Lefebvre A.H., Ballal D.R. Gas Turbine Combustion: Alternative Fuels and Emissions
Подождите немного. Документ загружается.

370 Gas Turbine Combustion: Alternative Fuels and Emissions, Third Edition
wall-cooling air. The temperature of this air is so low that all chemical reac-
tions are effectively frozen. Thus, the lm-cooling air emanating from the
primary zone normally contains signicant quantities of CO. Unless this CO
is subsequently entrained into the hot central core with sufcient time to
react to completion, it will appear in the exhaust gas.
9.4.1.5 Influence of Fuel Atomization
The main effect of mean drop size on CO emissions stems from its strong
inuence on the volume required for fuel evaporation. At low-power opera-
tion, where these emissions attain their highest concentrations, a signicant
proportion of the total combustion volume is occupied in fuel evaporation.
Consequently, less volume is available for chemical reaction.
9.4.2 unburned Hydrocarbons
UHC include fuel that emerges from the combustor in the form of drops
or vapor, as well as the products of the thermal degradation of the parent
fuel into species of lower molecular weight. They are normally associated
with poor atomization, inadequate burning rates, the chilling effects of lm-
cooling air, or any combination of these. The reaction kinetics of UHC forma-
tion are more complex than for CO formation, but it is generally found that
those factors that inuence CO emissions also inuence UHC emissions and
in much the same manner.
9.4.3 Smoke
Exhaust smoke is caused by the production of nely divided soot particles
in fuel-rich regions of the ame that, in conventional combustors, are always
close to the fuel spray. These are the regions in which recirculating burned
products move upstream toward the fuel injector, and local pockets of fuel
vapor become enveloped in oxygen-decient gases at high temperature. In
these fuel-rich zones, soot may be produced in considerable quantities.
Most of the soot produced in the primary zone is consumed in the high-
temperature regions downstream. Thus, from a smoke viewpoint, a combus-
tor may be considered to comprise two separate zones—the primary zone,
which governs the rate of soot formation, and the intermediate zone (and, on
modern high-temperature engines, the dilution zone also), which determines
the rate of soot consumption. The soot concentration actually observed in the
exhaust gas is the difference between two large numbers.
Analysis of the soot found in exhaust gases shows that it consists mostly
of carbon (96%) and a mixture of hydrogen, oxygen, and other elements. Soot
is not an equilibrium product of combustion except at mixture strengths far
richer than those employed in the primary zones of gas turbines. Thus, it
is impossible to predict its rate of formation and nal concentration from
Emissions 371
kinetic or thermodynamic data. In practice, the rate of soot formation tends
to be governed more by the physical processes of atomization and fuel–air
mixing than by kinetics.
9.4.3.1 Influence of Pressure
Problems of soot and smoke are always most severe at high pressures. There
are several reasons for this; some derive from chemical effects, whereas oth-
ers stem from physical factors that affect spray characteristics and hence the
distribution of mixture strength in the soot-forming regions of the ame. For
premixed kerosine/air ames, it is found that no soot is formed at pressures
below 0.6 MPa and equivalence ratios below 1.3.
One adverse effect of an increase in pressure is to extend the limits of
ammability, so that soot is produced in regions that, at lower pressures,
would be too rich to burn. Increased pressure also accelerates chemical reac-
tion rates, so that combustion is initiated earlier and a larger proportion of
the fuel is burned in the fuel-rich regions adjacent to the spray. With pressure
atomizers, reduced spray penetration is one of the main causes of smoke at
high pressures. At low pressures, the fuel is distributed across the entire
combustion zone, but at high pressures it tends to concentrate in the soot-
forming region just downstream of the fuel nozzle. Another adverse effect of
an increase in pressure is to reduce the cone angle of the spray. This encour-
ages soot formation, partly by increasing the mean fuel drop size, but mainly
by raising the mixture strength in the soot-forming zone. The total effect of
all these factors is that with pressure atomizers, smoke emission increases
steeply with pressure.
With airblast atomizers, the inuence of pressure on spray characteristics
is much less pronounced. Recent experimental studies carried out by Zheng
et al. [13] on a modern practical airblast atomizer, showed that spray cone angle
and spray volume are largely independent of pressure, provided that the air/
fuel ratio is kept constant, which corresponds to the normal engine situation at
power settings above idle. It was also observed that changes in pressure have
very little effect on the mean drop size in the spray. Thus, in contrast to pressure
atomizers, the spray characteristics of gas turbine airblast atomizers are largely
uninuenced by variations in ambient air pressure. This is the main reason
that combustors tted with airblast atomizers exhibit only small increases in
soot formation and smoke with an increase in combustion pressure.
9.4.3.2 Influence of Fuel Type
Fuel properties can inuence smoke production in two ways; rst by inducing
the formation of local fuel-rich regions, and second, by exerting variable resis-
tance to carbon formation. The former is controlled by physical properties,
such as viscosity and volatility, which affect the mean drop size, penetration,
and rate of evaporation of the fuel spray, whereas the latter relate to molecular
372 Gas Turbine Combustion: Alternative Fuels and Emissions, Third Edition
structure. It is well established that smoking tendency increases with an
increase in aromatic content of the conventional petroleum-based hydrocar-
bon fuel. This is because the polycyclic aromatic hydrocarbons (PAHs) form
the nuclei for the growth of the soot particles. Therefore, as discussed in
Chapter 10, if the fuel has no aromatic compounds (e.g., coal-derived fuels
produced by Fischer–Tropsch synthesis), it will produce virtually no soot,
as shown later in Figure 10.31. Also, hydrogen content is commonly used in
correlating rig and engine test data on various soot-related parameters such
as smoke emissions, ame radiation, and liner-wall temperature. However,
Chin and Lefebvre [14] have shown that a better index of sooting tendency
is the ASTM smoke point, which is obtained experimentally by burning the
test fuel in a wick lamp and slowly increasing the height of the ame until it
begins to smoke. The height of the ame in millimeters is the smoke point; the
higher this is, the lower the tendency of the fuel to soot formation.
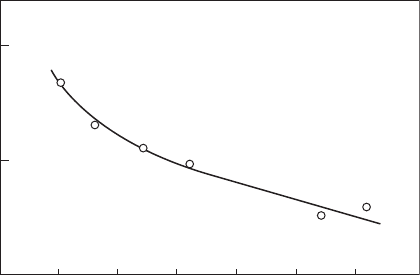
The correlation shown in Figure 9.4 was obtained from an analysis of
measurements of SN carried out on a Pratt & Whitney F100 combustor by
Russel [15]. The generally high quality of the data t obtained with this
and several other aircraft combustors led Chin and Lefebvre to conclude that
smoke point is superior to hydrogen content as a correlating parameter for
soot-related combustion phenomena.
9.4.3.3 Influence of Fuel Atomization
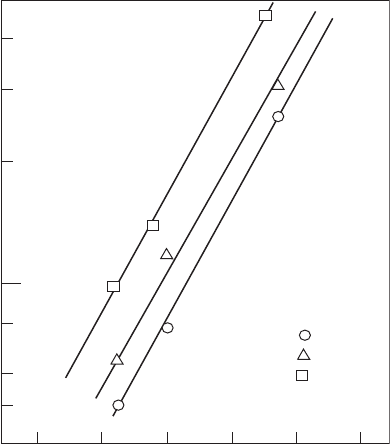
The inuence of fuel drop size on soot formation has been investigated by
Rink and Lefebvre [10] using the tubular combustor described above, sup-
plied with a kerosine fuel. Their results for a combustion pressure of 1.52 MPa
(15.5 atm) are shown in Figure 9.5. This gure shows that improvements
30
20
10
01020
Smoke point
Smoke number
30
F 100 cruise
P
3
= 1.12–1.18 MPa
FAR = 0.020
Figure 9.4
Correlation of smoke number with smoke point for an F100 combustor. (From Chin, J.S. and
Lefebvre, A.H., ASME Paper 89-GT-261, 1989. With permission.)
Emissions 373
in atomization quality inhibit soot formation. For example, at the highest
equivalence ratios, reducing the mean drop size from 110 to 30 µm effectively
halves the soot concentration. The importance of atomization quality to soot
formation and smoke stems from the fact that, as the fuel spray approaches
the ame front, heat transmitted from the ame starts to evaporate the drops.
The smallest droplets in the spray have time to evaporate completely ahead of
the ame front, and the resulting fuel vapors then mix with the combustion
air and burn in the manner of a premixed ame. However, the largest drops
in the spray do not have time to fully evaporate and mix completely with air
before being consumed by the ame. In consequence, they burn in the mode
of fuel-rich diffusion ames. Clearly, any increase in mean drop size will
increase the proportion of large drops in the spray. This, in turn, will raise
the proportion of fuel burned in diffusion-type combustion, as opposed to
premixed combustion.
In general, exhaust smoke decreases with mean drop size, but if improved
atomization should lead to a reduction in spray penetration, as occurs with
all types of pressure atomizers, the smoke output may actually go up because
of the local increase in fuel concentration. In fact, reduced spray penetration
is one of the main causes of smoke on high-pressure ratio engines tted with
dual-orice atomizers.
5.0
4.0
Fuel = kerosine
T
3
= 573 K
P
3
= 1.52 MPa
3.0
2.0
1.0
0.8
0.6
0.5
0.8 0.9 1.0
Equivalence ratio
1.1
SMD, µm
30
70
110
Soot emissions, g/m
3
1.2 1.3
Figure 9.5
Inuence of fuel mean drop size on soot formation. (From Rink, K.K. and Lefebvre, A.H.,
International Journal of Turbo and Jet Engines, 6(2), 113–22, 1989. With permission.)
374 Gas Turbine Combustion: Alternative Fuels and Emissions, Third Edition
9.4.4 Oxides of Nitrogen
Most of the nitric oxide (NO) formed in combustion subsequently oxidizes
to NO
2
. For this reason, it is customary to lump NO and NO
2
together and
express results in terms of NO
x
, rather than NO. It can be produced by four
different mechanisms: thermal NO, nitrous oxide mechanism, prompt NO,
and fuel NO.
9.4.4.1 Thermal Nitric Oxide
This is produced by the oxidation of atmospheric nitrogen in high-temperature
regions of the ame and in the postame gases. The process is endothermic
and it proceeds at a signicant rate only at temperatures above around 1850 K.
Most of the proposed reaction schemes for thermal NO utilize the extended
Zeldovich mechanism:
O
2
= 2O,
N
2
+ O = NO + N,
N + O
2
= NO + O,
N + OH = NO + H.
NO formation is found to peak on the fuel-lean side of stoichiometric.
This is a consequence of the competition between fuel and nitrogen for the
available oxygen. Although the combustion temperature is higher on the
slightly rich side of stoichiometric, the available oxygen is then consumed
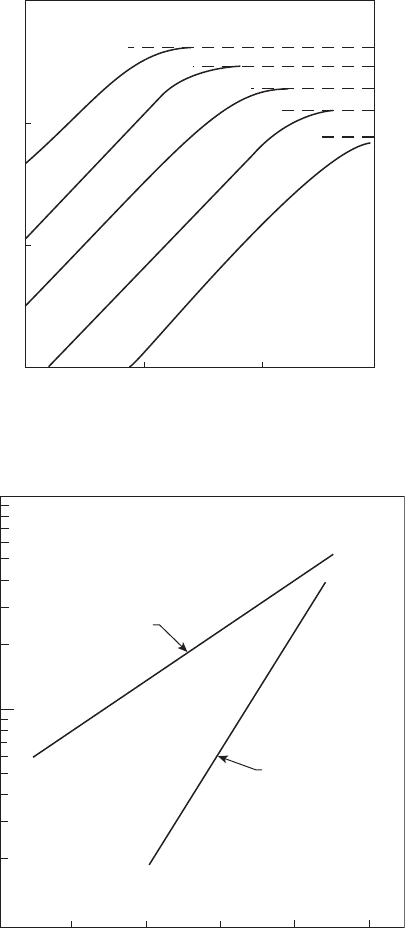
preferentially by the fuel. The exponential dependence of thermal NO on
ame temperature is demonstrated in Figure 9.6. This gure shows that NO
production declines very rapidly as temperatures are reduced, particularly
at normal combustor residence times of around 5 ms.
Figure 9.7 illustrates the exponential dependence of NO
x
on ame temper-
ature for both gaseous and liquid fuels. It is based on experimental data (not
shown in the gure) obtained by Snyder et al. [16] in their studies on the com-
bustion performance achieved when using a tangential entry lean-premixed
fuel nozzle. Of special interest in this gure is that the well-known differ-
ence in NO
x
emissions between liquid and gaseous fuels diminishes with
an increase in ame temperature, becoming negligibly small at the highest
levels of temperature. The reason for this is because when burning liquid
fuels there is always the potential for near-stoichiometric combustion tem-
peratures, and consequently high NO
x
formation, in local regions adjacent to
the fuel drops, although the average equivalence ratio throughout the com-
bustion zone may be appreciably less than stoichiometric. With an increase
Emissions 375
10
–2
10
–3
10
–4
10
–5
10
1
Time, ms
110
2
10
3
T = 2500 K
2400
2300
2200
NO, mass fraction
2100
φ = 1.0
Figure 9.6
NO
x
formation as a function of time and temperature; P = 1 MPa.
P = 1.44 MPa (14.2 atm)
T = 650–730 K
Gaseous fuel
(Methane)
Liquid fuel
(No 2 fuel oil)
50
100
40
30
20
10
NO
X
, ppm
5
4
3
2
1
1500 1600 1700
Flame temperature, K
1800 1900 2000
Figure 9.7
Dependence of NO
x
on ame temperature for liquid and gaseous fuels. (From Snyder,
T.S., Rosfjord, T.J., McVey, J.B., and Chiappetta, L.M., ASME Paper 94-GT-283, 1994. With
permission.)
376 Gas Turbine Combustion: Alternative Fuels and Emissions, Third Edition
in the equivalence ratio, the bulk ame temperature becomes closer to the
stoichiometric value, so that local conditions around the fuel drop have less
inuence on the overall combustion process and the NO
x
emissions begin
to approximate those produced by gaseous fuels when burning at the same
equivalence ratio.
9.4.4.1.1 Inuence of Inlet Air Temperature
As NO emissions are very dependent on ame temperature, an increase in
inlet air temperature would be expected to produce a signicant increase in
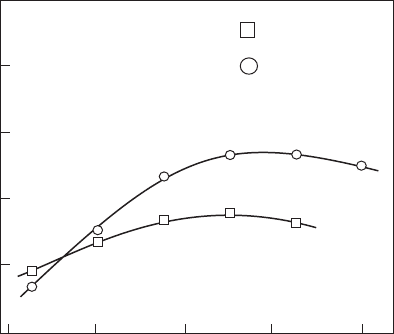
NO, and this is conrmed by the results shown in Figure 9.8 from Rink and
Lefebvre [17]. This gure contains data for a mean fuel drop size (SMD) of
110 microns, but similar results were obtained when the SMD was reduced
to 30 microns.
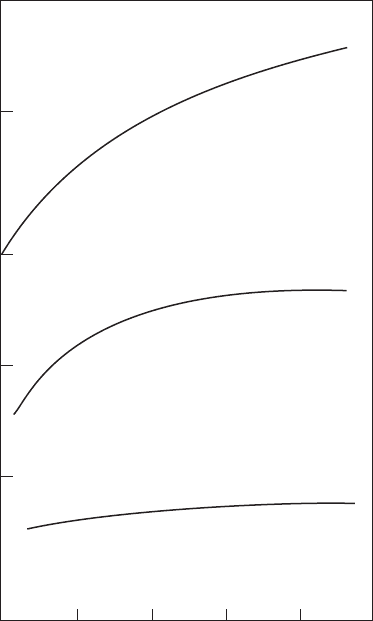
9.4.4.1.2 Inuence of Residence Time
Combustor residence time can also inuence NO
x
emissions, as shown in
Figure 9.9, which contains results obtained by Anderson [18] when using a
premix-prevaporize combustor supplied with premixed gaseous propane
fuel. It shows that NO
x
emissions increase with an increase in residence time,
except for very lean mixtures (ϕ ≅ 0.4), for which the rate of formation is so low
that it becomes fairly insensitive to time. Similar results showing the insensi-
tivity of NO
x
formation to residence time in lean-premixed combustion have
been obtained by Leonard and Stegmaier [19] and Rizk and Mongia [20].
750
Fuel = DF 2
P
3
= 0.76 MPa
SMD = 110 µm
600
450
300
150
0
0.4 0.6
Equivalence ratio
T
3
= 473 K
T
3
= 573 K
NO, ppm
0.8 1.0 1.2
Figure 9.8
Inuence of inlet air temperature on NO
x
formation. (From Rink, K.K. and Lefebvre, A.H.,
Combustion, Science and Technology, 68, 1–14, 1989. With permission.)
Emissions 377
These ndings have important practical implications to the design of lean-
premixed combustors.
The key points regarding thermal NO may be summarized as follows:
Thermal NO formation is controlled largely by ame temperature•
Little NO is formed at temperatures below around 1850 K•
For conditions typical of those encountered in conventional gas tur-•
bine combustors (high temperatures for only a few milliseconds), NO
increases linearly with time, but does not attain its equilibrium value
For very lean-premixed combustors (• ϕ < 0.5), NO formation is largely
independent of residence time
0.5
0.2
0.5
1.0
2.0
5.0
10.0
1.0 1.5
Residence time, ms
2.0 2.5
0.4
0.5
Equivalence ratio = 0.6
NO
X
, g/kg propane
3.0
Figure 9.9
Effect of residence time on NO
x
in a premixed fuel–air system. (From Anderson, D.N., ASME
Paper 75-GT-69, 1975. With permission.)
378 Gas Turbine Combustion: Alternative Fuels and Emissions, Third Edition
9.4.4.2 Nitrous Oxide Mechanism
According to Nicol et al. [21], this mechanism is initiated by the reaction
N
2
+ O = N
2
O,
and the nitrous oxide (N
2
O) formed is then oxidized to NO mainly by the
reaction
N
2
O + O = NO + NO,
but also by the reactions
N
2
O + H = NO + NH,
N
2
O + CO = NO + NCO.
9.4.4.3 Prompt Nitric Oxide
Under certain conditions, NO is found very early in the ame region—a fact
that is in conict with the idea of a kinetically controlled process. According
to Nicol et al. [21], the initiating reaction is
N
2
+ CH = HCN + N.
The balance of the prompt NO mechanism involves the oxidation of the
HCN molecules and N atoms. Under lean-premixed conditions, the HCN
oxidizes to NO mainly by a sequence of reactions involving HCN → CN →
NCO → NO. The N atom reacts mainly by the second Zeldovich reaction.
The inuence of pressure is of special interest and importance because
prompt NO can be a signicant contributor to the NO emissions produced in
lean premix (LPM) combustion [22]. Unfortunately, few data are available on
this effect. Fennimore’s [23] pioneering study of prompt NO in ethylene-air
ames over a range of pressures from 1 to 3 atm concluded that prompt NO
∝ P
0.5
. Later work by Heberling [24] over a much wider range of pressures
from 0.1 to 1.8 MPa showed that prompt NO was independent of pressure.
Altermark and Knauber [25] also concluded that NO
x
is independent of
pressure for equivalence ratios below 0.6. The practical implications of these
ndings are discussed below.
9.4.4.4 Fuel Nitric Oxide
Light distillate fuels contain less than 0.06% of organically bonded nitrogen
(usually known as fuel-bound nitrogen; FBN), but the heavy distillates may
contain as much as 1.8%. During combustion, some of this nitrogen reacts
Emissions 379
to form the so-called “fuel NO.” The fraction of nitrogen undergoing this
change increases only slowly with increasing ame temperature. As far as
gaseous fuels are concerned, natural gases contain little or no FBN, but some
is found in certain processes and low-Btu gases. Depending on the degree of
nitrogen conversion, fuel NO can represent a considerable proportion of the
total NO [26].
Nicol et al. [21] analytically examined the relative contributions of the vari-
ous mechanisms discussed above to the total NO
x
emissions produced by
a lean-premixed combustor burning methane fuel, for which the fuel NO
is zero. The results of their study show that at relatively high temperatures
of around 1900 K, and equivalence ratios of around 0.8, the contributions
are about 60% thermal, 10% N
2
O, and 30% prompt. With reductions in tem-
perature and equivalence ratio, the contributions made by N
2
O and prompt
NO increase signicantly until, at a temperature of 1500 K and an equiva-
lence ratio of around 0.6, the relative contributions to the total NO
x
emissions
become 5% thermal, 30% N
2
O, and 65% prompt. At the lowest equivalence
ratios (ϕ = 0.5–0.6), the major source of NO
x
is that formed by the N
2
O
mechanism. These results clearly have great importance to the design of
ultralow NO
x
lean-premixed combustors.
9.4.5 influence of Pressure on Oxides of Nitrogen Formation
Pressure effects on NO
x
formation are of special importance due to the
continual trend toward engines of higher pressure ratio to meet the
need for lower fuel consumption. Combustor testing at high pressures
is extremely expensive and it would, therefore, be highly convenient to
carry out combustion tests at low levels of pressure and then extrapo-
late the results obtained to high levels of pressure where NO
x
emissions
attain their highest values. Such extrapolation could be carried out with
condence if the relationship between NO
x
and pressure were accurately
known. Unfortunately, the experimental data obtained on different com-
bustor types are conicting in this regard. They vary from no effect of
pressure on NO
x
, to quite signicant increases in NO
x
with an increase
in pressure.
For conventional combustors, it is generally found that NO
x
∝P
n
, where n
has values ranging from around 0.5 to around 0.8. The results of Maughan
et al. [27] from a well mixed combustor supplied with natural gas fuel showed
an increase in n with an increase in exhaust gas temperature. For example,
raising the combustor outlet temperature from 1227 to 1310 K caused n to
increase from 0.38 to 0.51. Maughan et al. regard this result as evidence that
the lowest NO
x
levels result from the N
2
O and prompt mechanisms, which
dominate at low temperatures and are independent of pressure, whereas
the higher NO
x
levels associated with higher combustion temperatures are
due primarily to thermal NO
x
, which exhibits a square-root dependence
on pressure.
