Lima J.J.Pedroso, de (ed.). Nuclear Medicine Physics
Подождите немного. Документ загружается.


98 Nuclear Medicine Physics
TABLE 4.1
Examples of Some Radiopharmaceuticals Used in Molecular Imaging Studies and
Their Application
Radiopharmaceutical Physiological Process/Molecular Target Reference
H
2
15
O Blood flow (neurology) [3]
C
15
O Blood volume [4]
[
18
F]fluoromisonidazole (FMISO) Cellular hypoxia [5]
[
18
F]annexin V Apoptosis [6]
2-[
18
F]fluoro-2-deoxy-D-glucose (FDG) Oxidative metabolism [7]
3
-deoxy-3
-[
18
F]fluorothymidine (FLT) Proliferation (cell replication) [8]
[
11
C]choline Proliferation (membrane synthesis) [9]
2
-deoxy-2
-[
18
F]fluoro-5
-iodo-1β-
D-arabinofuranosyluracil (FIAU) Gene expression [10]
[
13
N]NH
3
Blood flow (cardiology) [11]
[
11
C]acetate Oxidative metabolism (cardiology) [12]
[
11
C]palmitate Fatty acid metabolism (cardiology) [13]
14-[
18
F]fluoro-
6-thia-heptadecanoic acid (FTHA) Fatty acid metabolism (cardiology) [14]
6-[
18
F]fluoro-L-DOPA (FDOPA) Dopamine biosynthesis [15]
[
11
C]methyl-methionine (MET) Proliferation (protein synthesis) [16]
O-(2-[
18
F]fluoroethyl)tyrosine (FET) Proliferation (protein synthesis) [17]
L-[3-
18
F]-α-methyl-tyrosine (FMT) Proliferation (protein synthesis) [18]
[
11
C]WAY1000635 Serotonin 5-HT
1A
receptor [19]
[
11
C-methyl]SCH 23390 Dopamine D
1
receptor [20]
[
11
C]raclopride (RAC) Dopamine D
2
receptor [21]
4.3 Tracer Molecule
A fundamental characteristic of a radiopharmaceutical is that it can access
the physiological compartment where the target is located. To achieve this,
two fundamental characteristics are needed: the availability of the tracer in
blood plasma and its distribution across the relevant biological barriers. The
majority of drugs are transported in blood bound to plasma fractions, either
proteic or lipidic. The extent of binding as well as the kinetics of the binding
process (fast or slow) determine the availability of the radiopharmaceutical
to enter the tissue. In addition, the passage of the radiopharmaceuticals into
the tissue depends on the existence of a transport mechanism or the pres-
ence of favorable physicochemical characteristics of the molecules for their
passive diffusion across the barriers. For example, in the central nervous sys-
tem, the main physical barrier involves the tight junctions of the capillary
endothelium (blood–brain barrier). The extent by which a certain compound
Radiopharmaceuticals 99
can cross this barrier can be estimated by multiplying the permeability of the
compound by the capillary surface (the so-called PS product). In this context,
the permeability of the barrier to a certain compound is a characteristic value
easily related by structure–function studies with physicochemical properties
such as lipophilicity, charge, and molecular size [22].
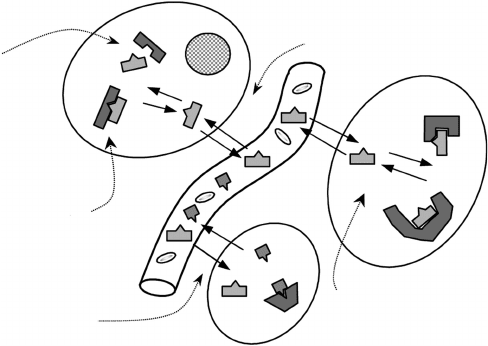
The maximization of the signal measured in vivo and, consequently, the via-
bility of a radiopharmaceutical for molecular imaging (Figure 4.2), ultimately
depend on the balance between the molecular interactions established with
the active site (specific signal) and all other interactions (nonspecific signal).
We can therefore write, assuming first-order kinetics that the tissue concen-
tration of a radiopharmaceutical C
t
is a function of its plasma concentration
C
p
over time:
C
t
(t) =
n
i=1
α
i
t
0
C
p
(τ)e
−β
i
(t−τ)
dτ. (4.2)
In this expression, components with a high value of α
i
correspond to
significant contributions to the observed signal, whereas β
i
values for the
specific signal that are significantly different from the β
i
values of the other
components allow us to separate the specific signal. A common situation is
when the β value of the specific process is much lower than those of all the
others (β →0). In this case, the protocol of the exam can allow sufficient time
for the activity due to nonspecific processes to decay before image acquisition
takes place. This is, for example, the case of the studies with 2-fluoro-deoxy-
D-glucose (see Section 4.7.2.1), in which an essentially irreversible specific
Selectivity
Blood–tissue
partition
Nonspecific
binding
Affinity
Metabolism
FIGURE 4.2
Overview of the main physiological processes involved in a molecular imaging study with radio-
pharmaceuticals. (Adapted from Physiological Imaging of the Brain with PET, Academic Press, San
Diego, pp. 51–56, 2001.)

100 Nuclear Medicine Physics
process (phosphorylation by hexokinase) and the definition of an appropri-
ate waiting time after the injection allow the quantification of the specific
process (oxidative metabolism).
Metabolism of the radiopharmaceuticals can be another obstacle for the
quantification of a specific component of the signal. In fact, the formation,
during the study, of metabolites that maintain the labeling are able to cross
relevant biological barriers and present affinity for molecular targets in the
region of interest can preclude the quantification of the specific binding of
the radiopharmaceutical to its specific site. The development of metabolism-
resistant radiopharmaceuticals has been one of the main concerns, especially
in the case of molecules that are present in several metabolic pathways (e.g.,
carbohydrates or amino acids).
4.4 Radionuclide Selection
The success of a radiopharmaceutical for in vivo imaging requires that the
labeling process has a minimal interference in the physicochemical char-
acteristics of the molecule. This requires, ideally, an isotopic substitution
or, in case this is not possible, the substitution by an atom or chemical
group that does not significantly change the in vivo behavior of the labeled
molecule.
In the case of PET, the existence of radioactive isotopes of carbon (
11
C,
t
1/2
= 20.4 min), nitrogen (
13
N, t
1/2
= 9.97 min), and oxygen (
15
O, t
1/2
=
122 s) that are positron emitters allows the labeling of virtually any organic
molecule. However, the relatively short half-lives of these nuclides signif-
icantly limit the complexity of the molecules to be labeled as well as the
range of processes that can be studied in vivo. In contrast, halogen positron
emitters presenthalf-lives that aremore appropriate for complex labeling pro-
cedures and more prolonged studies (
18
F, t
1/2
= 109.8 min,
76
Br, t
1/2
= 16 h,
or
124
I, t
1/2
= 4.2 days); but their presence is not very common in organic
molecules, and the introduction of a halogen atom frequently causes signifi-
cant changes in the physicochemical properties of the compounds. A notable
exception is fluorine-18, which has been used with great success as a sub-
stitute for the hydrogen atom that has a close Van der Waals radius and,
sometimes, even for hydroxyl groups with which it shares a similar electronic
configuration.
The substitution of hydrogen by Fluorine-18 is, as a consequence, the most
common form of nonisotopic labeling in PET, as the changes introduced in
a molecule by this substitution are minimal. The physicochemical changes
introduced in a molecule by a change of a certain chemical group are usu-
ally evaluated in a diagram similar to that presented in Figure 4.3 (Craig
plot) in which a series of chemical groups are plotted in terms of two main
physicochemical characteristics: lipophilicity (σ) and polarity (π). This dia-
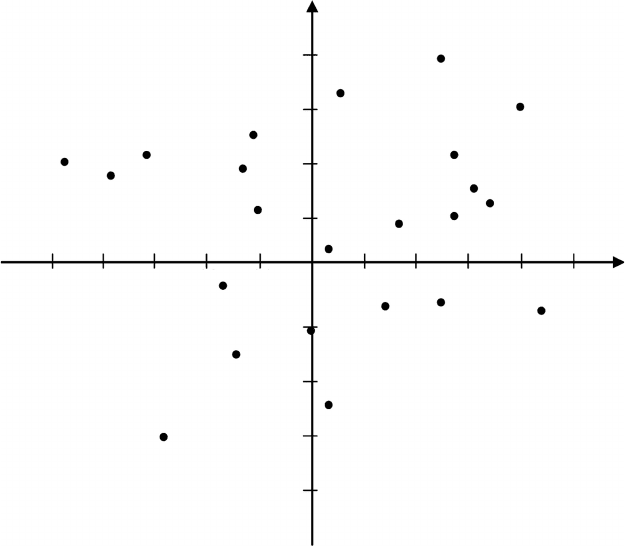
Radiopharmaceuticals 101
–1.00
–0.75
–0.50
OH
13
NH
2
11
CH
3
CONH
N(
11
CH
3
)
2
O
11
CH
3
11
CH
3
C
2
H
5
18
F
123
I
OCF
3
CF
3
SF
5
11
CF
3
SO
2
11
CH
3
SO
2
11
CH
3
CO
11
CO
2
H
11
CONH
SO
2
NH
2
11
CN
NO
2
CI
Br
[
11
C]t-but.
–0.25
2.01.61.20.80.4–0.4–0.8–1.2–1.6–2.0
0.25
0.50
0.75
1.00
σ
π
FIGURE 4.3
Craig plot for the two main physicochemical properties, charge (σ) and lipophilicity (π). Points
that are close in the diagram represent chemical groups with similar physicochemical profile. H,
by definition, is the origin. (Adapted from P. N. Craig. J. Med. Chem., 14(8): 682, 1971.)
gram allows us to identify which chemical group is the best to substitute at a
certain position of a molecule to minimize the effect of the substitution over
the biological activity of the compound.
As can easily be seen in Figure 4.3, fluorine is the element closest to the ori-
gin, which makes it an excellent substitute for hydrogen [by definition, σ(H) =
π(H) = 0], a fact that is the basis for the development of many PET radiophar-
maceuticals such as [
18
F]fluoro-2-deoxy-D-glucose ([
18
F]FDG). This is also
another considerable advantage of molecular imaging with PET compared
with that by single photon emission tomography (SPECT) as the most appro-
priate γ emitter,
123
I, for use in this would undoubtedly lead to considerable
modifications in the physicochemical characteristics of a molecule.
Similarly, we can conclude that the possible substitution of the OH group
by fluorine-18 would not be very favorable. As we can see, these two groups
are present in opposite quadrants of the diagram in Figure 4.3 (+σ/+π for
F−σ/−π for OH). Thehydroxylgroupis, therefore, muchbetter substituted by
the O-methyl groupand in fact, the labeling by the O-[
11
C]methyl groupis one
102 Nuclear Medicine Physics
TABLE 4.2
Positron Emitting Nuclides with Indication of Half-lives and an Example of a
Production Reaction
Nuclide t
1/2
%β
+
Reaction Nuclide t
1/2
%β
+
Reaction
11
C 20.4 m 99.8
14
N(p, α)
11
C
68
Ga 1.13 h 90 (Generator)
13
N 9.97 m 100
12
C(d,n)
13
N
68
Ge 270.8 d
69
Ga(p,2n)
68
Ge
15
O 122.24 s 99.9
14
N(d,n)
15
O
73
Se 7.1 h 65
75
As(p,3n)
73
Se
18
F 109.8 m 97
20
Ne(d,α)
18
F
75
Br 1.62 h 75.5
76
Se(p,2n)
75
Br
38
K 7.6 m 100
35
Cl(α,n)
38
K
76
Br 16 h 57
75
As(
3
He,2n)
76
Br
52
Fe 8.28 h 57
52
Kr(
3
He,3n)
52
Fe
82
Rb 1.26 m 96 (Generator)
55
Co 17.5 h 77
56
Fe(p,2n)
55
Co
82
Sr 25.6 d 100 Mo(p,spall)
82
Sr
62
Cu 9.74 m 98 (Generator)
86
Y 14.74 m 34
88
Sr(p,3n)
86
Y
62
Zn 9.22 h 93
63
Cu(p,2n)
62
Zn
89
Zr 3.27 d 25
89
Y(p,n)
89
Zr
64
Cu 12.7 h 18
64
Ni(p,n)
64
Cu
94m
Tc 53 m 72
94
Mo(p,n)
94m
Tc
66
Ga 9.5 h 57
67
Zn(p,2n)
66
Ga
124
I 4.18 d 25
124
Te(p,n)
124
I
Note: d: days; h: hours; m: minutes; s: seconds.
ofthe most widely used systems for the developmentof radiopharmaceuticals
labeled with carbon-11.
Table 4.2 presents a list of some of the main positron emitting nuclides
used in PET. Although the majority of the PET tracers have been developed
around the first four of these, there are positron emitters from elements across
the entire periodic table including, for example, isotopes of technetium and
iodine, elements that are usually associated with SPECT studies, as well as
nuclides available from reactors such as gallium-68 and rubidium-82.
Other important considerations involved in the selection of a positron emit-
ting nuclide include the percentage of β emission, the energy of the positron
(that influences its range in tissue), and its half-life, which should be long
enough to permit chemical synthesis and the exam to take place but not too
long to avoid exposing the patient to unnecessary doses of radiation after the
exam is finished.
4.5 Labeling Position
The selection of the labeling position in a radiopharmaceutical is critical, as it
can determine the fate of the emitting nuclide as the molecule is metabolized
inthe body.As anexample, Figure4.4indicates two possible labeling positions
for the molecule dihydroxyphenylalanine (DOPA), a marker of dopaminer-
gic function in Parkinson’s disease: [1-
11
C]DOPA [23] and [2-
11
C]DOPA [24].
In the first case (labeling on carbon 1), the action of the enzyme
L-amino
acid decarboxylase present in the target tissue causes the loss of the labeling
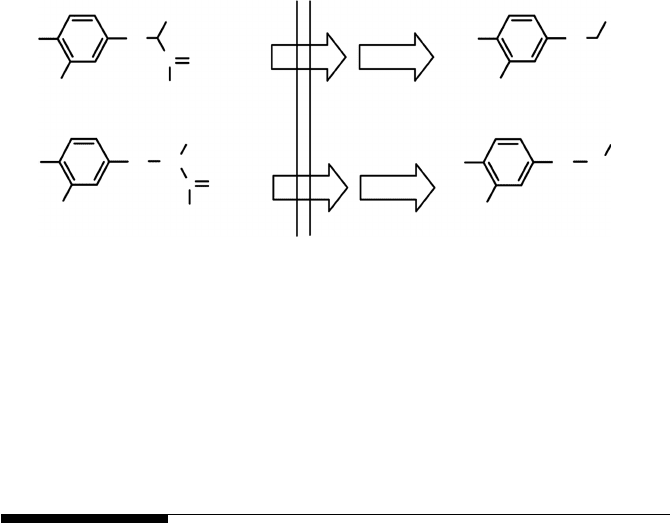
Radiopharmaceuticals 103
NH
2
NH
2
NH
2
NH
2
11
CH
2
CH
2
CO
2
11
CO
2
CH
2
CH
2
CH
2
[1-
11
C]DOPA
L-amino acid
decarboxylase
Dopamine
[2-
11
C]DOPA
[
11
C]Dopamine
HO
HO
HO
HO
11
C
11
C
O
OC
OH
OH
HO
HO
HO
HO
FIGURE 4.4
Fate of the emitting nuclide for two carbon-11 labeling positions of DOPA. Labeling in carbon 1
causes the loss of the labeling through
11
CO
2
whereas labeling in carbon 2 leads to the production
of [
11
C]dopamine.
with the production of
11
CO
2
. In the second case (labeling on carbon 2),
the same metabolic mechanism leads to the production of the dopaminergic
neurotransmitter [
11
C]dopamine.
4.6 Radiosynthesis
The specific nature of the radiopharmaceuticals imposes some conditions on
the synthesis process as well as on the necessary quality control procedures.
Additionally, considering that it will be applied in clinical studies, the final
productshould also be sterile, apyrogenic,and suitably formulated for human
use. Also for clinical reasons, the compounds have to be produced with very
high specific activity, which means that the entire synthesis process has to be
made within a very short time. In the case of nuclides with very short half-
lives, such as oxygen-15 and nitrogen-13 (t
1/2
< 10 min), their chemistry is
limited to very simple molecules (CO, H
2
O, and NH
3
) that do not have sig-
nificant requirements in terms of quality control. In the case of nuclides with
longer half-lives, such as carbon-11 (t
1/2
= 20.4 min), it is possible to syn-
thesize small organic molecules such as amino acids, carbohydrates, or fatty
acids and perform typical quality control procedures. For radionuclides with
longer half-lives, such as fluorine-18 (t
1/2
= 109.8 min), it is possible to have a
more complex chemistry and to include detailed quality control procedures.
In this latter case, it is also possible to distribute the radiopharmaceuticals
to centers that do not have a cyclotron available, a feature that facilitates the
clinical use of these compounds.
In order to minimize synthesis time, the process is optimized to include
the radionuclide in the last possible step. The process relies, therefore, on the
use of (cold) prevalidated precursor molecules that are added to the labeling

104 Nuclear Medicine Physics
agent (radionuclide). It is also common to use a large stoichiometric excess
of the cold reagents to compensate for the relative lack of the labeling agent
and to improve the general yield of the reaction.
In compounds labeled with positron emitters, the short half-lives also
require working with very high initial activities. The principles of radio-
protection, particularly considering the high-energy γ rays that result from
annihilation of the positrons (511 keV), require the use of automated synthesis
and dispensing modules housed in heavily shielded hot cells with a minimum
thickness of 75 mm of lead in their walls. This increasing automation of the
processes also helps in establishing reproducibility, allowing the optimiza-
tion of the synthesis conditions and facilitating production according to good
manufacturing practice (GMP) regulations.
4.7 PET Nuclides: Reactions and Radiopharmaceuticals
4.7.1 Carbon-11
Carbon-11 is usually produced by proton irradiation of a nitrogen target
through the reaction
14
N(p,α)
11
C. The presence of small quantities of oxy-
gen leads to the formation of
11
CO
2
, whereas the presence of hydrogen
leads to the formation of
11
CH
4
. After a purification stage, each of these
gases is propelled by a helium overpressure and is cryogenically trapped for
the radiolabeling of a variety of important chemical precursors (Table 4.3).
Of these, the most important is methyl iodide (
11
CH
3
I), which is used for
the radiolabeling of numerous radiopharmaceuticals that are based on this
nuclide.
TABLE 4.3
Examples of Chemical Species Labeled with Carbon-11 as Radiopharmaceuticals
Per Se or as Precursors for Other More Complex Molecules
Reaction Via Labeled Species
14
N(p,α)
11
C →
11
CO
2
→
11
CH
3
OH →
11
CH
3
I →
11
CH
3
OSO
2
CF
3
→ H
11
CHO
→ R
11
COOMgX → R
11
COOH
→ R
11
COCl
→ CH
3
11
COOLi →CH
3
11
COOH
→
11
CO
→
11
CH
4
→
11
CHCl
3
→
11
CH
2
N
2
→ H
11
CN →
11
CH
3
NH
2
→
11
CO(NH
2
)
2
→
11
CCl
4
→
11
COCl
2
Radiopharmaceuticals 105
4.7.2 Fluorine-18
Two distinct nuclear reactions are used to produce the two chemical main
forms of fluorine-18 used in PET (Table 4.4):
18
F
−
(nucleophilic fluorine) and
18
F
2
(electrophilic fluorine).
Fluoride ion is normally produced by the reaction
18
O(p,n)
18
F through
the cyclotron irradiation of
18
O-enriched water. The resulting
18
F
−
is then
trappedin ananionic exchange resinand extracted fromthe aqueous medium.
Since the fluoride ion is not very reactive in aqueous solution, it is taken to
dryness and resuspended in an aprotic solvent such as DMSO or acetonitrile.
A key factor for the production of reactive nucleophilic fluoride is the
choice of the counter ion. Potassium is normally used, although it is usu-
ally chelated in a poli-ether macrocyclic complex (Cryptand 222), which
increases the distance between both ions and therefore, helps the release of
the fluoride for nucleophilic substitution reactions. This is, in fact, the most
common radiolabeling mechanism with
18
F used, for example, for the syn-
thesis of [
18
F]FDG through the nucleophilic attack of F
−
on the precursor
tetra-O-acetyl-2-O-trifluoro-methanesulfonyl-β-
D-mannopyranose [25].
In contrast, the production of molecular fluorine is traditionally done by
the reaction
20
Ne(d,n)
18
F by deuteron bombardment of neon in a nickel tar-
get. Molecular fluorine is a very reactive species, and the activity produced
is trapped by adsorption onto the target walls. To remove this, we need to
flush the target with cold fluorine, which, by competition, will remove the
18
F
2
adsorbed onto the nickel walls of the target. The fluorine-18 obtained
is consequently heavily diluted with nonradiolabeled fluorine, which leads
to a very low specific activity of the radiopharmaceuticals produced [26].
This constraint limits the use of this synthesis pathway for the labeling of
most radiopharmaceuticals, although it is still used as the major path for the
TABLE 4.4
Examples of Chemical Species Labeled with [
18
F]fluoride and [
18
F]fluorine, in Most
Cases with the Objective of being Used as Hot Precursors for the Labeling of More
Complex Molecules
Reaction Via Labeled species
20
Ne(d,n)
18
F →
18
F
2
→ CH
3
COO
18
F
→ RSO
2
N
18
FR
N
O
18
F
18
O(p,n)
18
F →
18
F
−
→ Br
18
F
→ I(CH
2
)
n
18
F
→ CH
3
CH
18
FCOOCH
3
→
18
FArCHO
106 Nuclear Medicine Physics
production of a few radiotracers (e.g., [
18
F]DOPA) for which the specific
activity factor is not so critical.
4.7.2.1 Deoxyglucose Method
The method is based on the properties of 2-deoxy-
D-glucose (deoxyglu-
cose, DG), and it was originally developed to determine local glucose
utilization ex vivo in animals by autoradiography with carbon-14 (2-deoxy-
D-[1-
14
C]glucose) [27]. This method was later adapted for in vivo imaging
by labeling DG with positron emitters. Carbon-11 was tested (2-deoxy-
D-
[1-
11
C]glucose) [28], but it was the nonisotopic substitution of one of the
two hydrogens in position 2 by fluorine-18 (2-[
18
F]fluoro-2-deoxy-D-glucose
[
18
F]FDG) [6] that showed ideal characteristics to enable the determination
in vivo, in humans, the local energy metabolism (Figure 4.5).
Deoxyglucose shares with glucose the same passive transport mechanism
forentering cells (GLUTtransporters). Inthe cells, deoxyglucoseis alsometab-
olized by hexokinase through phosphorylation to produce deoxyglucose-
6-phosphate. This compound is effectively trapped within the cells due to
its high polarity and is considered, within a compartmental model, to be
in a different compartment with no direct access back to the blood pool
(Figure 4.6).
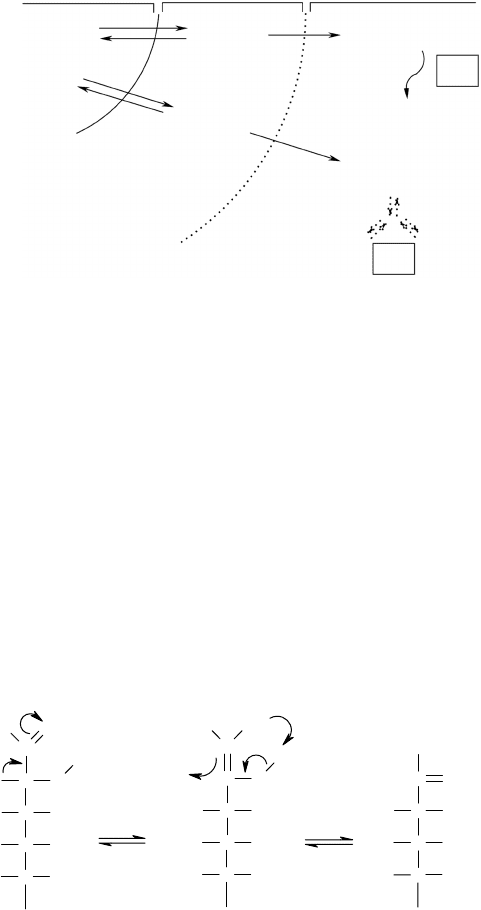
In normal glucose, phosphorylation by hexokinase is only the first step
of glycolysis, the initial step of the process of oxidative metabolism. The
glycolytic pathway then proceeds with the action of an enzyme called
phosphoglucose isomerase, which catalyzes the reaction that transforms
glucose-6-phosphate into fructose-6-phosphate. This reaction, which can be
easily understood using Fischer projections (Figure 4.7), relies on the forma-
tion of an enediol intermediate, which is essential for the conversion of the
aldol group of glucose into the keto group of fructose.
In the case of deoxyglucose, the absence of the hydroxyl group in the
β position makes this reaction impossible; and as a consequence, there is
accumulation of the metabolic intermediate deoxyglucose-6-phosphate. It
2-deoxy-D-[1-
14
C]glucose 2-deoxy-D-[1-
11
C]glucose 2-[
18
F] fluro2-deoxy-D-glucose
H
OH
H
H
OH
H
H
14
C
OH
O
H
H
OH
H
H
OH
H
H
11
C
OH
O
H
H
OH
H
H
OH
18
F
H
OH
O
H
CH
2
OH CH
2
OH CH
2
OH
FIGURE 4.5
Structural formulas of the different forms of deoxyglucose labeled with β-emitters: 2-deoxy-
D-[1-
14
C]glucose ([
14
C]DG) and β+: 2-deoxy-D-[1-
11
C]glucose ([
11
C]DG) and 2-[
18
F]fluoro-
2-deoxy-
D-glucose ([
18
F]FDG).
Radiopharmaceuticals 107
Glucose-6-phosphate
EM
DM
III III
Deoxyglucose-6-phosphate
Deoxyglucose
Deoxyglucose
Glucose
CO
2
+ H
2
O
C
M
C
M
*
C
E
*
C
P
*
C
E
Glucose
C
P
k
3
k
3
*
k
2
*
K
1
*
K
1
k
2
*
FIGURE 4.6
Three-compartment model for deoxyglucose labeled as a tracer for the determination of oxida-
tive metabolism. Compartment I: capillary lumen; compartment II: intact compounds in tissue;
compartment III: metabolic products. Cp, CE, and CM glucose concentrations in compartments
I, II, and III respectively. K1-k3, kinetic constants of passage between compartments. Variables
labeled with ‘∗’ are the same for deoxyglucose. EM, energetic metabolism; DM, deoxyglucose
metabolites.
should be noted that, although this chemical reaction is not possible, there
is evidence that deoxyglucose-6-phosphate binds effectively to phosphoglu-
cose isomerase [29], leading to a competitive inhibition of the binding of
glucose. This is, in fact, one of the mechanisms proposed for the toxic effect
of deoxyglucose when administered in higher doses, which is suggested to
cause an inhibition of glycolysis and the consequential development of symp-
toms similar to those of hypoglycemia. Although the simple reversion of
the phosphorylation reaction of deoxyglucose by hexoquinase is, as in the
case of glucose, highly unfavorable in terms of the energy balance, several
Glucose-6-phosphate Enediol intermediate Fructose-6-phosphate
C
C
C
OH
C
C
O
H
H
HHO
OHH
OHH
C
C
C
O
–
H
C
C
O
H
HHO
OHH
OHH
CH
2
OH
C
C
C
C
O
HHO
OHH
OHH
H
+
CH
2
OPO
3
2–
CH
2
OPO
3
2–
CH
2
OPO
3
2–
FIGURE 4.7
Schematic representation of the reaction catalyzed by the enzyme phosphoglucose isomerase
that converts glucose-6-phosphate in fructose-6-phosphate evidencing the enediol intermediate
fundamental to the formation of the final product.
