Lin S.D. Water and Wastewater Calculations Manual
Подождите немного. Документ загружается.

Wastewater Engineering 755
The molar ratio of ferric ion to orthophosphate is 1 to 1. Similarly to
alum, a greater amount of iron is required to precipitate phosphorus and
to react with alkalinity in wastewater. Its competing reaction with nat-
ural alkalinity is
FeCl
2
⭈ 6H
2
O ⫹ S Fe(OH)
3
T ⫹3Cl
–
⫹ 3CO
2
⫹ 6H
2
O (6.173)
The stoichiometric weight ratios of Fe:P is 1.8:1. Weight ratio of lime
(CaO) to phosphorus is 2.2:1. The reaction of Eq. (6.173) is slow.
Therefore, lime or some other alkali may be added to raise the pH and
supply hydroxyl ion for better coagulation. The reaction is
2FeCl
3
⭈ 6H
2
O ⫹ 3Ca(OH)
2
S 2Fe(OH)
3
T ⫹ 3CaCl
2
⫹ 12H
2
O (6.174)
Both ferric (Fe
3⫹
) and ferrous (Fe
2⫹
) ions are used in the precipitation
of phosphorus. Ferrous sulfate application for phosphorus removal is
similar to that of ferric sulfate.
Calcium ion reacts with phosphate ion in the presence of hydroxyl ion
to form hydroxyapatite. Lime usually comes in a dry form, calcium
oxide. It must be mixed with water to form a slurry (calcium hydroxide,
Ca(OH)
2
) in order to be fed to a wastewater. Equipment required for lime
precipitation includes a lime-feed device, mixing chamber, settling tank,
and pumps and piping.
Phosphorus removal in primary and secondary plants. In conventional pri-
mary and secondary treatment of wastewater, phosphorus removal is
only sparingly undertaken because the majority of phosphorus in
wastewater is in soluble form. Primary sedimentation removes only
5% to 10% of phosphorus. Secondary biological treatment removes
10% to 20% of phosphorus by biological uptake. The effectiveness of
primary and secondary treatment without (as in most conventional
plants) and with chemical addition for phosphorus removal as well as
for SS and BOD removals is presented in Table 6.22. Generally, the
3HCO
2
3
TABLE
6.22 Efficiencies of Primary and Secondary Treatment Without and with
Mineral Addition for Phosphorus and Other Constituents Removal
Phosphorus Suspended solids
removal % removal, % BOD removal, %
Treatment process Without With Without With Without With
Primary 5–10 70–90 40–70 60–75 25–40 40–50
Secondary
Activated sludge 10–20 80–95 85–95 85–95 85–95 85–95
Trickling filter 10–20 80–95 70–92 85–95 80–90 80–95
RBC* 8–12
*RBC ⫽ rotating biological contactor.
SOURCE: US EPA 1976

756 Chapter 6
total phosphorus concentration of 10 mg/L in the raw wastewater is
reduced to about 9 mg/L in the primary effluent and to 8 mg/L in the
secondary effluent.
Example 1: Alum is added in the aeration basin of a conventional activated-
sludge process for phosphorus removal. The effluent limit for total phosphorus
is 0.5 mg/L. The alum is dosed at 140 mg/L. The average total phosphorus con-
centrations are 10.0 and 9.0 mg/L respectively for the influent and effluent
(influent of the aeration basin) of the primary clarifier. Determine the molar ratio
of aluminum to phosphorus and the weight ratio of alum dosed to the phosphorus
content in the wastewater. Estimate the amount of sludge generated, assum-
ing 0.5 mg/L of biological solids are produced by 1 mg/L of BOD reduction and
using the following given data for the aeration basin (with alum dosage).
Influent BOD ⫽ 148 mg/L
Effluent BOD ⫽ 10 mg/L
Influent TSS ⫽ 140 mg/L Effluent
TSS ⫽ 12 mg/L
Also estimate the increase of sludge production of chemical–biological treat-
ment compared to biological treatment, assuming 30 mg/L for both TSS and
BOD in the conventional system.
solution:
Step 1. Compute the molar ratio of Al to P
Molecular wt of Al
2
(SO
4
)
3
⭈ 18H
2
O ⫽ 27 ⫻ 2 ⫹ 3(32 ⫹ 16 ⫻ 4) ⫹ 14(2 ⫹ 16)
⫽ 666
Alum dosage ⫽ 140 mg/L
Aluminum dosage ⫽ 140 mg/L (2 ⫻ 27/594)
⫽ 12.7 mg/L
Molar ratio of Al:P ⫽ 12.7 mg/L : 9.0 mg/L
⫽ 1.4 : 1
Step 2. Compute the weight ratio of alum dosed to P in the influent
(6.174)
Step 3. Estimate sludge residue for TSS removal
TSS removed ⫽ 140 mg/L ⫺ 12 mg/L ⫽ 128 mg/L
Step 4. Estimate biological solids from BOD removal
Biological solids ⫽ 0.5 (148 mg/L ⫺ 10 mg/L)
⫽ 69 mg/L
Alum dosed
P in the influent
5
140” mg/L
9.0 mg/L
5
15.6
1

Wastewater Engineering 757
Step 5. Estimate organic P in biological solids
Assuming organic P in biological solids removed is 2% by weight,
P in biological solids ⫽ 69 mg/L ⫻ 0.02
⫽ 1.4 mg/L
Step 6. Compute P removed by alum precipitation
P removed by alum ⫽ P in (influent ⫺ biological solids ⫺ effluent)
⫽ (9.0 ⫺ 1.4 ⫺ 0.5) mg/L
⫽ 7.1 mg/L
Step 7. Compute AlPO
4
precipitate
Note: M.wt. ⫽ molecular weight
Step 8. Compute unused alum
Refer to Eq. (6.167) to find alum used
Unused alum ⫽ alum dosed – alum used ⫽ 140 mg/L – 76.3 mg/L
⫽ 63.7 mg/L
Step 9. Compute Al(OH)
3
precipitate
Referring to Eq. (6.172), the unused alum reacts with natural alkalinity.
5 14.9
mg/L
5
63.7 mg/Ls2 3 s27 1 17 3 3dd
666
AlsOHd
3
precipitate
5
sunused alumds2 3 m.wt. of AlsOHd
3
d
M.wt. of alum
5 76.3 mg/L
5
7.1 mg/L 3 666
2 3 31
Alum used 5
sP removeddsM.wt. of alumd
2 3 M.wt. of P
5 27.9 mg/L
5
7.1 mg/L s27 1 31 1 16 3 4d
31
AlPO
4
precipitate 5
sP removed by alumd sM.wt. of AlPO
4
d
sM.wt. of Pd
758 Chapter 6
Step 10. Compute total sludge produced in the secondary clarifier
Total sludge ⫽ TSS removed ⫹ biological solids ⫹ AlPO
4
T ⫹ Al(OH)
3
T
⫽ (128 ⫹ 69 ⫹ 27.9 ⫹ 14.9) mg/L
⫽ 239.8 mg/L
Step 11. Estimate sludge generated from the conventional activated-sludge
system
TSS removal ⫽ 140 mg/L – 30 mg/L ⫽ 110 mg/L
Biological sludge ⫽ 0.5 (148 mg/L – 30 mg/L)
⫽ 59 mg/L
Total sludge ⫽110 mg/L ⫹ 59 mg/L
⫽ 169 mg/L
Step 12. Increase of sludge production by chemical–biological process over
biological process
Increase ⫽ 243.8 mg/L – 169 mg/L
⫽ 74.8 mg/L
% increase ⫽ 74.8 mg/L ⫻ 100%/169 mg/L
⫽ 44.3%
Example 2: A wastewater has a soluble phosphorus concentration of 7.0 mg/L
as P. Determine the stoichiometric quantity of ferric chloride required to
remove P completely.
solution:
Step 1. Calculate the gram molecular weights
Equation (6.169) shows that 1 mole of ferric chloride reacts with 1 mole of
orthophosphate to be removed. The pertinent gram molecular weights for
FeCl
3
and P are:
FeCl
3
⫽ 55.845 ⫹ 35.453 ⫻ 3 ⫽ 162.204 (g)
P ⫽ 30.975 g
Step 2. Calculate the stoichiometric amount (Y ) of FeCl
3
required
Y ⫽ 7.0 mg/L (162.204 g/30.974 g)
⫽ 36.66 mg/L
Notes: In practice, the actual FeCl
3
dose would be 1.5 to 3.0 times the stoi-
chiometric amount. Likewise, the alum dose would be 1.3 to 2.5 times the sto-
ichiometric amount due to side reaction, solubility products limitations, and
wastewater quality variations. Jar tests on wastewater are needed for deter-
mining the actual amount.
Wastewater Engineering 759
Phosphorus removal by mineral addition to secondary effluent. Generally,
an alum dosage of about 200 mg/L is required for phosphorus removal
from typical municipal raw wastewater and a dosage of 50 to 100 mg/L
is sufficient for secondary effluent. Iron salts have little application
because of residual iron remaining in the treated wastewater. An Al : P
molar ratio of 1 : 1 to 2 : 1 is required. The optimum pH for alum
treatment is near 6.0, whereas that for iron is near 5.0 (Recht and
Ghassemi, 1970). The pH of high alkalinity waters may be reduced
either by using high dosages of alum or by adding supplementary
dosages of sulfuric acid. Anionic polyelectrolytes (coagulant aids) can
be used to enhance P removal. If higher phosphorus removal is
required, a filtration process must be used to achieve 0.1 mg/L of
residual P.
The surface overflow rates for settling tanks range from 24 to 58
m
3
/(m
2
⭈ d) (580 to 1440 gal/(d ⭈ ft
2
)). Filtration rates are 0.08 to 0.20
m
3
/(m
2
⭈ min) (2 to 5 gal/(min ⭈ ft
2
)) (US EPA, 1976).
Example: Estimate the daily liquid alum requirement for phosphorus
removal from the secondary effluent which has an average design flow of
1 Mgal/d (3785 m
3
/d) and an average phosphorus concentration of 8 mg/L.
Assume that the specific weight of alum is 11.1 lb/gal and alum contains
4.37% of aluminum.
solution:
Step 1. Calculate the mass of the incoming P loading rate
Load ⫽ 1 Mgal/d ⫻ 8 mg/L ⫻ 8.34 lb/(Mgal ⭈ mg/L)
⫽ 66.7 lb/d
Step 2. Determine aluminum in mol/d required
Atomic weights of phosphorus and aluminum are 31 and 27, respectively.
P load ⫽ 66.7 lb/d ⫼ 31 lb/mol
⫽ 2.15 mol/d
Usually a molar ratio of Al : P ⫽ 2 : 1 is used, refering to Eq. (6.167)
Al required ⫽ 2.15 mol/d ⫻ 2
⫽ 4.3 mol/d
Step 3. Determine liquid alum required
Mass of Al ⫽ 4.3 mol/d ⫻ 27 lb/mol
⫽ 116.1 lb/d

760 Chapter 6
Note: Or this can be solved simply as:
Al required ⫽ 66.7 lb/d ⫻ 54/31
⫽ 116.1 lb/d
Using liquid alum having 4.37% of Al, with 11.1 lb/gal
⫽ 239 gal/d ⫽ 0.166 gal/min
⫽ 0.628 L/min
Phosphorus removal by lime treatment of secondary effluent. For phosphorus
removal, lime can be added to the primary sedimentation tank and to
the secondary effluent. Lime treatment of wastewater is essentially the
same process as is used for lime softening of drinking water, but with a
different purpose. While softening may occur, the primary objective is
to remove phosphorus by precipitation as hydroxyapatite (Eq. (6.171)).
During phosphorus precipitation, a competing reaction of lime with
alkalinity will occur. This reaction results in calcium removal, an action
of softening which has a very important effect on the phosphorus
removal efficiency of the process. This reaction may occur in two ways:
Ca(OH)
2
⫹ Ca(HCO
3
)
2
S 2CaCO
3
T ⫹ 2H
2
O (6.175)
Ca(OH)
2
⫹ NaHCO
3
S CaCO
3
T ⫹ NaOH ⫹ H
2
O (6.176)
Another reaction may occur to precipitate magnesium hydroxide:
Mg
2⫹
⫹ 2OH
–
S Mg(OH)
2
T (6.177)
In the two-stage lime treatment process, recarbonation after the first
stage is required to reduce the pH and to precipitate excess lime as cal-
cium carbonate by adding carbon dioxide. The chemical reaction is
Ca
2⫹
⫹ CO
2
⫹ 2OH
–
S CaCO
3
⫹ H
2
O (6.178)
Lime can be added to secondary effluent by single-stage or two-stage
treatment. For the single-stage process (Fig. 6.36), lime is mixed with
feed wastewater to raise the pH (10–11); this is then followed by floc-
culation and sedimentation. The clarified water is adjusted to lower pH
with carbon dioxide and is filtered through a multimedia filter to pre-
vent postprecipitation of calcium carbonate before discharge. The set-
tled lime sediment may be disposed of to a landfill or may be recalcined
for recovery of lime. In recovery, the sediment is thickened, dewatered
by centrifuge or vacuum filter, calcined, and then reused.
Liquid alum required 5
116.1 lb/d
0.0437s11.1 lb/gald
Al required
P load
5
2 3 27
31
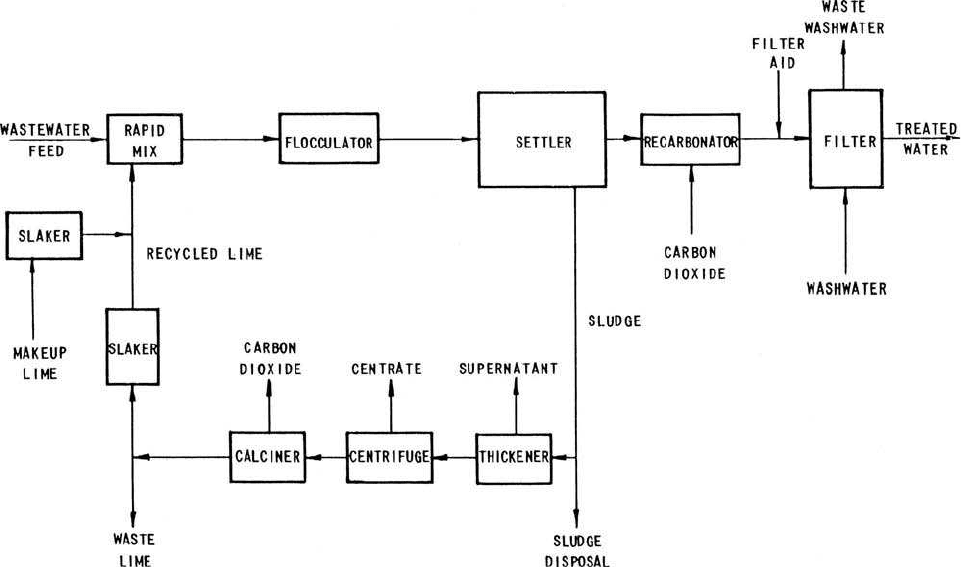
Figure 6.36 Single-stage lime treatment process for phosphorus removal (source: US EPA, 1976 ).
761
762 Chapter 6
For a typical two-stage process (Fig. 6.37), in the first-stage clarifier
of the two-stage process, sufficient lime is dosed to raise the pH above
11 to precipitate the soluble phosphorus as hydroxyapatite, calcium car-
bonate, and magnesium hydroxide.
The calcium carbonate precipitate formed in the process acts as a
coagulant for suspended solids removal. The excess soluble calcium is
removed in the second clarifier as a calcium carbonate precipitate by
adding carbon dioxide gas to reduce the pH to about 10. The calcium car-
bonate settles out in the secondary (or tertiary) clarifier. To remove the
residual levels of phosphorus and suspended solids, the secondary (or
tertiary) clarified effluent is filtered, then discharged. Usually pH is
reduced to about 8.0 and 8.5 in first-stage recarbonation and further
reduced to 7.0 in second-stage recarbonation. Fifteen minutes’ contact
time in the recarbonation basin is recommended.
Typical hydraulic loading rates for chemical treatment clarifiers are
32 to 61 m
3
/(m
2
⭈ d) (800 to 1500⬚ gal/(d ⭈ ft
2
)).
Example 1: From the results of jar tests, calcium concentration drops from
56 to 38 mg/L when the pH is reduced from 10 to 7 by addition of carbon diox-
ide. Determine the carbon dioxide dose rate required for recarbonation.
solution:
Step 1. Determine theoretical Ca : CO
2
ratio, using Eq. (6.178)
Ca
2⫹
⫹ CO
2
⫹ 2OH
–
S CaCO
3
T ⫹ H
2
O
then
CO
2
: Ca ⫽ (12 ⫹ 2 ⫻ 16) : 40
⫽ 1.1 : 1
Step 2. Calculate CO
2
theoretical requirement
CO
2
required : Ca reduction ⫽ 1.1 : 1
CO
2
⫽ (56 – 38) mg/L ⫻ 1.1
⫽ 19.8 mg/L
≅ 20 mg/L
Step 3. Calculate field requirement of CO
2
A safe factor of 20% should be added to calculated dosage to compensate for
inefficiency in absorption.
CO
2
required ⫽ 20 mg/L ⫻ 1.2
⫽ 24 mg/L
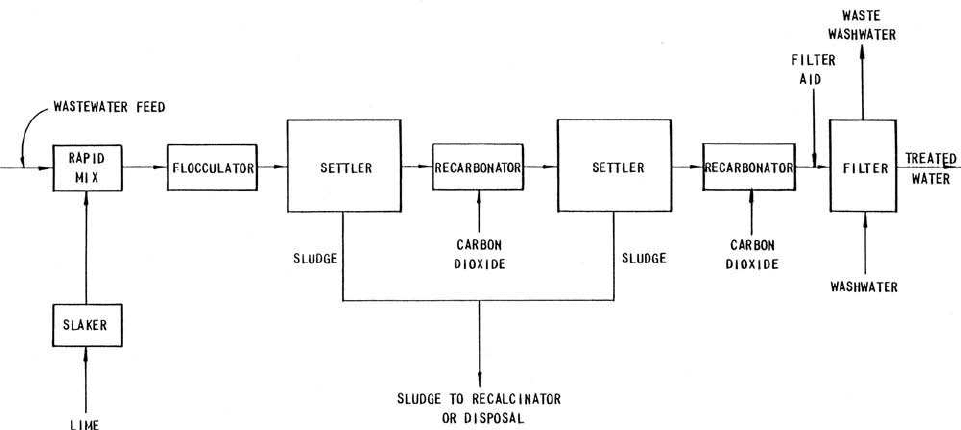
Figure 6.37 Two-stage lime treatment process for phosphorus removal (source: US EPA, 1976 ).
763
Example 2: A single-stage lime treatment system is used as advanced treat-
ment for phosphorus removal. Estimate the residue mass and volume gen-
erated in the clarifier under the following conditions.
Influent design flow ⫽ 37,850 m
3
/d (10 Mgal/d)
Lime dosage as Ca(OH)
2
⫽ 280 mg/L
Influent as P ⫽ 8 mg/L
Influent Ca
2⫹
⫽ 62 mg/L
Influent Mg
2⫹
⫽ 3 mg/L
Effluent as P ⫽ 0.6 mg/L
Effluent Ca
2⫹
⫽ 20 mg/L
Effluent Mg ⫽ 0 mg/L
Influent suspended solids ⫽ 20 mg/L
Effluent suspended solids ⫽ 2 mg/L
Specific gravity of residue ⫽ 1.07
Moisture content of residue ⫽ 92%
solution:
Step 1. Compute the mass of Ca
5
(PO
4
)
3
(OH) formed
(a) Calculate the moles of P removed
⫽ 0.239 ⫻ 10
–3
mol/L
⫽ 0.239 mM/L
where mM ⫽ millimol
(b) Calculate the moles of Ca
5
(PO
4
)
3
(OH) formed
From Eq. (6.171), 3 moles of P removed will form 1 mole of Ca
5
(PO
4
)
3
(OH)
Mole of Ca
5
(PO
4
)
3
(OH) formed ⫽ 1/3 ⫻ 0.239 mM/L
⫽ 0.080 mM/L
(c) Calculate the mass of Ca
5
(PO
4
)
3
(OH) formed
Mass of 1 mole Ca
5
(PO
4
)
3
(OH) ⫽ 40 ⫻ 5 ⫹ 30.97 ⫻ 3 ⫹ 16 ⫻ 13 ⫹ 1 g/mol
⫽ 501.9 g/mol
⫽ 501.9 mg/mM
Mass of Ca
5
(PO
4
)
3
(OH) formed ⫽ 0.08 mM/L ⫻ 501.9 mg/mM
⫽ 40.2 mg/L
Mole of P removed 5
8 mg/L 2 0.6 mg/L
30.974 g/mol 3 1000 mg/g
PO
32
4
PO
32
4
764 Chapter 6
