ 17 ⫻ 2) mg/mM
⫽ 7.2 mg/L
Step 4. Calculate the total mass of residue removed as a result of the lime
application
(a) Mass of residue due to lime dosage
Chemical mass ⫽ sum of mass from Ca
5
(PO
4
)
3
(OH), CaCO
3
, and Mg (OH)
2
⫽ (40.2 ⫹ 443.5 ⫹ 7.2) mg/L ⫻ 37,850 m
3
/d
⫽ 490.9 g/m
3
⫻ 37,850 m
3
/d
⫽ 18,580,000 g/d
⫽ 18,580 kg/d
766 Chapter 6
(b) Calculate the mass of SS removed
Mass of SS ⫽ (20 – 2) mg/L ⫻ 37,850 m
3
/d
⫽ 681 kg/d
(c) Calculate the total mass of residues
Total mass ⫽ mass of chemical residue ⫹ mass of SS
⫽ 18,580 kg/d ⫹ 681 kg/d
⫽ 19,261 kg/d
Step 5. Determine total volume V of residue from lime precipitation process
Given: specific gravity of residue ⫽ 1.07, moisture content of residue ⫽ 92%, then
Solid content ⫽ 8%
⫽ 225 m
3
/d
⫽ 7945 ft
3
/d
Phosphorus removal by biological processes. Numerous biological meth-
ods of removing phosphorus have been developed. Integrated biological
processes for nutrient removal from wastewater use a combination of
biological and chemical methods to bring phosphorus and nitrogen con-
centrations to below the effluent standards. Some patented (17 years)
integrated biological processes have been installed in the United States.
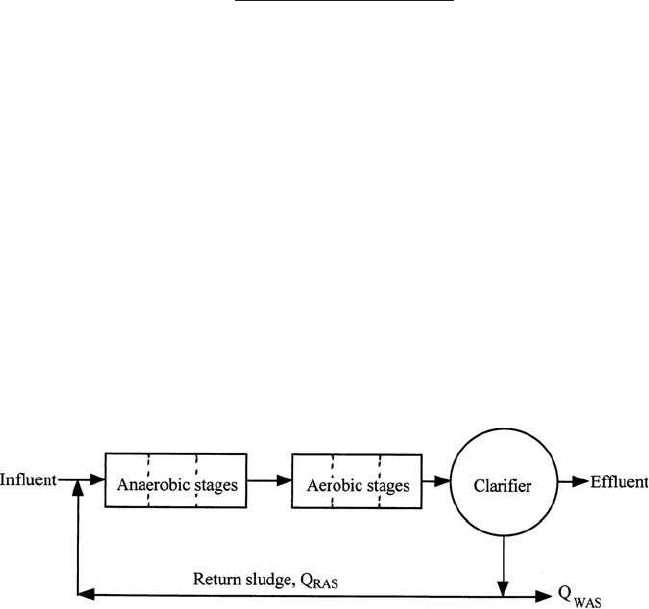
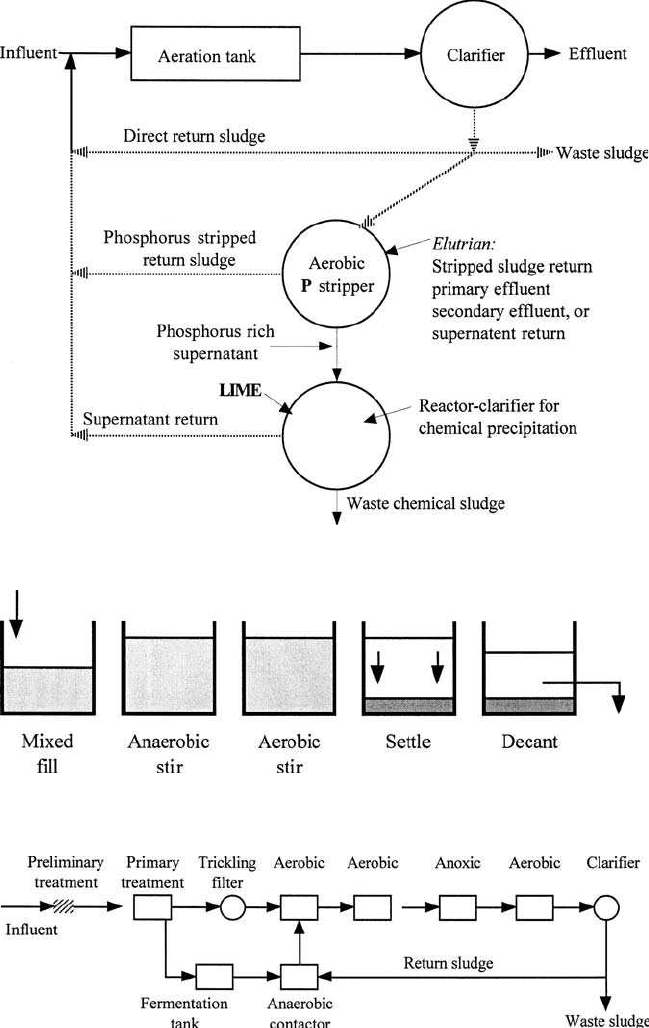
The A/O process (Fig. 6.38) for mainstream P removal, Phostrip process
(Fig. 6.39) for sidestream P removal, sequencing batch reactor (SBR,
Fig. 6.40), sidestream fermentation process (OWASAnitrification, Fig. 6.41),
and chemical polishing are used for phosphorus removal. Combined
removal of phosphorus and nitrogen by biological methods includes the
V 5
19,261 kg/d
1000 kg/m
3
3 1.07 3 0.08
Figure 6.38 A/O process.
Wastewater Engineering 767
Figure 6.39 PhoStrip process.
Figure 6.40 Sequencing batch reactor for removal of phosphorus and carbonaceous
BOD.
Figure 6.41 OWASA nitrification process.
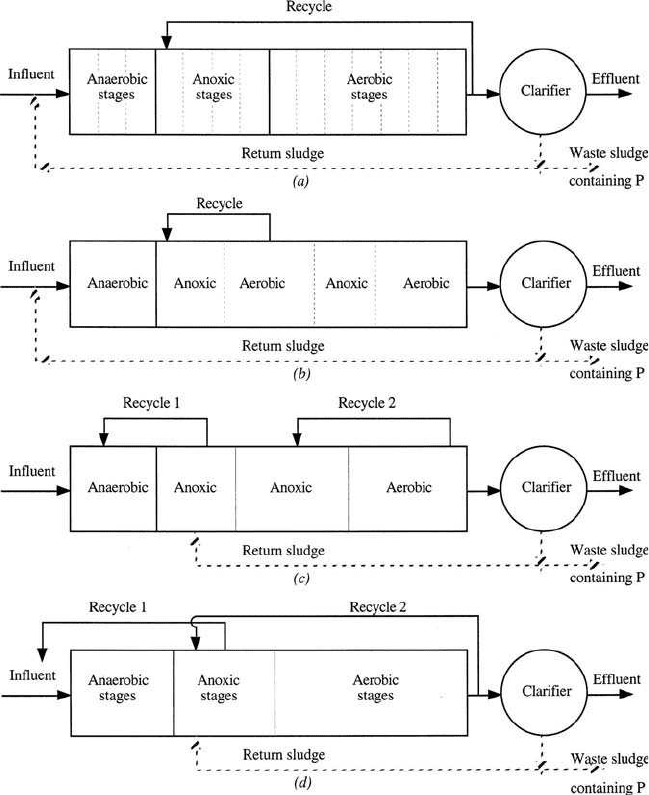
Figure 6.42 Combined biological nitrogen and phosphorus removal processes: (a) A
2
/O
process; (b) five-stage Bardenpho process; (c) UCT process; and (d) VIP process (source:
Metcalfe and Eddy, Inc. 1991).
A
2
/O process (Fig. 6.42a), the modified (5-stage) Bardenpho process
(Fig. 6.42b), the University of Cape Town (UCT) process (Fig. 6.42c), the
VIP process (Virginia Institute Plant in Norfolk, Virginia) (Fig. 6.42d),
PhoStrip II process (Fig. 6.43), SBR (Fig. 6.44), and phased isolation
ditch. For nitrogen control, biological denitrification includes the
Wuhrmann process (Fig. 6.45), Ludzack–Ettinger process (Figs. 6.46
768 Chapter 6
Wastewater Engineering 769
769 Chapter 3
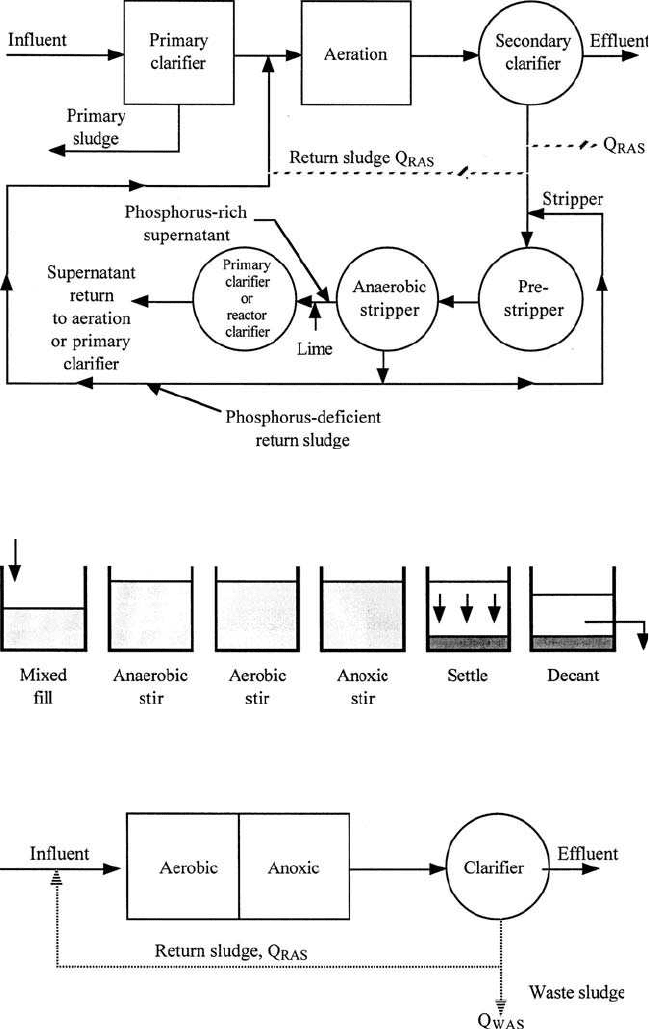
Figure 6.43 PhoStrip II process for phosphorus and nitrogen removal.
Figure 6.44 Sequencing batch reactor for carbon oxidation plus phosphorus and nitrogen
removal.
Figure 6.45 Wuhrmann process for nitrogen removal.
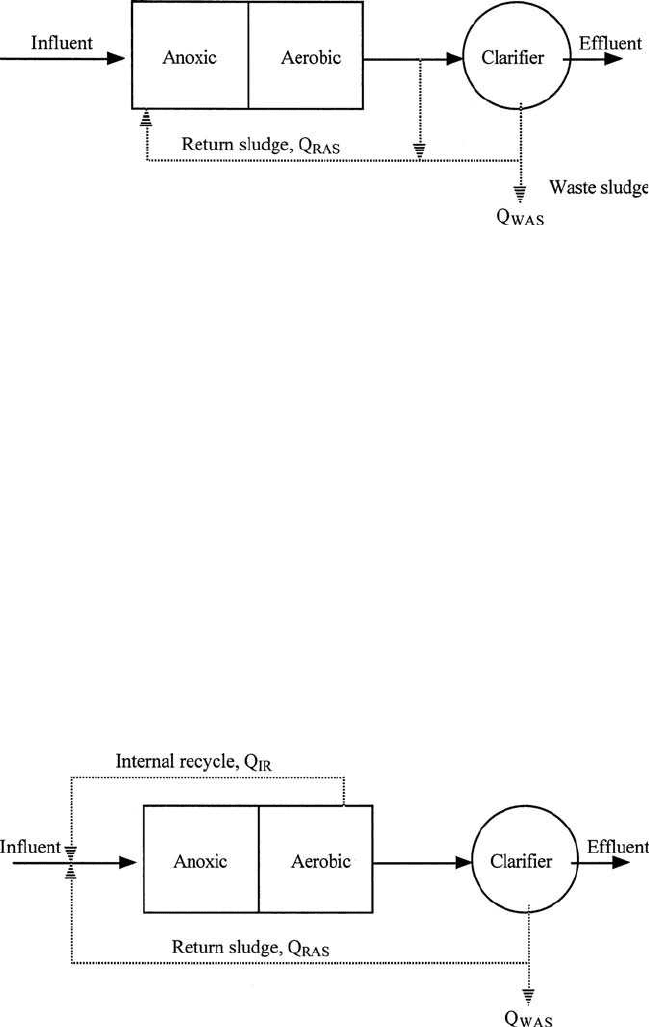
770 Chapter 6
and 6.47), Bordenpho (4-stage) process, oxidation ditch, phase isolation
ditch, dual-sludge process (Fig. 6.48), triple-stage process (Fig. 6.49),
denitrification filter, RBC, and fluidized bed process. The basic theory,
stoichiometry, kinetics, design considerations, and practice of integrated
systems in the US are discussed in detail elsewhere (WEF and ASCE
1991b, Metcalf and Eddy, Inc. 1991).
Biological processes for nutrient removal feature the exposure of alter-
nate anaerobic and aerobic conditions to the microorganisms so that their
uptake of phosphorus will be above normal levels. Phosphorus is not only
utilized for cell synthesis, maintenance, and energy transport, but is also
stored for subsequent use by the microorganisms. The sludge produced con-
taining phosphorus is either wasted or removed through a sidestream
(return sludge stream). The alternate exposure of the microorganisms to
anaerobic and aerobic (oxic) conditions in the main biological treatment
is called the mainstream process. Adesign example (Ex. 15.2) is illustrated
Figure 6.46 Ludzack–Ettinger process for nitrogen removal.
Figure 6.47 Modified Ludzack–Ettinger process for nitrogen removal.
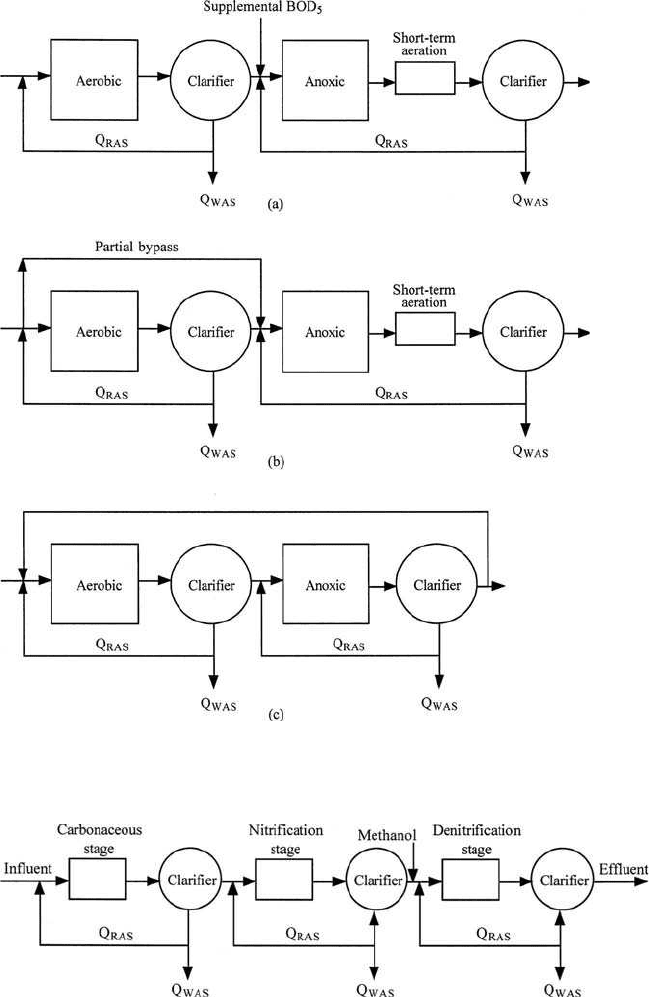
Wastewater Engineering 771
Figure 6.48 Dual-sludge processes for nitrogen removal (source: Grady and
Lim, 1980).
Figure 6.49 Triple-sludge process for nitrogen removal.
772 Chapter 6
by WEF and ASCE (1991b); this uses the activated-sludge kinetic model
developed by Lawrence and McCarty (1970), combined with empirical
equations for complete denitrification using a four-stage Bardenpho
process.
28.3 Nitrogen control
Nitrogen (N) in wastewater exists commonly in the form of organic,
ammonia, nitrite, nitrate, and gaseous nitrogen. The organic nitrogen
includes both soluble and particulate forms. The soluble organic nitro-
gen is mainly in the form of urea and amino acids. The main sources are
from human excreta, kitchen garbage, and industrial (food processing)
wastes. Typical domestic wastewater contains 20 mg/L of organic nitro-
gen and 15 mg/L of inorganic nitrogen.
Environmental effects. Nitrogen compounds, particularly ammonia, will
exert a significant oxygen demand through biological nitrification and
may cause eutrophication in receiving waters. Ammonia (unionized)
can be toxic to aquatic organisms and it readily reacts with chlorine
(affecting disinfection efficiency). A high nitrate (NO
3
) level in water sup-
plies has been reported to cause methemoglobinemia in infants. The
need for nitrogen control in wastewater effluents has generally been
recognized.
Treatment process. Many treatment processes have been developed
with the specific purpose of transforming nitrogen compounds or remov-
ing nitrogen from the wastewater stream.
Conventional processes. In conventional treatment processes, the con-
centration of inorganic nitrogen is not affected by primary sedimen-
tation and is increased more than 50% to 24 mg/L after secondary
(biological) treatment. The overall primary and secondary treatment
removes 25% to 75% (5 to 15 mg/L) of organic nitrogen. Typically,
about 14% and 26% of total nitrogen from raw wastewater are
removed by conventional primary and secondary treatment processes,
respectively.
Biological processes remove particulate organic nitrogen and trans-
form some to ammonium and other inorganic forms. A fraction of the
ammonium present will be assimilated into organic materials of cells.
Soluble organic nitrogen is partially transformed to ammonium by
microorganisms, with 1 to 3 mg/L of organic nitrogen remaining insol-
uble in the secondary effluent.
Advanced processes. Advanced wastewater treatment processes
designed to remove wastewater constituents other than nitrogen often
remove some nitrogen compounds as well. Removal is usually limited
to particulate forms, and the removal efficiency is not high.
Tertiary filtration removes the suspended organic nitrogen from the
secondary effluent. However the majority of nitrogen is inorganic
(ammonium). Reverse osmosis and electrodialysis can be used as a ter-
tiary process for ammonium removal. Their effectiveness is 80% and
40%, respectively. In practice, RO and electrodialysis are not used for
wastewater treatment.
Chemical coagulation for phosphorus removal also removes particu-
late organic nitrogen. The process for nitrogen control may be divided
into two categories, i.e. nitrification and nitrification–denitrification,
depending on the quality requirements of wastewater effluent. The
nitrification process is the oxidation of organic and ammonia nitro-
gen (NH
3
-N) to nitrate, a less objectionable form; it is merely the con-
version of nitrogen from one form to another form in the wastewater.
Denitrification is the reduction of nitrate to nitrogen gas, constitut-
ing a removal of nitrogen from wastewater. Nitrification is used only
to control NH
3
-N concentration in the wastewater. Nitrification–
denitrification is employed to reduce the total level of nitrogen in the
effluent.
There are several methods of nitrogen control. They are biological
nitrification–denitrification, break-point chlorination, selective ion
exchange, and air (ammonia) stripping. This section is mainly devoted
to biological nitrification or biological technologies, since most processes
have been discussed previously in Chapter 5.
Biological nitrification can be achieved in separate stage processes
following secondary treatment (most cases) or in combination for
carbon oxidation–nitrification and for carbon oxidation–nitrification–
denitrification. The secondary effluent with its high ammonia con-
tent and low BOD provides greater growth potential for the nitrifiers
relative to the heterotrophic bacteria. The nitrification process is
operated at an increased sludge age to compensate for lower tem-
perature. After the nitrifiers have oxidized the ammonia in the aer-
ation tank, the activated sludge containing a large fraction of nitrifiers
is settled in the final clarifier for return to the aeration tank and for
waste.
Nitrification reaction. Biological nitrification is an aerobic autotrophic
process in which the energy for bacterial growth is derived from the oxi-
dation of inorganic compounds, primarily ammonia nitrogen. Autotrophic
nitrifiers, in contrast to heterotrophs, use inorganic carbon dioxide
instead of organic carbon for cell synthesis. The yield of nitrifier cells per
unit of substrate metabolized is many times smaller than that for het-
erotrophic bacteria.
Wastewater Engineering 773
774 Chapter 6
Although a variety of nitrifying bacteria exist in nature, the two
genera associated with biological nitrification are Nitrosomonas and
Nitrobacter. The oxidation of ammonia to nitrate is a two-step process
requiring both nitrifiers for the conversion. Nitrosomonas oxidizes
ammonia to nitrite, while Nitrobacter subsequently transforms nitrite
to nitrate. The respective oxidation reactions are as follows:
Ammonia oxidation:
⫹ 1.5O
2
⫹ 2HC N ⫹ 2H
2
CO
3
⫹ H
2
O (6.179)
Nitrite oxidation:
N ⫹ 0.5O
2
N (6.180)
Overall reaction:
⫹ 2O
2
⫹ 2HC ⫹ 2H
2
CO
3
⫹ H
2
O (6.181)
Note: The above three equations are essentially the same as Eqs. (1.87),
(1.88), and (1.89), respectively.
To oxidize 1 mg/L of NH
3
-N, theoretically 4.56 mg/L of oxygen is
required when synthesis of nitrifiers is neglected. As ammonia is
oxidized and bicarbonate is utilized; nitrate is formed and carbonic
acid is produced. The carbonic acid will further depress the pH of the
wastewater. Theoretically, alkalinity of 7.14 mg/L CaCO
3
is con-
sumed for each mg/L of NH
3
-N oxidized. The destruction of alkalin-
ity and increase of carbonic acid results in a drop in pH of the
wastewater.
Reviews of the synthesis and energy relationship associated with
biological nitrification are available elsewhere (Painter, 1970, 1975;
Haug and McCarty, 1972; US EPA, 1975). Overall oxidation and
synthesis–oxidation reactions of ammonia are presented below (US
EPA, 1975c):
55NH
4
⫹
⫹ 76O
2
⫹ 109HCO
3
⫺
: C
5
H
7
NO
2
⫹ 54NO
2
⫺
⫹ 57H
2
O ⫹ 104H
2
CO
3
Nitrosomonas (6.182)
and
400NO
2
⫺
⫹ NH
4
⫹
⫹ 4H
2
CO
3
⫹ HCO
3
⫺
⫹ 195O
2
: C
5
H
7
NO
2
⫹ 3H
2
O ⫹ 400NO
2
⫺
Nitrobacter (6.183)
NO
2
3
Nitrifiers
h
O
2
3
NH
1
4
O
2
3
Nitrobacter
h
O
2
2
O
2
2
Nitrosomonas
h
O
2
3
NH
1
4
