Lima J.J.Pedroso, de (ed.). Nuclear Medicine Physics
Подождите немного. Документ загружается.


108 Nuclear Medicine Physics
mechanisms may occur to diminish the accumulation of deoxyglucose-6-
phosphate in tissue.
In cancer cells, there is usually an increase in the GLUT expression that
favors an increased uptake of glucose into the tissue. There arealso several key
enzymes in glycolysis that have their activity enhanced in tumor processes,
including hexokinase, the first glycolytic enzyme. These two processes lead
to an increase in deoxyglucose uptake and consequent entrapment, which
provide the physiological basis for the widely successful use of [
18
F]FDG in
oncology.
4.8 SPECT Radionuclides
Currently,
99m
Tc is the most widely used diagnostic radionuclide and is
responsible for more than 80% of diagnostic scans done each year in hospitals
and clinics. However,a large number of other radioisotopes have found appli-
cation, both in diagnostics and in therapy [30–34]. Table 4.5 summarizes some
clinically used radionuclides, their physical characteristics, and their produc-
tion methods. The most important properties of a radionuclide to be used
in clinical applications are its half-life and decay mode, the energy of the
emitted radiation, and, at least as important, the cost and ease of its produc-
tion. Radionuclide half-lives should be long enough to allow production of
the corresponding radiopharmaceutical and to perform the diagnostic scan
but not so long as to increase the patient dosimetry without diagnostic ben-
efits. Its energy should be appropriate for the detection system; for example,
for SPECT cameras the energy of the gamma rays should be between 100
and 250 keV. Outside this range, the images will have poor quality. For lower
energies, most of the photons will be stopped inside the patient’s body, result-
ing in poor statistics, while at the same time increasing the patient’s radiation
dose. At high energies, as a consequence of the low selectivity of the photons
TABLE 4.5
Physical Data for the Most Widely Used Nuclear Medicine Radionuclides and
Their Production Processes
Nuclide t
1/2
Main Emissions (keV) Production
Techenetium-99m 6.01 h γ 141 Generator (
99
Mo/
99m
Tc )
Iodine-123 13.2 h γ 159 Cyclotron (
124
Te(p, 2n)
123
I)
Iodine-131 8.04 d γ 364, β 606 Nuclear fission
Thalium-201 73.1 h γ 167, 135; X68 −82
203
Tl(p,3n)
201
Pb →
201
Tl
Gallium-67 78.3 h γ 300, 181, 93 Cyclotron (
68
Zn(p, 2n)
67
Ga)
Indium-111 2.81 d γ 245, 171 Cyclotron
111
Cd(p,n)
111
In
Xenon-133 5.25 d γ 81; β 46; X 30–36 Nuclear fission
Krypton-81m 13.3 s γ 190 Generator (
81
Rb/
81
Kr)
Radiopharmaceuticals 109
by the collimator, there will be an increase in the scattering and, thus, a loss
of image quality.
Thedecay scheme is also a very importantcriterion to choosean appropriate
radionuclide: For therapeutic purposes, β
−
or α emission is desirable; whereas
for imaging diagnosis, particle emission is a big disadvantage, as it only con-
tributes to the absorbed radiation dose without providing any benefit for the
imaging. A good example is
131
I, which decays by β
−
emission to an excited
nuclear state of
131
Xe, which, in turn, decays by emitting gamma radiation of
various energies, including 384, 637, 284, and 80 keV.
4.8.1 Technetium-99m: Production, Coordination Chemistry,
and Radiopharmaceuticals
In the Periodic Table of elements, technetium (Tc) is the element 43, a transition
metal. Its name comes from the Greek technetos, meaning artificial.
As indicated, technetium in the form of one of its isotopes (Tc-99m) is, by
far, the most widely used radioisotope in NM. This is due both to its excellent
nuclear properties and also to the fact that it can be produced in situ and daily
by a generator (see below).
Radionuclide generators are based on a relatively long-lived parent that
decays to a daughter nuclide, which is itself radioactive but with a short
half-life [72]. The system will be useful for routine applications only if there
is a straightforward method to separate the daughter radionuclide from its
parent. The most common generator system is the Mo-Tc-99m [73], which
is used daily in every NM Service around the world. However, there are
other generator systems, such as Rb-81/Kr-81m, Sr-82/Rb-82, and of growing
importance due to the increasing use of Ga-68 in PET imaging, the generator
system Ge-68/Ga-68 [75–77].
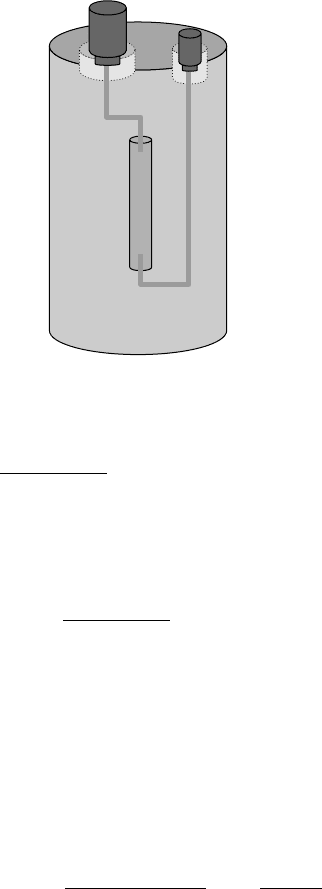
The commercial generator Mo-99/Tc-99m (Figure 4.8) contains radioactive
molybdate (sodium molybdate), adsorbed in an aluminum oxide column. The
99m
Tc, which is continuously formed inside the column, and the molybdate
have different chemical affinities for the alumina, which allows their separa-
tion by column elution with saline solution. The reaction inside the column
follows the scheme:
99
MoO
−
4
(adsorbed in the column) →
99m
TcO
−
4
(free in solution) (4.3)
From consideration of the half-life ratio between
99
Mo (t
1/2
= 66 h) and
99m
Tc (t
1/2
= 6 h), we can easily conclude that this leads to a transient equilib-
rium, with the time or activity curves for the parent or daughter radionuclides
given by
A
[Mo]
= A
0
[Mo]
e
−λ
{Mo}
t
(4.4)
110 Nuclear Medicine Physics
Eluent
Eluted
Column
Lead
protection
FIGURE 4.8
Schematic representation of the
99
Mo-
99m
Tc generator.
A
[Tc ]
= A
0
[Mo]
λ
[Mo]
λ
[Tc ]
−λ
[Mo]
(e
−λ
[Mo]
t
−e
−λ[Tc ]t
) +A
0
[Tc ]
e
−λ
[Tc ]
t
(4.5)
For the case where the initial technetium-99m activity equals zero (after one
elution), the above equations take the form:
A
[Tc ]
= A
0
[Mo]
λ
[Mo]
λ
[Tc ]
−λ
[Mo]
(e
−λ
[Mo]
t
−e
−λ
[Tc ]
t
) (4.6)
The two curves involved can be represented by the diagram given in
Figure 4.9.
The upper curve for
99m
Tc corresponds to the direct application of these
equations; whereas the lower curve takes into account the fact that only 87%
of the decays produce 140 keV γ rays.
It is straightforward to demonstrate that the time at which the maximum
technetium activity is obtained corresponds to
t
[Tc ]
max
=
1.44
t
1/2[Mo]
t
1/2[Tc ]
t
1/2[Mo]
−t
1/2[Tc ]
ln
t
1/2[Mo]
t
1/2[Tc ]
. (4.7)
This corresponds to ca. 22.8 h, which is the main reason that the
99
Mo–
99m
Tc
generators are normally eluted the same time each day to allow an almost
ideal accumulation of pertechnetate. Combining this process with the decay
scheme of the
99m
Tc permits determination of the conditions of maximum
activity in the presence of
99
Tc (Figure 4.10).

Radiopharmaceuticals 111
0
0.2
0.4
0.6
0.8
1
0 20406080100
Time (h)
99
Mo
99m
Tc
Activity
FIGURE 4.9
Activity/time diagrams for
99
Mo and
99m
Tc in the
99
Mo–
99m
Tc generator.
Tc-99m emits 140 keV γ rays with 89% abundance, which makes it almost
ideally suited for imaging with gamma cameras while being safe from the
pointof view ofthe radiation dose given to the patient. It has a veryconvenient
half-life of 6 h, which allows the preparation and quality control of the
99m
Tc-
radiopharmaceuticals and the possibility of performing even a complicated
imaging protocol.
The existence of lyophilized cold kits containing appropriate formulations
for
99m
Tc complexation makes it easy to obtain
99m
Tc radiopharmaceuticals
just by addition of
99m
Tc in the pertechnetate form (
99m
TcO
−
4
) to the kit.
The
99m
Tc is eluted from the generator as an aqueous solution of sodium
pertechnetate (Na
99m
TcO
4
) and has an oxidation number of +7. The
99m
Tc
7+
is unreactive with most of the ligands and needs to be reduced. Tin chlo-
ride and HCl are among the most commonly used agents to reduce the
pertechnetate ion. After being reduced in the presence of suitable ligands,
the technetium bears an oxidation number ranging from +1 to +6, depend-
ing on the reductant and ligand characteristics and the coordination reaction
conditions.
Although the possibility that technetium can exist in all these oxidation
states may make it difficult to control the reactions and also increases the
lability of the complexes formed, on the other hand, it does offer more pos-
sibilities for the modification of the structure and properties of complexes
99
Mo
99m
Tc
99
Tc
66 h
6, 01 h
2, 12 × 10
5
years
Ru (stable)
99m
Tc
FIGURE 4.10
Production and decay scheme of
99m
Tc .
112 Nuclear Medicine Physics
through the choice of the appropriate ligands [35]. A further important char-
acteristic of these technetium compounds is the possibility of the presence of
isomeric structures: geometric isomers, epimers, enantiomers, and diasteri-
oisomers. The presence of isomers, which is more frequent with technetium
oxo-complexes, may have an important impact on the biological properties
of the radiopharmaceuticals, as these isomers often have differences in their
lipophilicity and, as a consequence, in their biodistribution profile [35].
To sum up, technetium can produce a large number of complexes display-
ing different structures, oxidation states, with coordination numbers ranging
from 4 to 7, which introduces a very rich chemistry and a huge number of
synthetic pathways.
A large variety of
99m
Tc-radiopharmaceuticals have been developed and
approved by FDA for diagnosis, whereas a myriad of new ones are at the
preclinical phase. There is virtually no organ function or disease process, from
neuroreceptor imaging to oncology that cannot be visualized in NM using a
99m
Tc-based tracer [57]. Table 4.6 summarizes some of the new developments
in this area. For more information about
99m
Tc-based radiopharmaceuticals
and
99m
Tc chemistry, the readeris referred to excellent comprehensivereviews
and textbooks [31,35,36].
As with other metal-based radiopharmaceuticals,
99m
Tc radiopharma-
ceuticals can be classified in two categories: “technetium essential” and
“technetium tagged.” Technetium essential radiopharmaceuticals are those
in which the Tc is an integral part of the radiopharmaceutical and for which
the molecule would not be delivered to its target in the absence of the Tc. Their
in vivo behavior depends only on their chemical–physical properties, such as
size, charge, and lipophilic or hydrophilic character. The latter class, tech-
netium tagged radiopharmaceuticals, includes those in which the targeting
moiety (e.g., antibody, peptide, and hormone) has been labeled with
99m
Tc,
either directly or by using a bifunctional chelate; and their in vivo local-
ization is mediated by biological interactions with, for example, receptors
or proteins. A bifunctional chelator bears chelator groups that are able to
coordinate the metal, while, at the same time, having functional groups
which allow the radiopharmaceutical to be recognized by a target biologi-
cal molecule. The introduction of a chelator in a bioconjugated molecule can
have a profound effect on its biodistribution, and this will increase with a
decrease of the size of the biomolecule; this will be more pronounced for
small peptides than for larger antibodies. To minimize this effect, it is usual
to introduce a “spacer” to separate the chelator from the bioactive part of the
molecule [45].
Considering
99m
Tc compounds, we can include the following radiopharma-
ceuticals in the first group (where their in vivo behavior depends only on the
complex structure):
99m
Tc-D,L-HM-PAO (Ceretec) and
99m
Tc-LL-ECD (
99m
Tc
coordinatedto N, N
-1,2-ethylenediylbis-L-cysteine diethyl ester—Neurolite),
both of which are used to determine brain–blood flow; the complexes
99m
Tc-
DTPA and
99m
Tc-MAG3 (Technescan) used to evaluate kidney function; and

Radiopharmaceuticals 113
TABLE 4.6
Examples of
99m
Tc-Labeled Ligands as Diagnostic Radiopharmaceuticals
Radiopharmaceutical Chemical and Biological characteristics Application Reference
99m
Tc-TRODAT-1
99m
Tc complex in which a diaminodithiol ligand is complexed with the metal and a
tropane analog is derivatized from one nitrogen. It presents two diastereoisomers syn
on the coordination to TcO
3+
. The human images of
99m
Tc-TRODAT-1 showed
localization at the basal ganglia, which correlates with the binding to the dopamine
transporters (DAT).
Imaging of dopamine
transporters
[37]
99m
Tc BMS 181321 Nitroimidazole-derivatized TcO
3+
amine oxime complexes. Hypoxic tissues can be
differentiated from normal tissues on the basis of their redox profile inside the cell. The
radiopharmaceutical should enter the cell and be reduced in a hypoxic environment.
Hypoxia targeting [38]
99m
Tc-sestamibi A monocationic hexakis-isonitrile (2-methoxy-2-methyl-1-propyl) isonitrile, where
techenetium is present with oxidation number+1. This is a very rare Tc oxidation state,
but the complex is very kinetically stable. The
99m
Tc-sestamibi is a myocardium
perfusion agent, but it was also demonstrated that the
99m
Tc-sestamibi is transported
out of tumor cells expressing MDR by the Pgp glycoprotein, allowing its use for MDR
evaluation.
Evaluation of
myocardium
perfusion; MDR
detection
[39,40]
99m
Tc-annexin V Annexin V labeled with
99m
Tc(CO)
3
(OH
2
)
3
. The annexin V is a protein that binds to the
phosphatidylserine residues present at the membrane of apoptotic cells. It was
demonstrated that the
99m
Tc labeling using the tricarbonile does not modify the cell
affinity of annexin V.
Apoptosis tracer [41]
99m
Tc-P829 Radiopharmaceutical that binds to the somatostatin receptors overexpressed in a large
variety of tumors. The labeling using the precursor
99m
Tc(CO)
3
(OH
2
)
3
does not need
the presence of a reductor species, an advantage as it protects the disulfide peptide
bond that is essential to its receptor recognition.
Neuroendocrin tumor
detection
[42]
99m
Tc- RP419/DMP444 Peptide bearing the RGD (-Arg-Gly-Asp) sequence; it binds to the l GPIIb/IIIa receptors
expressed in the activated platelets, the first step in the thrombus formation.
Thrombosis detection [43]
99m
Tc-ciprofloxacin The ciprofloxacin is an antibiotic. It is not known the coordination mechanism of the
technetium to this ligand and the radiolabeling yield is only ca. 40%.
Bacterial infection
diagnosis
[44]
Note: MDR, multidrug resistance.
114 Nuclear Medicine Physics
99m
Tc-sestamibi (
99m
Tc-hexakis-isonitrile) (Cardiolite) and
99m
Tc-tetrafosmin
([
99m
Tc-(1,2-bis((bis-ethoxyethyl)phosphino)ethane)]
+
) (Myoview) as myoc-
ardium perfusion agents. Pertechnetate itself (as eluted from the generator)
has diverse uses in NM, such as thyroid and parathyroid scintigraphy and
imaging of the salivary glands. Figure 4.11 shows the structures of some of
these technetium-99m complexes. As examples of
99m
Tc-bioconjugates (the
second group mentioned), we can include
99m
Tc-annexin V, for apoptosis
evaluation [38], the
99m
Tc-monoclonal antibody used in CEA-Scan [46], or
some peptide somatostatin analogs labeled with
99m
Tc [42].
N
NN
NN
N
H
Tc
Tc
Tc
S
SS
O
O
O
O
R = CH
2
CH
2
OCH
3
tetrofosmin
RR
RR
RR
RR
P
P
P
P
R
R
R
N
N
N
C
C
C
C
C
C
Tc
N
N
N
R
R
R
R = CH
2
C(CH
3
)
2
OCH
3
sestamibi
O
O
Tc
O
N
O
COOH
ECD
EtO
2
C
CO
2
Et
O
N
N
MAG3
HMPAO
–1
+1
+1
FIGURE 4.11
Chemical structure of some
99m
Tc radiopharmaceuticals:
99m
Tc-Sestamibi,
99m
Tc-tetrafosmin,
99m
Tc-L,L-ECD,
99m
Tc-D,L-HM-PAO, and
99m
Tc-MAG3.
Radiopharmaceuticals 115
4.8.2 Gallium and Indium: Radioisotopes and Their
Coordination Chemistry
There are three gallium radionuclides (
66
Ga,
67
Ga, and
68
Ga) and two indium
radionuclides (
111
In,
113
In) with suitable physical characteristics for use in
gamma scintigraphy or PET [30].
The isotope
67
Ga (t
1/2
= 78.1 h) is produced in a cyclotron by the nuclear
reaction
68
Zn(p,2n)
67
Ga starting with a
68
Zn-enriched sample. The
67
Ga
obtained is then separated either by solvent extraction or by an ionic exchange
process.
68
Ga, a positron emitter, is produced by a
68
Ge or
68
Ga generator and decays
by positron emission in 89% yield. The maximum energy of this positron is
1.899 keV, whereas the average energy per disintegration is 740 keV. The long
half-life of
68
Ge (t
1/2
= 280 days) makes it possible to obtain
68
Ga in situ,
without a cyclotron, although it is necessary to have an efficient method of
separation of
68
Ga from
68
Ge.
68
Ga has a very convenient half-life of 68 min
compatible with the biokinetics and biological half-lives of a lot of low or
average molecular weight radiopharmaceuticals, such as peptides or oligonu-
cleotides [47], thus opening the possibility of developing lyophilized cold kit
formulations. The chemical properties of
68
Ge and
68
Ga are different enough
to permit an efficient separation of the two radionuclides. In the literature,
two main strategies are found to isolate
68
Ga from
68
Ge:
1. Using organic matrices bearing phenolic groups that form very stable
complexes with Ge(IV) and which allow the elution of
68
Ga
3+
as
68
GaCl
−
4
by using HCl as eluent. An alternative methodology uses
an N-methylglucamine-based polymer and a 0.1 M trisodium citrate
solution as eluent [48].
2. With inorganic oxides matrices, such as Al
2
O
3
, SnO
2
,Sb
2
O
5
, ZrO
2
,
and TiO
2
, eluted with HCl or EDTA [49]. Recently, a generator has
been introduced in the market where the
68
Ge or
68
Ga separation
is done on a TiO
2
column using a 0.1 M HCl solution as eluent
(Cyclotron Co, Obninsk, Russia). The reader is directed to excellent
reviews concerning this subject [50,51].
66
Ga is also a positron emitter (t
1/2
= 9.45 h) and is produced in a cyclotron
by the
66
Zn(p,2n)
66
Ga reaction. However, there are very few examples of
application of this radionuclide.
The radioisotope
111
In (t
1/2
= 67.2 h) is produced in a cyclotron, starting
from
111
Cd,
111
Cd(p,n)
111
In and decays by electron capture producing 173 keV
(89%) and 247 keV (95%) photons. The separation of the
111
In from the starting
material,
111
Cd, follows processes similar to those used to isolate
67
Ga.
113m
In (t
1/2
= 1.7 h) obtained with the generator
113
Sn/
113m
In emits γ radi-
ation with energy 393 keV. However, nowadays this radionuclide has been
replaced for most of its applications by
99m
Tc.
116 Nuclear Medicine Physics
The coordination chemistry of gallium and indium is of considerable great
interest, mostly due to the potential use of their radioisotopes in radio-
pharmacy [31,45,52]. Gallium and indium are metals belonging to the III B
group of the periodic table and under physiological conditions they only
exist in the +3 oxidation state. This fact is determinant for their radiochem-
istry. These two metals, together with boron and aluminum, are classified
as “hard acids” [53], and they prefer to form chemical bonds with ionic and
nonpolarizable Lewis bases such as nitrogen and oxygen atoms (carboxylate,
phosphonate, and amino groups). The coordination chemistries of gallium
(III) and iron (III) are very similar; and, as we will see, this fact is crucial in
the use and preparation of gallium radiopharmaceuticals. The coordination
chemistry of In (III), having a larger ionic radius, is comparable with that of
Y (III) and lanthanides.
In solution, the hydrated cations Ga (III) and In (III) are only enough stable
under acidic conditions, hydrolyzing at higher pH values and leading to the
insoluble hydroxides Ga(OH)
3
and In(OH)
3
:
M(H
2
O)
3+
6
+H
2
O → M(OH)(H
2
O)
2+
5
+H
3
O
+
; (4.8)
M(OH)(H
2
O)
2+
5
+H
2
O → M(OH)
2
+(H
2
O)
+
4
+H
3
O
+
; (4.9)
M(OH)
2
+(H
2
O)
+
4
→ M(OH)
3
(s) +H
3
O
+
+3H
2
O. (4.10)
As an example, in a gallium(III) solution containing 1 mCi/mL (which
corresponds to a
68
Ga concentration of 4 ×10
−10
M and to a
67
Ga concen-
tration of 2.5 ×10
−8
M), Ga(OH)
3
precipitation occurs for pH ≥ 3 if there are
no stabilizing ligands present in solution [54]. Even though it is possible to
use gallium-68 hydroxide-based colloids for liver–spleen PET imaging [55],
normally these hydrolysis reactions prevent the preparation of gallium radio-
pharmaceuticals. In contrast with what happens with In(OH)
3
, the hydroxide
Ga(OH)
3
is amphoteric; and in addition to being soluble in acids, it is soluble
and at basic pH through the reaction,
Ga(OH)
3
(s) +OH
−
→ Ga(OH)
−
4
, (4.11)
which allows the redissolution of Ga(OH)
3
.
For their use as radiopharmaceuticals, the compounds of gallium and
indium must be thermodynamically stable at physiological pH values and/or
kinetically inert on the timescale of the procedures used in NM.
4.8.2.1 Gallium and Indium Ligands
On the basis of their structural properties, one can establish two main classes
of ligands suitable for coordinating In
3+
or Ga
3+
: linear chain ligands and
macrocyclic ligands. In addition to the metal coordinating groups, many of
the ligands belonging to the two classes possess other functional groups

Radiopharmaceuticals 117
HOOC
HOOC
HOOC
HOOC
NNN
NNN
COOH
COOH
Bz-NHCSNH-mAb
(a) (b)
COOH
COOH
COOH
CONH-mAb
FIGURE 4.12
Structures of the bifunctional chelators (a) SCN-BzDTPA (octadentate) and (b) cDTPA (hepta-
dentate) (Adapted from M. W. et al., Inorg. Chem., 25:2772–2781, 1986.). The arrow shows the
coupling site.
(e.g., –NH
2
or –COOH), which allow the functionalization with a macro-
molecule. DTPA (diethylenetriaminepentaacetate) is an example of a widely
used linear chain chelator in radiopharmacy, such asfor metal radiolabeling of
monoclonal antibodies [56] and peptides [57]. The amino acid conjugation is
normally achieved through the cyclical bis-anhydride derivative of the DTPA
(cDTPA) [58]. This method has the inconvenience of sacrificing one of the car-
boxylate groups of the DTPA to form an amide bond with the lysine residue
of the lateral chain of the antibody such that only seven coordinate sites of
the DTPA stay available for coordination to the metal, thus lowering the ther-
modynamic stability of the chelate. For this reason, the use of another deriva-
tive of DTPA, l-(p-isothiocyanatobenzyl)-diethylenetriaminepentaacetate (p-
SCN-Bz-DTPA), has been proposed, where the linking group to the antibody
uses one carbon of the skeleton of the DTPA(Figure4.12) [59]. However,DTPA
only forms a complex that is sufficiently stable to permit its use in vivo with the
In
3+
. Another linear chelator has, therefore, been proposed for studies with
gallium: the desferrioxamine-B (DFO) (Figure 4.13), which is used as a bifunc-
tional chelator with high yield for
67/68
Ga
3+
labeling [60]. In addition, DFO
presents a –NH
2
group, which is available for conjugation with biomolecules,
such as peptides or antibodies.
(CH
2
)
5
(CH
2
)
5
(CH
2
)
5
(CH
2
)
5
(CH
2
)
5
(CH
2
)
5
COOH
NH
2
OH
O
OH
O
O
N
N
N
N
N
O
O
OOH
N
FIGURE 4.13
Structure of the desferrioxamine-B (DFO), a bifunctional chelator coupled to biomolecules via
the −NH
2
group or a succinyl spacer (Adapted from P.M. Smith–Jones, et al., J. Nucl. Med., 35:
317–325, 1994.).
