Kutz M. Handbook of materials selection
Подождите немного. Документ загружается.


598 CHARACTERIZATION AND IDENTIFICATION OF PLASTICS
Fig. 3 Digital programmable viscometer. (Courtesy Brookfield Engineering Laboratories)
polymer melt processing operations. A rheometer is a precision instrument that
provides the accuracy and reproducibility necessary for polymer characterization
test.
A quality control test to qualify the incoming material is quick, simple, and
accurate. The capillary rheometer can be used to assure that a material complies
with the flow tolerances set for that grade of material. A quality control graph
of a polymer flow curve can be generated with upper and lower critical viscosity
limits over a wide range of shear rates by running multiple tests on a standard
material. The thermal stability of a polymer and resulting processing limitations
are of extreme importance to a processor. Capillary rheometer can accurately
predict the thermal stability of the polymer. Materials such as polyvinyl chloride
(PVC) can begin to cross-link and exhibit a dramatic increase in viscosity if left
in the process too long. This can lead to rejected or failure-prone parts and
wasted time and materials. The capillary rheometer allows the residence time
capabilities of materials to be studied at wide range of processing temperatures
and shear rates so the optimal processing temperature can be determined. The
data generated from capillary rheometer measurements can be used to study the
processibility of regrind material as well as process optimization. The capillary
rheometer consists of an electrically heated cylinder, a pressure ram, temperature
controllers, timers, and interchangeable capillaries. The plunger can be moved

1 MATERIAL CHARACTERIZATION TESTS 599
at a constant velocity, which translates to a constant shear rate. The force to
move the plunger at this speed is recorded, which determines the shear stress.
Alternately, a weight or constant pressure can be applied to the plunger, which
generates a constant shear stress, and the velocity of the plunger can be deter-
mined by cutting and weighing the output. Shear rate can be calculated by
knowing the melt density.
The sample material is placed in the barrel of the extrusion assembly, brought
to temperature, and forced out through a capillary. The force required to move
the plunger at each speed is detected by a load cell. Shear stress, shear rate, and
apparent melt viscosity are calculated. Shear stress versus shear rate and apparent
viscosity versus apparent shear rate curves are plotted.
1.3 Viscosity Tests
Viscosity is defined as the property of resistance to flow exhibited within the
body of a material expressed in terms of a relationship between the applied
shearing stress and the resulting rate of strain in shear. In the case of ideal or
Newtonian viscosity, the ratio of shear stress to shear rate is constant. Plastics
typically exhibit non-Newtonian behavior, which means that the ratio varies with
the shearing stress. There are two different aspects of viscosity. Dynamic or
absolute viscosity, best determined in a rotational type of viscometer with a small
gap clearance, is independent of the density or specific gravity of the liquid
sample and is measured in poises (P) and centipoises (cP). Kinematic viscosity,
usually determined in some form of efflux viscometer equipped with a capillary
bore or small orifice that drains by gravity, is strongly dependent on density or
specific gravity of the liquid and is measured in stokes (S) and centistokes (cS).
The relationship between two types of viscosity is
stokes
⫻ specific gravity ⫽ poises
The measurement and control of rheological properties are usually performed
with simple devices called ‘‘viscometers’’ or ‘‘viscosimeters,’’ which do not mea-
sure true viscosities of either the dynamic or kinematic type but make relative
flow comparisons. Viscosity of plastic materials is measured in three basic ways,
employing principles involving liquid deformation due to various forces.
1. Downward rate of gravity flow through capillary bores and small orifices
(capillary viscometers).
2. Upward speed of a trapped air bubble.
3. Torque developed by the liquid drag between moving and stationary sur-
faces.
The first method is basically used for thermoplastic materials. The latter two
are more commonly used for thermosetting materials, plastisols, and organosols
and are discussed in Section 1.2.
1.4 Gel Permeation Chromatography
Quite often traditionally used tests such as melt index or viscosity tests do not
provide enough information about the processibility of the polymer. Such tests

600 CHARACTERIZATION AND IDENTIFICATION OF PLASTICS
only measure an average value and tell us nothing about the distribution that
makes up the average. Take, for instance, average daily temperatures reported
for a particular city. These reports can be very misleading since they lack more
useful information about extreme high and extreme low temperatures. In a very
similar manner, the melt index and viscosity tests relate very well to the average
molecular weight of the polymer but fail to provide the necessary information
about the molecular weight distribution of the polymer. Two batches of resin
may have the same melt index, which simply indicates that their viscosity av-
erage molecular weights are similar. Their molecular weight distributions, the
number of molecules of various molecular weights that make up their averages,
can be significantly different. If an excessively high-molecular-weight fraction
is present, the material may be hard and brittle. Conversely, if an excessive
amount of low-molecular-weight fraction is present, the material may be soft or
sticky. When evaluating the nature of the incoming plastics material is extremely
important to a processor, he must look for a more reliable technique that will
provide the necessary information, to qualify the material, such as the measure-
ment of molecular weight distribution. The molecular weight distribution is the
single most fundamental property of the polymer. The molecular weight distri-
bution not only provides basic information regarding the processibility of the
polymer but also gives valuable information for predicting its mechanical prop-
erties.
Gel permeation chromatography (GPC) is the method of choice for determin-
ing the molecular weight distribution of a polymer. This technique has gained
wide acceptance among the plastic material manufacturers and the processors
because of its relatively low cost, simplicity, and its ability to provide accurate,
reliable information in a very short time. GPC reveals the molecular weight
distribution of a polymer compound. It detects not only the resin-based mole-
cules such as polymer, oligomer, and monomer but most of the additives used
in plastic compounds and even low-level impurities. The molecular weight dis-
tribution curve is plotted for a well-characterized standard material, and the
profile of the curve is compared with the test sample. In this manner, batch-to-
batch uniformity can be checked quickly as a means of quality assurance.
The separation of polymer molecules by GPC is based upon the differences
in their ‘‘effective size’’ in solution. (Effective size is closely related to molecular
weight.) Separation is accomplished by injecting the polymer solution into a
continuously flowing stream of solvent that passes through highly porous, tiny,
rigid gel particles closely packed together in a tube. The pore size of the gel
particles may vary from small to very large. As the solution flows through the
gel particles, molecules with small effective size (low molecular weights) will
penetrate more pores than molecules with larger effective sizes and, therefore,
take longer to emerge than the larger molecules. If the gel covers the right range
of molecular sizes, the result will be a size separation with the largest molecules
exiting the gel-packed tubes (columns) first.
A test sample is prepared by dissolving a small amount of polymer in the
solvent and filtering the solution to remove the undissolved impurities. The next
step is to select the proper size columns, connect them, set the sensitivity setting
on the detectors, and allow the instrument to equilibrate. A trial analysis is done
by injecting the polymer solution into the instrument. The chromatogram is

1 MATERIAL CHARACTERIZATION TESTS 601
Fig. 4 GPC equipment. (Courtesy Waters Corporation)
carefully analyzed. If the chromatogram shows all the desired information, the
final analysis is carried out. If not, the operating parameters, such as column
size, flowrate, and number of columns are optimized. The trial step is repeated
before proceeding to the final analysis.
During the final analysis, as the sample flows through the column, the mol-
ecules are separated according to size by a simple mechanical effect. Because
of their smaller size, the smaller molecules enter into the gel pores more readily
and, therefore, take extra time to reach the bottom of the column. The various
molecular weight species are separated by the difference in travel time through
the column and pass through a detector in descending order of size. The detector
measures the concentration of each molecular size and plots the molecular
weight distribution of the sample on a strip chart. Commercially available GPC
equipment is shown in Fig. 4.
A GPC curve can reveal a great deal of information. The regions of the
chromatogram can generally be easily and quickly correlated with the molecular
weights of the components of the polymer. The most direct—and sometimes the
most informative—use of the GPC curve is in the comparison of different ma-
terials by overlaying their chromatograms. Differences in molecular weight dis-
tributions, peak shapes, shifts, and tailing are readily observable. Comparisons
of additives and other lower molecular weight species are straightforward.
Frequently, a master chromatogram representing the acceptable range of GPC
profiles is established with samples from ‘‘good’’ batches. All subsequent
batches are then chromatographed and compared with the master curve as a
rapid quality control method. A number of correlations between the GPC curve
and the physical and processing behaviors of a polymer have been developed.
602 CHARACTERIZATION AND IDENTIFICATION OF PLASTICS
Fig. 5 Thermal analysis of plastics. (Courtesy Perkin-Elmer Corporation)
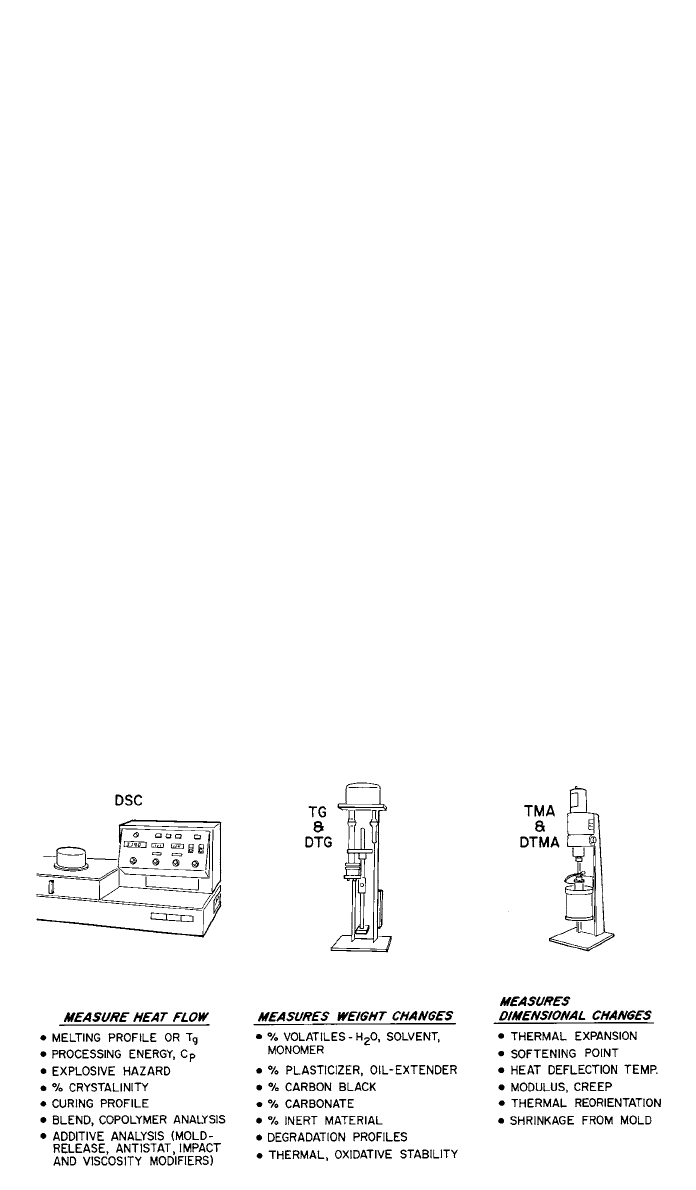
1.5 Thermal Analysis Techniques
Thermal analysis (TA) consists of a family of analytical techniques in which a
property of the sample is monitored against time or temperature while the tem-
perature of the sample is programmed. Properties include weight, dimension,
energy take-up, differential temperature, dielectric constant, mechanical modulus
evolved gases, and other, less common attributes. The application of thermal
analysis is widespread within the polymer and elastomer industries. Thermal
analyzers are used in industry to qualify a material for fitness of use and to
troubleshoot processing problems. Thermal analysis consists of three primary
techniques that may be used individually or in combination:
1. Differential scanning calorimetry (DSC)
2. Thermogravimetric analysis (TGA)
3. Thermomechanical analysis (TMA)
The maximum benefit of thermal analysis can be gained by using combination
of all three techniques to characterize a polymer. Figure 5 shows the three most
common techniques and the test methods for each. The following sections de-
scribe the more common techniques in terms of analytical capability and stan-
dard methodology.
Differential Scanning Calorimetry (ASTM D3417, ASTM D3418)
Differential scanning calorimetry (DSC), the most widely used thermal analysis
technique, is a technique in which the heat flowrate to the sample (differential
power) is measured while the temperature of the sample, in a specified atmo-
sphere, is programmed. Because all materials have a finite heat capacity, heating
or cooling a sample specimen results in a flow or heat in or out of the sample.
1 MATERIAL CHARACTERIZATION TESTS 603
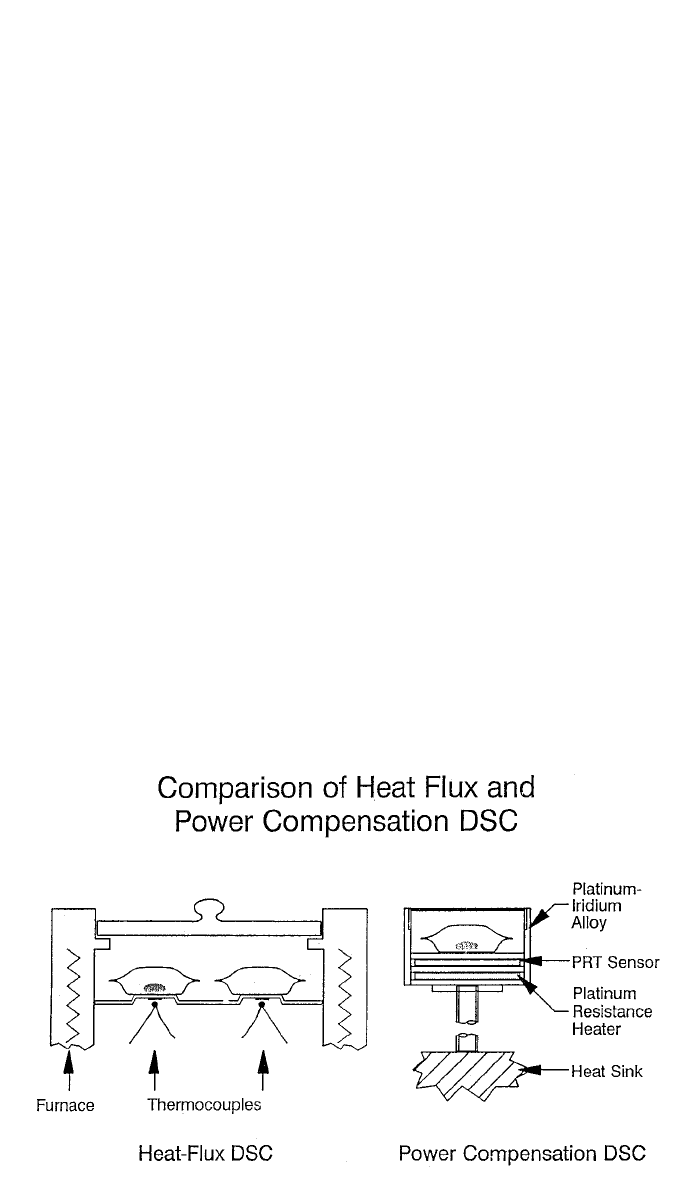
Fig. 6 Two most common types of DSC measuring cells. (Courtesy Perkin-Elmer Corporation)
The two most common types of commercial DSC measuring cells are shown in
Fig. 6. The heat flux DSC employs a disk containing sample and reference
positions that are heated by a common furnace. The differential heat flow to the
sample is proportional to the temperature difference that develops between
sample and reference junctions of a thermocouple. The power compensations
approach controls a temperature enclosure around the sample and reference in-
dividually. Through amplified feedback from platinum resistance thermometers,
it records the differential energy flow necessary to maintain the sample on the
specified temperature program. With either approach the output is heat flow,
normally expressed in milliwatts, watts/gram (normalized) or watts/gram-degree
(specific heat units).
The test procedure is simple. A small quantity of sample, usually 5–10 mg,
is weighed out into a inert capsule (usually made of aluminum). The encapsu-
lated sample is placed in the DSC sample holder or onto the sample platform
of a DSC cell disk. In the attached control module or computer the operator
selects a temperature range and heating rate or perhaps a more complex tem-
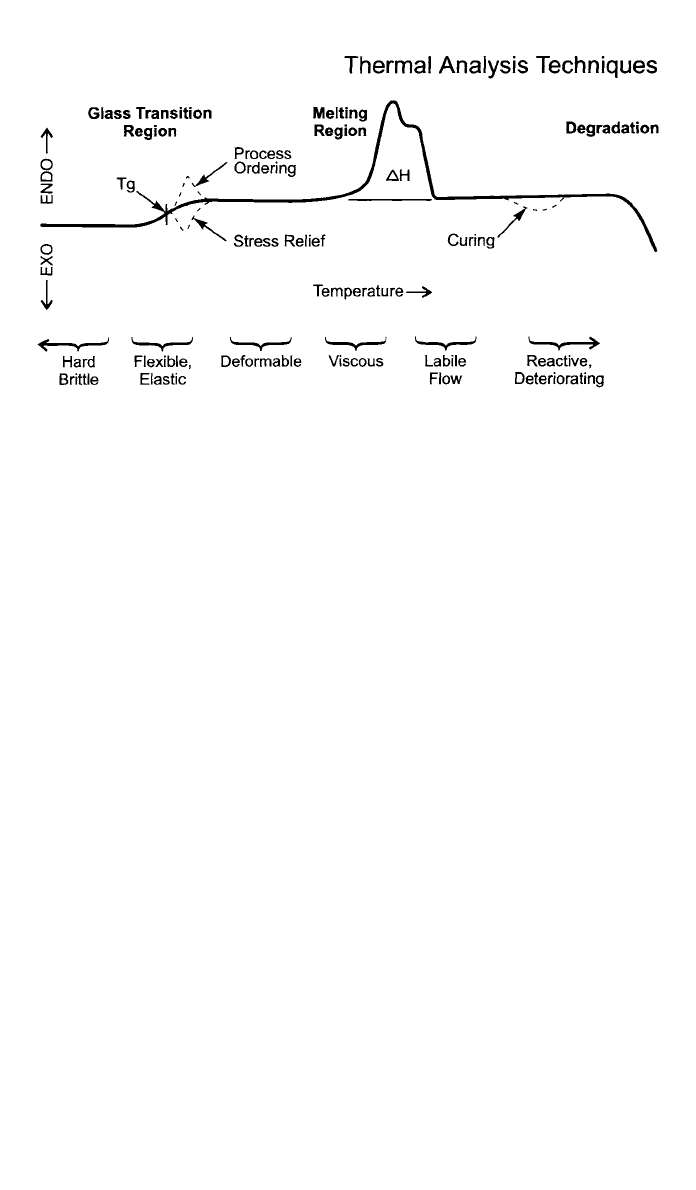
perature program. The test is started. (A hypothetical DSC curve showing both
endothermic and exothermic changes in a polymer is shown in Fig. 7.) Initially,
constant energy input is required to heat the sample at a constant rate. This
establishes a baseline. At a transition point, the sample requires either more or
less energy depending on whether the change is endothermic or exothermic. For
example, when the glass transition point is reached, the heat capacity increases.
The midpoint is taken as the glass transition temperature, T
g
. Adding plasticizers
to a formulation lowers T
g
. When a polymer reaches the melting point, it requires
more energy (endothermic) to melt the crystalline structure. The area of the peak
in units of energy is the enthalpy of fusion, the heat of melting. The temperature
dependence of the peak and its shape give information about degree of crystal-
linity, the molecular weight distribution, degree of branching, copolymer blend
604 CHARACTERIZATION AND IDENTIFICATION OF PLASTICS
Fig. 7 Typical DSC thermogram. (Courtesy Perkin-Elmer Corporation)
ratio, and/or processing history. Often quality procedures involve comparing the
melting profile to that of a standard ‘‘good’’ material.
When a sample cures, more energy is usually released, and the change is
exothermic. The area of the curing peak is proportional to the number of cross-
links that were formed. This indicates degree of cure. The shape of the curing
curve can be analyzed to obtain the reaction kinetic parameters.
Thermogravimetric Analysis (TGA)
Thermogravimetric analysis is a test procedure in which changes in weight of a
specimens is progressively heated. The sample weight is continuously monitored
as the temperature is increased either at a constant rate or through a series of
steps. The components of a polymer or elastomer formulation volatilize or de-
compose at different temperatures. This leads to a series of weight loss steps
that allow the components to be quantitatively measured. A typical high-
performance apparatus consists of an analytical balance supporting a platinum
crucible for the specimen, the crucible situated in a electric furnace. Variations
in instrumentation include horizontally mounted furnaces and top loading bal-
ances.
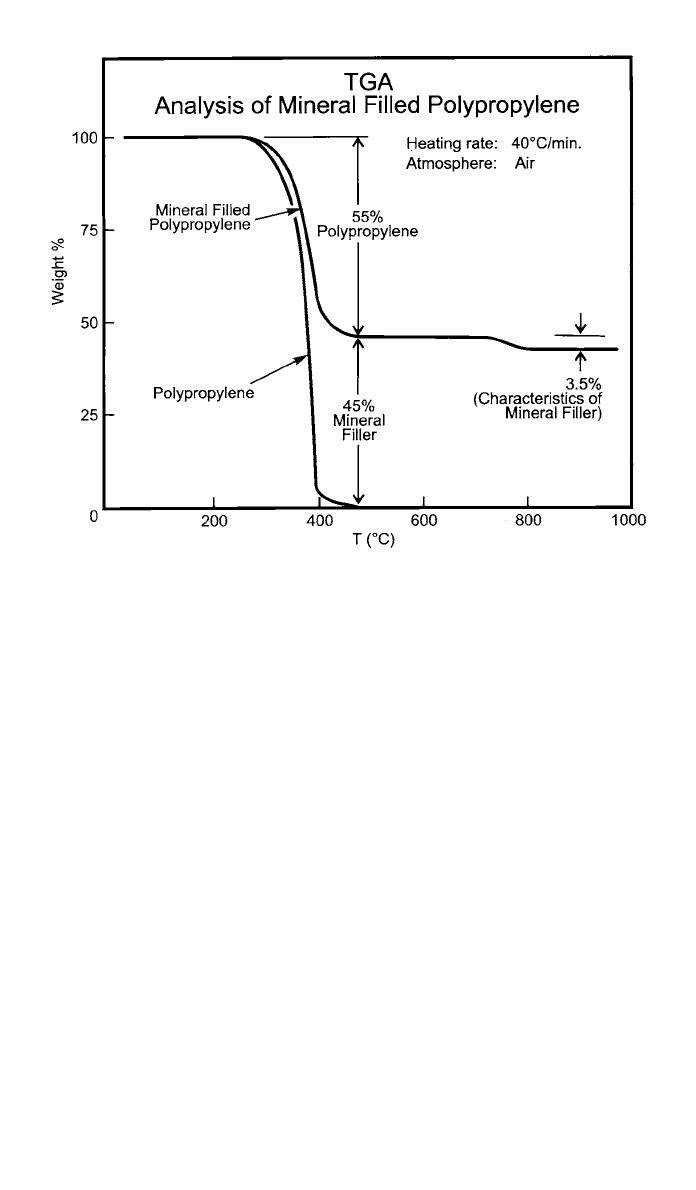
A simple thermal separation can be seen in the determination of mineral filler
in polypropylene (Fig. 8). The sample is heated in an air atmosphere to com-
pletely decompose the polypropylene such that the remainder is mineral filler.
The amount of volatiles (here, none), polymer, and filler is obtained by analysis
of the weight loss curve. The small weight loss (3.5%) occurring over the tem-
perature range of 750–800
⬚C is characteristic of the particular mineral filler used
in this sample. An unfilled polypropylene thermogram is also run to compare
with the filled formulation. TGA is very useful in characterizing polymers con-
taining different levels of additives by measuring the degree of weight loss. TGA
can also be used to identify the ingredients of blended compounds according to
the relative stability’s of individual components. The thermal stability of a pol-
ymer can be obtained through a kinetic analysis of the decomposition profile.
1 MATERIAL CHARACTERIZATION TESTS 605
Fig. 8 Typical TGA thermogram. (Courtesy Perkin-Elmer Corporation)
Thermomechanical Analysis (TMA)
When a sample is heated, its dimensions change because of thermal expansion,
stress reorientation, and deformation under an imposed stress. Thermomechan-
ical analysis consists of measuring these properties using a constant force on
the sample. Under a no-load or fixed load, it measures dimensional change in
the vertical direction as the sample temperature is controlled. TMA equipment
(Fig. 9) consists of a probe mechanically connecting three entities: (1) a force
transducer to control the force applied to the sample, (2) a position transducer
to measure the displacement, and (3) a temperature-controlled sample specimen.
Samples to be examined are cut to a defined geometry and then deformed by
the TMA probe in a defined manner. Sample deformation includes compression,
tension, and bending geometries. Once mounted, the sample is surrounded by a
furnace and monitored by a thermocouple.
A typical TMA thermal curve is shown in Fig. 10. Here a fused silica rod is
used as a compression probe. The probe is placed onto the sample, and the
thermal expansion or contraction is recorded as a function of temperature. The
polymer used in the test contains a chemical blowing agent. Over the first 60
⬚C,
the TMA probe is slightly pushed up by the expansion of the polymer solid. As
the melting point is reached, the lightly loaded probe penetrates the sample at
a rate related to the viscosity. This penetration is later reversed by the expansion
of the foam as the blowing agent is released. Polymer characterization through
the use of TMA is accomplished by determining the glass transition temperature,
the coefficient of expansion, and the elastic modulus. TMA data also correlates
with the Vicat softening point and the heat distortion temperature.
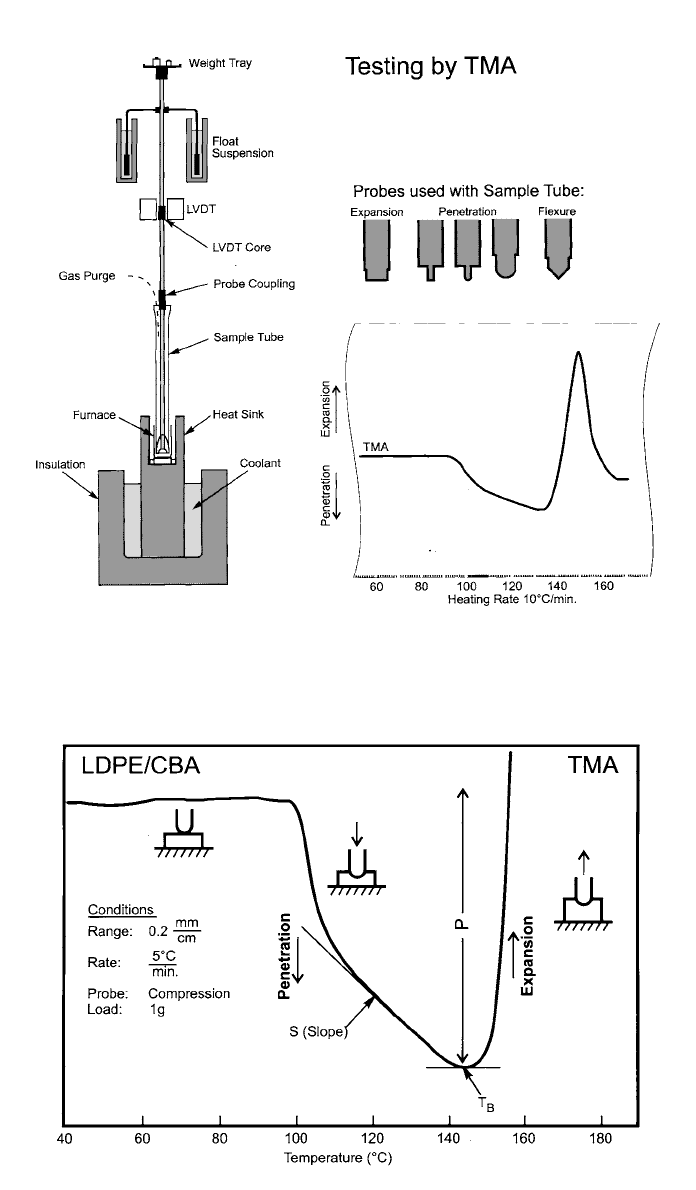
606 CHARACTERIZATION AND IDENTIFICATION OF PLASTICS
Fig. 9 Testing by TMA (schematic). (Courtesy Perkin-Elmer Corporation)
Fig. 10 Typical TMA thermal curve. (Courtesy Perkin-Elmer Corporation)

2 IDENTIFICATION ANALYSIS OF PLASTIC MATERIALS 607
1.6 Spectroscopy
Infrared spectroscopy is one of the most widely used material analysis tech-
niques for over 70 years. An infrared spectrum represents a fingerprint of a
sample with absorption peaks that correspond to the frequencies of vibrations
between the bonds of the atoms making up the material. Because each different
material is a unique combination of atoms, no two compounds produce the exact
same infrared spectrum. This fact allows a positive identification of polymeric
materials. By studying the size of the peaks in the spectrum, one can also de-
termine the amount of material present.
A newly developed technique called Fourier transform infrared (FT-IR) spec-
trometry overcomes the limitations encountered with the traditional infrared
technique. The original infrared instruments were of dispersive type, which sep-
arated the individual frequencies of energy emitted from infrared source using
a prism or grating. These instruments measured each frequency individually,
making the entire process painfully slow. Modern FT-IR instruments can process
as many as 100 samples per day compared to only 2–4 samples per day. FT-IR
spectroscopy is fast, precise, and simple, requiring a very small amount of sam-
ple for a successful amalysis. In infrared spectroscopy, IR radiation is passed
through a sample. Some of the infrared radiation is absorbed by the sample and
some of it is passed through (transmitted). The resulting spectrum represents
molecular absorption and transmission, creating a molecular fingerprint of the
sample. Figure 11 graphically illustrates this process.
The sample analysis process is quite simple with the use of modern spec-
trometer coupled with a powerful computer. Infrared energy is emitted from a
glowing black-body source. This beam passes through an interferometer where
the ‘‘spectral encoding’’ takes place. The resulting interferogram signal then exits
the interfermeter. Next, the beam enters the sample compartment where it is
transmitted though or reflected off the surface of the sample. This is where the
specific frequencies of energy, which are uniquely characteristic of the sample,
are absorbed. The beam finally passes through a detector and the signal is sent
to a computer where a mathematical technique called the Fourier transformation
takes place. The infrared spectrum is displaced on the CRT for analysis and
interpretation.
The computer is equipped with a collection of thousands of known polymer
and additive FT-IR spectra for easy comparison and identification. A spectral
library search can identify a polymer, additive, or a contaminant within minutes,
making the entire process very fast and efficient. Figure 12 illustrates the entire
FT-IR process.
2 IDENTIFICATION ANALYSIS OF PLASTIC MATERIALS
Plastic products are manufactured using a variety of processing techniques and
materials. It is practically impossible to identify a plastic material or product by
a visual inspection or a simple mechanical test. There are many reasons that
necessitate the identification of plastics. One of the most common reasons is the
need to identify plastic materials used in competitive products. Defective prod-
ucts returned from the field are quite often put through rigorous identification
analysis. Sometimes it is necessary to identify a finished product at a later date
