Lennarz W.J., Lane M.D. (eds.) Encyclopedia of Biological Chemistry. Four-Volume Set . V. 4
Подождите немного. Документ загружается.

Signal peptide
hTSHR
1 MRPADLLQLVLLLDLPRDLGG-- - 21
hFSHR
1 MALLLVSLLAFLSLGSG------- 17
hLHR
1 MKQRFSALQLLKLLLLLQPPLPRA 24
N-flanking Cysteine-rich sequence
hTSHR 22 ---MGC SSPPC E C HQEED - - FRVTC KDIQRI PSLPPSTQT- - - 56
hFSHR
18 -----
C
HHR I
C
H
C
SNRVFL-----
C
QESKVTEIPSDLPRNAI E 50
hLHR 25 L R E A L C P-EPC N C VPDG- - ALR- -C PGPTAGLTR--------- 53
Leucine-rich repeats
hTSHR 57 L KL I ETHLRT IPSHAFSNLPNISRI YVS I -DVTL QQLESHSFYNL SKVTHIEIRNTRNL T 115
hFSHR 51 L RFVLTKLRVIQKGAFSGFGDLEKIEISQNDV-L EV I EADVFSNL PKLHE I R IEKANNL L 109
hLHR 54 L SLAYLPVKV I PSQAFRGLNEVIKI E I SQIDS- L ER I EANAFDNL LNLSEI LIQNTKNL R 112
hTSHR 116 Y IDPDALKEL PLL KFLGI FNTGLKMFPDLTKVYSTDI FF I L E I T D N P Y M - T S I P V N A F Q G 174
hFSHR
110 Y I N P E A F Q N
L
PN
L
QYLL I SNTGI KHLPD-VHK I HSLQKVL
L
DIQDNINIHT-IERNSFVG 167
hLHR 113 Y I E P G A F I N L PRL KYLS ICNTGIRKFPDVTKVFSSESNF I L E I CDNLH I TT - IPGNAFQG 171
hTSHR 175 L CNETLTL K L YNNGFTSVQGYAFNGTKL DAVYL NKNKYL TVIDKDAFGGVYSGPSLL D V S 234
hFSHR 168 L SFESVI L
W
L
NKNGIQEIHNCAFNGTQ
L
DE
L
N
L
SDNNN
L
EELPNDVFHGASG-PVI
L
D I S 226
hLHR 172 M N N E S V T L K L YGNGFEEVQSHAFNGTTL TSL E L KENVHL EKMHNGAFRGATG-PKTL D I S 230
hTSHR
235 Q T S V T A
L
PSKG
L
EH
L
KE
L
IARNT
W
T L KKL PLSLSFLHL T R 274
hFSHR
227 R T R I H S
L
PSYG
L
EN
L
KK
L
RARSTYN
L
KK
L
PTLEKLVA
L
M E 266
hLHR
231 S T K L Q A
L
PSYG
L
ESIQR
L
I ATSSYS
L
KK
L
PSRETFVN
L
L E 270
C-flanking Cysteine-rich sequence
hTSHR
275 ADLSYPSH
CC
AFKNQKKIRGILESL---------MCNESSMQSLRQRKSVNALNSPLHQE 325
hFSHR 267 ASLTYPSHCCAFAN
W
RRQI SEL -HP ICNKS I LRQEVDYMTQTRGQRSSLAE- - - - - - - - - 316
hLHR 271 A T L T Y P S H CCAFRNLPTKEQNFSHS---------ISENFSKQCESTVRKVS--------- 312
hTSHR
326 YEENLGDSIVGYKEKSKFQDTHNNAHYYVFFEEQEDEI IGFGQELKNPQEETLQAFDSHY 385
hFSHR 317 -----------------------------------------DNESSYS- -RGFDMTYTEF 333
hLHR
313 -----------------------------------------NKTLYSSMLAESELSG
W
D Y 331
hTSHR 386 D Y T I C GDSEDMVC TPKSDEFNPC EDI 411
hFSHR
334 D Y D L
C
NEVVDVT
C
SPKPDAFNP
C
EDI 359
hLHR 332 E Y G F C -LPKTPRC APEPDAFNPC ED I 356
FIGURE 1 Alignments of the amino acid sequences of the human TSHR, FSHR, and LHR. The deduced amino acid sequences for each of the three glycoprotein hormone receptors of human origin
are shown.
Transmembrane re
g
ion
I II
hTSHR 412 MGYKFLRIVV
W
FVSLLALLG
N
VFVLLILLTSHYKLNVPRFLMCN
L
AFA
D
F C M G M Y L L L I A 471
hFSHR
360 M G Y N I L R V L I
W
FISILAITG
N
I IVLV I LTTSQYKLTVPRFLMCN
L
AFA
D
LCIGIYLLLIA 419
hLHR
357 M G Y D F L R V L I
W
LINILAIMGN MTVLFVLLTSRYKLTVPRFLMCNL SFAD F C M G L Y L L L I A 416
III
hTSHR
472 SVDLYTHSEYYNHAID
W
QTGPGC NTAGFFTVFASELSVYTLTVITLER
W
YAI T F A M R L D R 531
hFSHR
420 SVDIHTKSQYHNYAID
W
QTGAGC DAAGFFTVFASELSVYTLTAI TLER
W
HT I T H A M Q L D C 479
hLHR 417 SVDSQTKGQYYNHA I D
W
QTGSGC STAGFFTVFASELSVYTLTVI TLER
W
HT I T Y A I H L D Q 476
IV V
hTSHR 532 KIRLRHACAIMVGG
W
VCCFL LALLPLVGI SSYAKVS ICLPMDTETPLALAYIVFVLTLNI 591
hFSHR 480 KVQLRHAASVMVMG
W
I FAFAAALFPI FGI SSYMKVSICLPMDIDSPLSQLYVMSLLVLNV 539
hLHR 477 K L R L R H A I L I M L G G
W
LFSSL IAMLPI VGVSNYMKVS ICFPMDVETTLSQVYILTILILNV 536
VI
hTSHR
592 V A F V I V C C C
Y
VK I Y I TVRNPQYNPGDKDTK I AKRMAVL I FTDF I CMA
P
I S F Y A L S A I L N K 651
hFSHR 540 L A F V V I C G C Y IHIYLTVRNPNIVSSSSDTRI AKRMAML I FTDFLCMAP I SFFA I SASLKV 599
hLHR 537 V A F F I I C A C Y IKIYFAVRNPELMATNKDTKIAKKMAILIFTDFTCMAP I SFFA I SASFKV 596
VII
hTSHR
652 PLITVSNSKI LLVLFYPLNSCA
NP
FL
Y
AIFT 682
hFSHR 600 PL I TVSKAK I LLVLFHPINSCANPFLY AIFT 630
hLHR
597 PLITVTNSKVLLVLFYPINSCA
NP
FL
Y
AIFT 627
Helix VIII
Hx VIII
hTSHR
683 KAFQRDVFI LLSKFGI
C
699
hFSHR 631 KNFRRDFF I LLSKCG-C 646
hLHR 628 K T F Q R D F F L L L S K F G C C 644
C terminus
hTSHR 700 KRQAQAYRGQRVPPKNSTD IQVQKVTHDMRQGLHNMEDVYEL IENSHLTPKKQGQISEEY 759
hFSHR
647 YEMQAQIYRTETSSTVHNTHPRNGHCSSAPRVTNGSTY I LVPLSHLAQN- - - - - - - - - - - 695
hLHR 645 KRRAELYRRKDFSAYTSNCKNGFTGSNKPSQSTLKLSTLHCQGTALLDKTRYTEC- - - - - 699
hTSHR 760 M Q T V L 764
hFSHR
-----
hLHR -----
Conserved cysteine Transmembrane domains Intracellular loops Extracellular loops
Conserved leucine Highly conserved residues within rhodopsin-like GPCRs
FIGURE 1 (continued)
Interestingly, the ligands for LGR7 and LGR8, relaxin
and/or Leydig cell insulin-like peptide, are structurally
homologous to insulin rather than to the glycoprotein
hormones.
Genomic Organization of the
Glycoprotein Hormone Receptors
The genes for these three receptors have a similar
structure. The LHR gene has 11 exons and the TSHR
and FSHR genes each have 10 exons. The last exon
codes for a small portion of the N-terminal extracellular
domain and all of the serpentine region, including the
C-terminal cytoplasmic tail. The other exons are small
and they are spliced together to form most of the
N-terminal extracellular domain.
The TSHR, LHR, and FSHR genes map to human
chromosomes 14q31, 2p21, and 2p21 –16, respectively.
Activation of the Glycoprotein
Hormone Receptors
GPROTEINS AND CELLULAR PATHWAYS
ACTIVATED BY THE GLYCOPROTEIN
HORMONE RECEPTORS
The identity of the G proteins activated by the FSHR has
not been directly examined. The TSHR has been shown
to activate all four families of G proteins and the LHR
activates at least three or perhaps all four families as
well. All of these receptors activate the cAMP and
inositol phosphate/diacylglycerol signaling cascades.
For each of the glycoprotein hormones, the EC
50
for
cAMP accumulation is lower than the EC
50
for inositol
phosphate accumulation. Moreover, activation of the
cAMP pathway is detectable even at low receptor
densities, whereas activation of the inositol phosphate/
diacylglycerol pathway is generally detectable only at
high receptor densities.
It is generally agreed that cyclic AMP is the principal
mediator of the actions of the glycoprotein hormones on
the differentiated functions of their target cells. The
functional consequences of the activation of the inositol
phosphate/diacylglycerol pathway are not fully under-
stood. In addition to controlling the differentiated
functions of target cells, the glycoprotein hormones
also control their proliferation. The three glycoprotein
hormones can each activate the extracellular regulated
kinase 1/2 cascade (ERK1/2) in their target cells and, at
least in the case of the LHR and the TSHR, this
activation seems to be mediated by cAMP.
MECHANISM OF ACTIVATION
Although it is clear that binding specificity is provided
by the large extracellular domains of the receptors and
the
b
-subunits of the hormones, little else is known
about how this event leads to receptor activation. Three
models have been proposed. In one model the common
a
-subunit of the glycoprotein hormones is thought to
directly interact with and activate the transmembrane
region of the receptor. In another model hormone
binding is thought to induce a conformational change
in the extracellular domain of the receptor,
thus allowing it to interact with and activate the
transmembrane region. The last model proposes that
the extracellular domain constrains a constitutive
activity intrinsic to the transmembrane domain
and that hormone binding to the extracellular
domain relieves this inhibition. This latter model has
received strong experimental support when tested with
the TSHR.
Naturally Occurring Mutations
of the Glycoprotein
Hormone Receptors
Both activating (gain-of-function) and inactivating
(loss-of-function) mutations of the glycoprotein hor-
mone receptor genes have been identified in patients
with particular endocrine disorders. Activating
mutations of these receptors results in the constitutive
activation and unregulated elevation of intracellular
cAMP in the absence of bound agonist. Most activating
mutations have been found in the serpentine regions
of the receptors; however, activating mutations of
the hTSHR have also been found in the extra-
cellular domain. Germ-line mutations resulting in
constitutive activation of the glycoprotein hormone
receptors are inherited in an autosomal dominant
manner.
Loss-of-function mutations refer to a number of
different types of mutations that ultimately cause
decreased target cell responsiveness to hormone.
These include mutations that impair coupling to
G protein, hormone binding, and/or cell surface
expression. The majority of loss-of-function mutations
of the glycoprotein hormone receptors result in
decreased cell surface expression of the mutant receptor
due to the intracellular retention of the misfolded
mutant. Therefore, loss-of-function mutations have
been identified in all regions of the glycoprotein
hormone receptors. Mutations of the glycoprotein
hormone receptors resulting in a loss-of-function are
inherited in an autosomal recessive manner.
184
TSH, LH AND FSH RECEPTORS
TSHR
A large number of activating mutations of the hTSHR
have been identified in individuals with autonomously
functioning thyroid adenomas (somatic mutations) or
autosomal dominant nonautoimmune hyperthyroidism
(germ-line mutations).
A number of loss-of-function hTSHR mutations have
been identified in individuals with nonautoimmune
congenital hypothyroidism, where the degree of
hypothyroidism correlates with the extent of target cell
unresponsiveness to TSH.
LHR
Numerous activating mutations of the hLHR have been
identified in boys with gonadotropin-independent pre-
cocious puberty (testotoxicosis). In these cases, the
constitutively active hLHRs inappropriately stimulate
testosterone production in Leydig cells under conditions
of prepubertal levels of LH. Only one hLHR mutation,
D578H, has been found to also cause Leydig cell
adenomas. Unlike the other hLHR activating mutations,
D578H was somatic rather than germ-line in origin.
Although it was initially postulated that D578H may
activate a pathway(s) different from those activated by
the other constitutively active hLHRs, such differences
have not yet been observed. Therefore, as with the
hTHSR, tumor formation by D578H may be due to its
somatic origin.
Many different loss-of-function hLHR mutations
have been identified in genetic males with micropenis
(partial loss-of-function) or pseudohermaphroditism
(severe loss-of-function). In the latter case, the individual
presents with female external genitalia but fails to
undergo breast development or menstruation at the time
of puberty. Female siblings with homozygous or
compound heterozygous loss-of-function LHR
mutations are infertile.
FSHR
Only one naturally occurring activating mutation of the
hFSHR has been reported. This mutation was found in a
hypophysectomized male receiving testosterone sup-
plementation who exhibited normal spermatogenesis.
Studies of this mutant in cell culture, however, have led
to disparate results on whether or not it is truly
activating. Surprisingly, activating FSHR mutations
have not been found in granulosa cell tumors examined
thus far. The paucity of naturally occurring activating
mutations of the hFSHR may reflect the lack of a readily
detectable pathophysiological state in males or females
arising from a constitutively active hFSHR and/or to the
relative resistance of the hFSHR to mutation-induced
constitutive activity.
The first loss-of-function hFSHR mutation was
identified in a population of Finnish women with
primary amenorrhea due to ovarian failure. Cells
expressing the recombinant form of this hFSHR mutant
display no responsiveness to FSH. Surprisingly, men
homozygous for this mutation were not infertile,
although their fertility may have been impaired some-
what. Therefore, although FSH plays an important role
in spermatogenesis, these observations raised ongoing
debates regarding its absolute requirement. Since then,
other loss-of-function mutations of the hFSHR have
been identified in women with varying degrees of
FSH resistance.
SEE ALSO THE FOLLOWING ARTICLES
G Protein-Coupled Receptor Kinases and Arrestins †
Glycoproteins, N-linked
GLOSSARY
chorionic gonadotropin (CG) A hormone secreted by the placenta
that is nearly identical to the pituitary hormone LH and is, there-
fore, recognized by the LH receptor. Also, choriogonadotropin.
follicle-stimulating hormone (FSH) A pituitary hormone recognized
by the FSH receptor. Also, follitropin.
glycoprotein hormones The structurally related hormones TSH, LH,
CG, and FSH. The glycoprotein hormone receptors are the LH,
FSH, and TSH receptors.
gonadotropins LH, CG, and FSH; members of the glycoprotein
hormone family that act upon the gonads. The gonadotropin
receptors are the LH and FSH receptors.
G protein-coupled receptors (GPCRs) A class of membrane receptors
that contain seven transmembrane regions and mediate their
actions through the activation of G proteins.
luteinizing hormone (LH) A pituitary hormone recognized by the LH
receptor. Also, lutropin.
thyroid-stimulating hormone (TSH) A pituitary hormone recognized
by the TSH receptor. Also, thyrotropin.
FURTHER READING
Ascoli, M., Fanelli, F., and Segaloff, D. L. (2002). The lutropin/
choriogonadotropin receptor, a 2002 perspective. Endocr. Rev. 23,
141–174.
Davies, T., Marians, R., and Latif, R. (2002). The TSH receptor reveals
itself. J. Clin. Invest. 110, 161–164.
Hsu, S. Y., and Hsueh, A. J. (2000). Discovering new hormones,
receptors and signaling mediators in the genomic era. Mol.
Endocrinol. 14, 594–604.
Latronico, A., and Segaloff, D. L. (1999). Naturally occurring
mutations of the luteinizing-hormone receptor: Lessons learned
about reproductive physiology and G protein-coupled receptors.
Amer. J. Hum. Genet. 65, 949– 958.
Paschke, R., and Ludgate, M. (1997). The thyrotropin receptor in
thyroid diseases. N. Engl. J. Med. 337, 1675– 1681.
Rapoport, B., Chazenbalk, G. D., Jaume, J. C., and McLachlan, S. M.
(1998). The thyrotropin (TSH) receptor: Interaction with TSH and
autoantibodies. Endocr. Rev. 19, 673–716.
Simoni, M., Gromoll, J., and Nieschlag, E. (1997). The follicle-
stimulating hormone receptor: Biochemistry, molecular biology,
physiology, and pathophysiology. Endocr. Rev. 18, 739–773.
TSH, LH AND FSH RECEPTORS 185
Themmen, A. P. N., and Huhtaniemi, I. T. (2000). Mutations of
gonadotropins and gonadotropin receptors: Elucidating the physi-
ology and pathophysiology of pituitary-gonadal function. Endocr.
Rev. 21, 551–583.
BIOGRAPHY
Deborah L. Segaloff is a Professor of Physiology and Biophysics and
Mario Ascoli a Professor of Pharmacology in the Roy J. and Lucille
A. Carver College of Medicine at the University of Iowa in Iowa City,
Iowa Dr. Segaloff’s laboratory cloned the cDNA for the rat LHR, the
first glycoprotein hormone receptor to be cloned. Since then, Dr.
Segaloff’s and Dr. Ascoli’s laboratories have performed extensive
studies on the regulation and the mechanisms of activation and
desensitization of the gonadotropin receptors.
Dario Mizrachi is a Postdoctoral Associate in Dr. Segaloff’s laboratory.
As a Lalor Fellow, Dr. Mizrachi developed homology models of the
glycoprotein hormone receptors for use in predicting the structural
arrangements of the receptors.
186 TSH, LH AND FSH RECEPTORS

Tight Junctions
Shoichiro Tsukita
Kyoto University, Kyoto, Japan
The tight junction (TJ) or zonula occludens is one mode of
cell-to-cell adhesion in vertebrate epithelial and endothelial
cellular sheets, and is located at the most apical part of their
lateral membranes. The existence of separate fluid compart-
ments with different molecular compositions is of particular
importance for the development and maintenance of multi-
cellular organisms. These compartments are delineated by
various cellular sheets, which function as barriers to maintain
the distinct internal environment of each compartment. Within
these sheets, individual cells are mechanically linked to each
other to maintain the structural integrity of the sheet, and the
intercellular space between adjacent cells is sealed to prevent
the diffusion of solutes through the intercellular space. TJs are
directly involved in this intercellular sealing.
Structure
ELECTRON MICROSCOPIC IMAGE
On ultrathin section electron microscopy, tight junctions
(TJs) appear as a zone where plasma membranes of
neighboring cells focally make complete contact
(Figure 1D). On freeze-fracture electron microscopy,
TJs are visualized as a continuous, anastomosing
network of intramembranous particle strands
(TJ strands or fibrils) and complementary grooves.
THREE-DIMENSIONAL IMAGE
Detailed electron microscopic observations led to our
current understanding of the three-dimensional
structure of TJs (Figure 1C); each TJ strand is associated
laterally with another TJ strand in apposing membranes
of adjacent cells to form “paired” TJ strands, where the
intercellular space is completely obliterated.
Molecular Architecture
INTEGRAL MEMBRANE PROTEINS
Three distinct types of integral membrane proteins are
localized at TJs: claudin, occludin, and JAM (Figure 2).
Claudin with a molecular mass of , 23 kDa is a major
constituent of TJ strands, and bears four transmembrane
domains. In mice and humans, claudins comprise a
multigene family consisting of 24 members. When each
claudin species is overexpressed in mouse fibroblasts,
claudin molecules are polymerized within the plasma
membranes to reconstitute paired TJ strands. As many
distinct species of claudins are coexpressed in individual
cells, heterogeneous claudin species are copolymerized
to form individual TJ strands as heteropolymers, and
between adjacent TJ strands claudin molecules adhere to
each other in both homotypic and heterotypic manners,
except in certain combinations.
Occludin, a , 65 kDa integral membrane protein,
also bears four transmembrane domains, but does not
show any sequence similarity to claudins. Occludin is
incorporated in TJ strands in situ, but TJ strands can be
formed without occludin. JAM with a single trans-
membrane domain associates laterally with TJ strands,
while not constituting the strands per se. The physio-
logical functions of occludin and JAM remain elusive.
PERIPHERAL MEMBRANE PROTEINS
Claudins are packed densely within the TJ strands.
Therefore, their cytoplasmic surface appears similar to a
brush consisting of numerous short carboxyl-terminal
cytoplasmic tails of claudins. Interestingly, most of
these tails end in valine at their carboxyl termini,
suggesting that these carboxyl termini directly bind to
PDZ domains. Therefore, the cytoplasmic surface of
TJ strands can be regarded as a magnetic bar that
strongly attracts and recruits many PDZ-containing
proteins.
Indeed, three related PDZ-containing proteins,
ZO-1 (, 220 kDa), ZO-2 (, 160 kDa), and ZO-3
(, 130 kDa), are concentrated at TJs. These molecules
all have three PDZ domains (PDZ1 to 3), one SH3
domain, and one GUK (guanylate kinase-like) domain in
this order from their NH
2
-termini indicating that they
belong to membrane-associated guanylate kinase-like
homologues (MAGUKs). Among these three PDZ
domains, PDZ1 domain binds directly to the COOH
termini of claudins.
Encyclopedia of Biological Chemistry, Volume 4. q 2004, Elsevier Inc. All Rights Reserved. 187

In addition to these three TJ-specific MAGUKs,
several other PDZ-containing proteins are recruited to
the cytoplasmic surface of TJ strands. These proteins are
multidomain proteins, and may function as adapters at
the cytoplasmic surface of TJs together with non-
MAGUK proteins such as cingulin and symplekin,
which recruit various proteins including cytoskeletal as
well as signaling molecules.
Functions
BARRIER FUNCTION
Claudins are directly involved not only in the formation
of TJ strands but also in their barrier function in simple
epithelia, not only for solutes but also for pathogens
such as bacteria. Furthermore, TJs are not simple
barriers; they show ion and size selectivity and their
barrier function varies significantly in tightness depend-
ing on the cell-type and physiological requirements. Such
regulated and diversified permeability of TJs is required
for dynamically maintaining the environment of each
compartment. To explain the material transport across
TJs, aqueous pores (or paracellular channels) have been
postulated to exist within paired TJ strands. It is now
widely believed that the combination and mixing ratios
of claudins within individual paired strands determine
the tightness and selectivity of these aqueous pores.
FENCE FUNCTION
TJ strands are heteropolymers of integral membrane
proteins, claudins, within plasma membranes, and
continuously encircle the top of individual epithelial/
endothelial cells to delineate the border between the
apical and basolateral membrane domains. Therefore, it
is believed that TJ strands act as a “fence,” limiting the
lateral diffusion of lipids and proteins between the apical
and basolateral membrane domains. Indeed, when
fluorescently labeled lipids are inserted into the outer
leaflet of the apical membrane of cultured epithelial cells,
these lipids are retained on the apical surface. In contrast,
the importance of TJs in the asymmetric distribution of
integral membrane proteins remains controversial.
OTHER POSSIBLE FUNCTIONS
As previously mentioned, various types of proteins
including PDZ-containing proteins are recruited to the
cytoplasmic surface of TJ strands to form a huge
macromolecular complex. This macromolecular com-
plex is expected to play central roles in the intracellular
signaling of epithelial cells, being involved in the
regulation of their proliferation and morphogenesis,
i.e., contact inhibition of cell growth and epithelial cell
polarization, respectively.
SEE ALSO THE FOLLOWING ARTICLE
MDR Membrane Proteins
GLOSSARY
apical surface The free surface of epithelial cells facing the luminal
space or external environment.
endothelial cells Thin, flattened cells of mesoblastic origin that are
arranged in a single layer lining the blood vessels and some body
cavities (e.g., those of the heart).
FIGURE 1 Structure of tight junctions. (A) Schematic drawing of
simple epithelial cells. The junctional complex is located at the most
apical part of lateral membranes (encircled). (B) Electron micrograph
of the junctional complex in mouse intestinal epithelial cells. TJ, tight
junction (encircled); AJ, adherens junction; DS, desmosome. (C)
Schematic drawing of tight junctions. Individual tight junction strand
within plasma membranes associates laterally with another strand in
apposing membranes to form a paired strand. (D) Ultra-thin sectional
view of tight junctions. At kissing points of TJs (arrowheads), the
intercellular space is obliterated. Bars, 200 nm (b); 50 nm (d).
FIGURE 2 Integral membrane proteins localized at tight junctions.
Membrane folding models of mouse claudin-1, occludin, and JAM.
188 TIGHT JUNCTIONS
epithelial cells Closely packed cells, arranged in one or more layers,
that cover the outer surfaces of the body or line any internal cavities
or tubes (other than the blood vessels, heart, and serous cavities).
freeze-fracture replica electron microscopy An electron microscopic
method that uses metal replicas to visualize the interior of cell
membranes. This technique provides a convenient way of visualiz-
ing the distribution of large integral membrane proteins as
intramembranous particles on the plane of a membrane.
PDZ domain Protein–protein interaction domain first described in
proteins PSD-95, DLG, and ZO-1.
SH3 domain Src homology region 3, a protein domain present in
various signaling proteins.
FURTHER READING
Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., and Walter, P.
(2002). Molecular Biology of the Cell. Garland Publishing,
New York.
Cereijido, M., and Anderson, J. (2001). Tight Junctions. CRC Press,
New York.
Gonzalez-Mariscal, L., Betanzos, A., Nava, P., and Jaramillo, B. E.
(2003). Tight junction proteins. Prog. Biophys. Mol. Biol. 81,
1–44.
Schneeberger, E. E., and Lynch, R. D. (1992). Structure, function, and
regulation of cellular tight junctions. Am. J. Physiol. 262,
L647–L661.
Tsukita, Sh., Furuse, M., and Itoh, M. (2001). Multifunctional strands
in tight junctions. Nat. Rev. Mol. Cell. Biol. 2, 285–293.
BIOGRAPHY
Shoichiro Tsukita is a Professor in the Department of Cell Biology at
Kyoto University, Kyoto, Japan. His principal research interests are in
cell–cell adhesion and cytoskeletons. He holds M.D. and Ph.D. from
the University of Tokyo. His group established a new isolation
procedure for intercellular junctions and identified occludin and
claudins.
TIGHT JUNCTIONS 189

Toll-Like Receptors
Himanshu Kumar, Kiyoshi Takeda and Shizuo Akira
Osaka University, Osaka, Japan
Toll-like receptors (TLRs) are the family of receptor molecules
that recognizes microbe-specific conserved molecular motifs.
Recognition of these molecular motifs by innate cells of innate
immune system activates downstream signaling cascades to
induce secretion of cytokines, chemokines, and expression of
costimulatory molecules. These effects subsequently instruct
the major component of adaptive immune system that is B and
T cells to exhibit pathogen-specific antimicrobial activity. At
the end of the twentieth century, this family of receptors has
been discovered and till date ten TLR molecules are known.
Innate Immune System
Innate immunity acts as the first line of host defense
against microbial pathogens. It is conserved from flies to
mammals. Innate immunity is dependent on both
cellular components such as monocytes, macrophages,
neutrophils, dendritic cells, and endothelial cells, and
humoral components such as C-reactive protein (CRP),
lysozyme, and complements. The cellular component of
innate immunity recognizes a wide range of pathogen-
associated molecular motifs by virtue of pattern
recognition receptors (PRR). There are two types of
PRRs. One type of PRRs, such as CR3 and C-type
lectins, facilitate receptor-mediated phagocytosis. Other
PRRs, such as TLRs, trigger the activation of innate
immunity. It has recently been shown that toll-like
receptor (TLRs) are the most important PRRs in
triggering the activation of innate immunity.
TLRs and their Ligands
Toll receptor was first described in context of the dorso-
ventral development of Drosophila embryo. Toll and its
mammalian homologues (TLRs) are type I membrane
proteins, harboring an ectodomain consisting of leucine-
rich repeats (LRRs) and one or two cysteine rich regions.
The intracellular domain of toll receptors is structurally
similar to that of the IL-1 receptor. Therefore, it is called
Toll/IL-1R (TIR) domain. This region of TLRs is
required for homophilic and heterophilic interaction of
TLRs. The TIR domain further interacts with several
TIR domain-containing adaptors such as MyD88,
TIRAP/Mal, and TRIF. To date, ten TLRs (TLR1–10)
have been identified and shown to recognize different
classes of pathogen-derived molecular motifs (Table I).
Distribution of TLRs
Distribution of these TLR molecules in different cells
and tissue type is shown in Table II.
Intracellular Signal Transduction
through TLR
The molecular mechanisms by which TLRs trigger
activation of innate immunity have been elucidated
through analysis of signaling pathways. Intracellular
signal transduction initiates from the TIR domain of
TLRs. The TIR domain of TLRs interacts with the TIR
domain (present in the C terminus) of MyD88 adaptor
protein. MyD88 contains a death domain at the N
terminus. When ligands are bound to TLRs, MyD88
recruits IRAK to TLRs through the interaction of death
domains and leads to phosphorylation of IRAK, which
has serine/threonine kinase activity. IRAK then binds
TRAF-6 to form the IRAK–TRAF6 complex. This
complex activates the NF
k
B transactivator that induces
transcription of inflammatory cytokine genes. This
complex also activates the JNK pathway and generates
the active AP-1 transactivator as shown in Figure 1.In
addition to this MyD88-mediated component of signal-
ing pathway, a MyD88-independent component leading
to IFN-
b
production has been identified in TLR3 and
TLR4 signaling pathways.
MYD88-DEPENDENT
SIGNALING PATHWAY
Stimulation of MyD88-deficient macrophages with
known TLR ligands induced no production of inflamma-
tory cytokines. This indicates that MyD88 plays a pivotal
role in the TLR-mediated production of inflammatory
Encyclopedia of Biological Chemistry, Volume 4. q 2004, Elsevier Inc. All Rights Reserved. 190
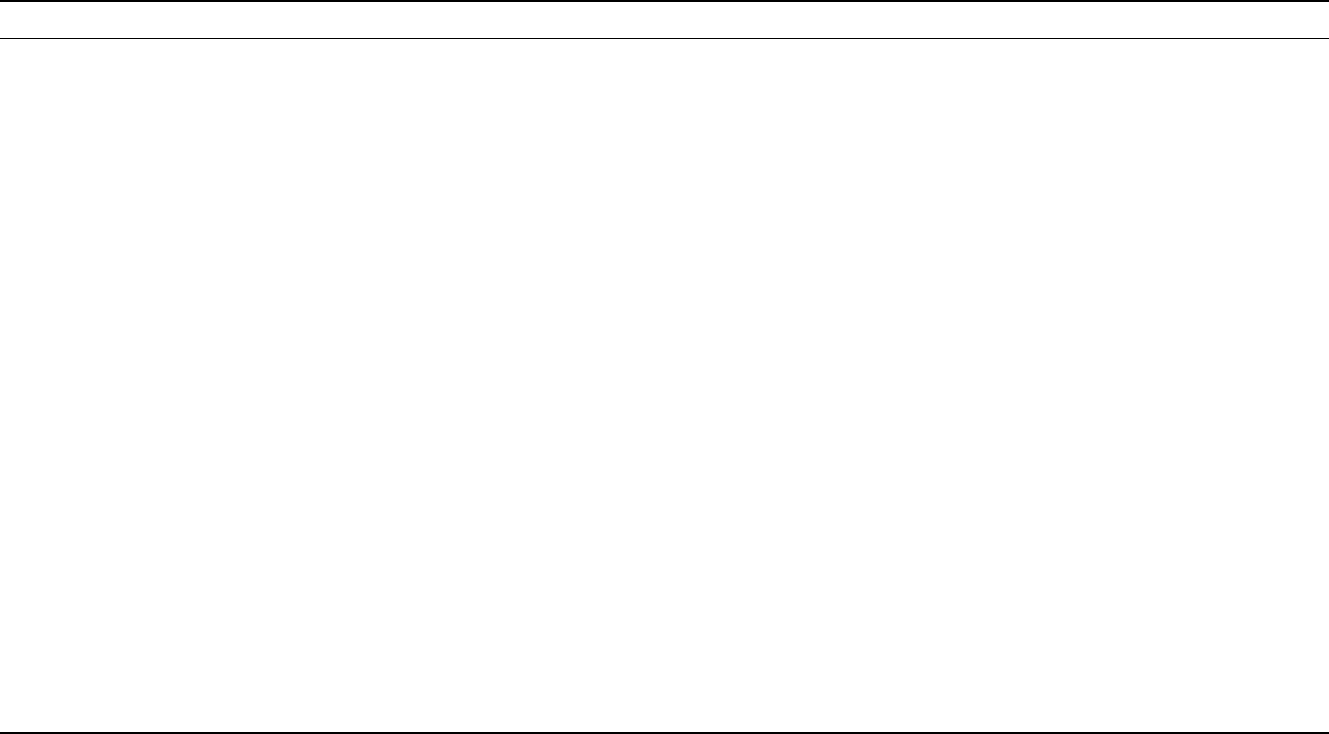
TABLE I
Description of Toll-Like Receptor
TLR Gene (Human) Functional receptor Ligands of TLR Immune response
TLR1 4p14 Heterodimers of TLR2-TLR1 Tri-acyl lipopeptides (bacteria, Mycobacteria), soluble factors,
Neisseria meningitis
Induce secretion of inflammatory
cytokine(s)
TLR2 4q32 Heterodimers of TLR2-TLR1
and heterodimer of TLR2-TLR6
Lipoprotein/lipopeptides (different pathogen),
peptiodoglycan (gram-positive bacteria),
lipoteichoic acid (gram-positive bacteria),
lipoarabinomannan (Mycobacteria),
a phenol soluble modulin (Staphylococcus aureus),
glycoinositolphospholipids
(Trypanosoma cruzi), glycolipid (Treponema maltophilum),
porins (Neisseria), zymosan (fungi), HSP70 (host),
atypical LPS (Leptospira interrogans), atypical LPS
(Porphyromonas gingivalis)
Induce secretion of inflammatory
cytokine(s)
TLR3 4q35 Not known Double-stranded RNA (mainly from virus) Induce type I interferon (IFN-
a
/IFN-
b
)
TLR4 9q32-33 LPS-LPS binding protein complex
associate with CD14 and MD-2,
in B-cell TLR4 associate with
RP105 and MD-1
LPS (gram-negative bacteria, taxol (plant), HPS60 (host),
HPS70 (host), fusion protein (RSV),
HPS60 (Chlamydia pneumoniae), fibronectin (host),
envelope proteins (MMTV), type III repeat
extra domain A of fibronectin (host), oligosaccharides
of hyaluronic acid (host), polysaccharides fragments
of heparin sulfate (host) and fibrinogen (host)
Induce secretion of inflammatory
cytokine(s) and chemokines
TLR5 1q33.3 Not known 55 kDa monomer obtained from bacteria flagella (flagellin) Induce inflammatory cytokine(s)
TLR6 4p14 Heterodimer of TLR2-TLR6 Di-acyl lipopeptides (mycoplasma) Induce secretion of inflammatory
cytokine(s)
TLR7 Xp22 Not known Synthetic compounds like imidazoquinoline
(imquimod (R-848), resiquimod (R848)),
loxoribine and bropirimine
Induce secretion of inflammatory
cytokine(s)
TLR8 Xp22 Not known Ligand(s) not known Not known
TLR9 3p21.3 TLR9 (localized in endosomal
compartment)
Unmethylated CpG DNA (Mycobacteria and Bacteria) Activate Th1 type immune
response, Induce proliferation
of B-cell, activate macrophage
and DCs
TLR10 Not known Not known Ligand(s) not known Not known
