Kutz M. Handbook of materials selection
Подождите немного. Документ загружается.

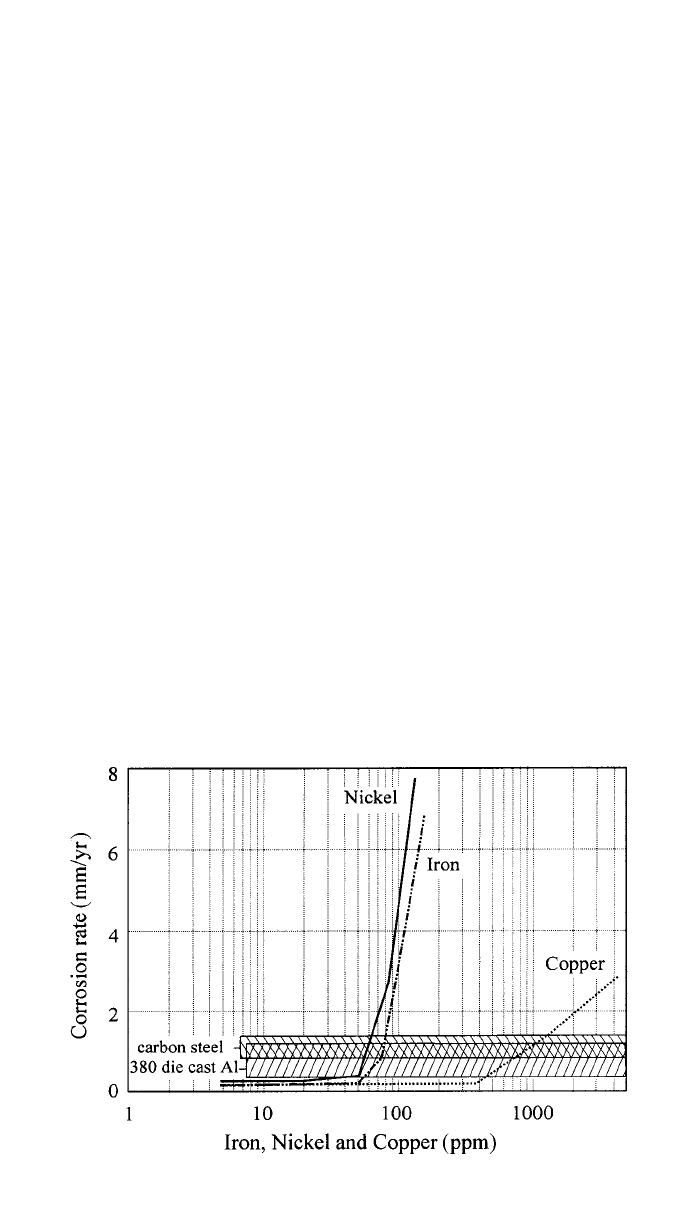
3 CORROSION 279
Fig. 8 Influence of Ni, Fe, and Cu impurities on the corrosion rate of die-cast AZ91 in the
standard salt spray test.
47
(Reprinted with permission from SAE paper number 850417 䉷 1985
Society of Automotive Engineers, Inc.)
hydrogen overvoltage. Noble impurities such as Fe, Cu, and Ni promote micro-
galvanic corrosion and show tolerance limits above which corrosion rate rapidly
increases
44,45
(see Fig. 8). Controlling the level of contamination below these
limits reduces the corrosion rate of Mg alloys containing Al by orders of mag-
nitude, making them very competitive. For example, high-purity AZ91 shows
lower salt spray corrosion rate than die cast 380 Al and cold-rolled steel
46,47
(see
Fig. 8). The individual tolerance limits depend on the specific alloy composition,
and a summary can be found in Ref. 27 for common high-purity alloys. For die-
cast high-purity AZ91 the ASTM specification B94
48
recommends: Fe ⬍ 50
ppm, Ni
⬍ 20 ppm, Cu ⬍ 300 ppm. Mg alloys containing Zr can be considered
to be of high purity because the highly reactive nature of Zr ensures that im-
purities present in the melt precipitate as compounds before any Zr can dissolve
in the Mg matrix.
49
The tolerant limits are important for recycled alloys as well.
During recycling care has to be taken to avoid contamination and secondary Mg
alloys aiming structural application have to be brought within the ASTM spec-
ifications.
The detrimental effect of noble metals decreases as follows: Ni
⬎ Fe ⬎ Cu.
Ni and Cu are usually not a problem because of their very low content in the
primary production. Fe is of most concern, especially due to the risk of Fe pick
up from carbon steel pots and casting molds. The solubility limit of Fe in Mg
is low, and it precipitates as Al
3
Fe, which acts as an effective cathode. The
detrimental effect of these precipitates is reduced by small additions of Mn (up
to 1%) to the melt (e.g., as MnCl
2
).
45,50
Manganese forms several Al–Mn–Fe
intermetallic particles, which can be removed after settling, and reduces the
effect of the remaining Fe containing particles compared to Al
3
Fe.
36,50,51
How-
ever, Mn should not be alloyed in excess of that necessary to reduce the Fe
content to the appropriate level because Fe-free Al–Mn particles also exhibit

280 CORROSION AND OXIDATION OF MAGNESIUM ALLOYS
some cathodic activity, particularly the Mn-rich ones. In addition to the maxi-
mum limit for Fe, specifications for high-purity alloys include requirements on
the Fe/Mn ratio (e.g., 0.032 and 0.021 for AZ91D and AM60B, respectively).
48
Poor corrosion resistance is also associated with the presence of nonmetallic
inclusions such as chlorides and oxides.
38
The development in the Mg foundry
practice, which includes the use of gas mixtures (e.g., SF
6
–air–CO
2
), to protect
the melt against excessive oxidation and the elimination of fluxes reduced sub-
stantially this concern. For a number of alloys in both cast and wrought condi-
tions, the critical concentration of chloride for pit initiation was reported to range
between 2
⫻ 10
⫺
3
and 2 ⫻ 10
⫺
2
M NaCl.
52
Only if impurities are controlled, other approaches such as the beneficial
presence of alloying elements and the use of favorable heat treatments or RS
become important.
Alloying
Keeping in mind the electrochemical properties of magnesium, it is clear that
measures to enhance corrosion resistance by alloying require the use of elements
with electrochemical potential close to that of magnesium (in order to avoid
microgalvanic processes) and ability to help formation of the passive film. The
majority of the elements conventionally alloyed to magnesium do not improve
significantly corrosion resistance, e.g., addition of Zn and Si. Mn and Zr con-
tribute significantly by reducing the effects of the impurities. The most important
benefit is provided by Al, which is simultaneously the most important alloying
element of Mg. RE and alkaline metals such as Ca are very attractive in regard
to the electrochemical potential, and efforts are being done to develop alloys or
to modify current alloys with small amounts of these elements.
Aluminum. Commercial Mg–Al alloys contain typically 2–10% of Al. In
chloride environments there is a significant decrease in the corrosion rate, in-
dependent of the alloy type, as the Al is increased up to 4%. Further increase
in the alloyed Al results in a smoother improvement of the corrosion resistance
51
(see Fig. 9).
Alloying with Al results in the precipitation of Mg
17
Al
12
(

phase), in partic-
ular along grain boundaries (see Fig. 10a). In addition, there is some evidence
for an Al gradient in the matrix, i.e., a decrease from the vicinity of the

phase
toward the bulk.
33,35
Mg
17
Al
12
precipitates and Al-rich coring zones act as a
barrier against the extension of local corrosion, enhancing the corrosion resis-
tance of Al containing alloys.
The beneficial effect of the

phase results from two effects:
33
(1) It is elec-
trochemically nobler than the Mg matrix while having small cathodic activity
as compared to Al–Mn–Fe intermetallics. (2) It is corrosion resistant over a
wide pH range (4–14) by combining the passive properties of Mg in alkaline
solutions with those of Al in neutral and slightly acidic media. The polarization
curve of Mg
17
Al
12
in 5% NaCl saturated with Mg(OH)
2
shows an anodic current
plateau
54
suggesting the formation of a partially protective film. The preferential
corrosion of the anodically more active bulk matrix (see Fig. 10b) might be the
reason for the ‘‘honeycomb’’-type morphology observed on corroded Al con-
taining alloys.
50,51
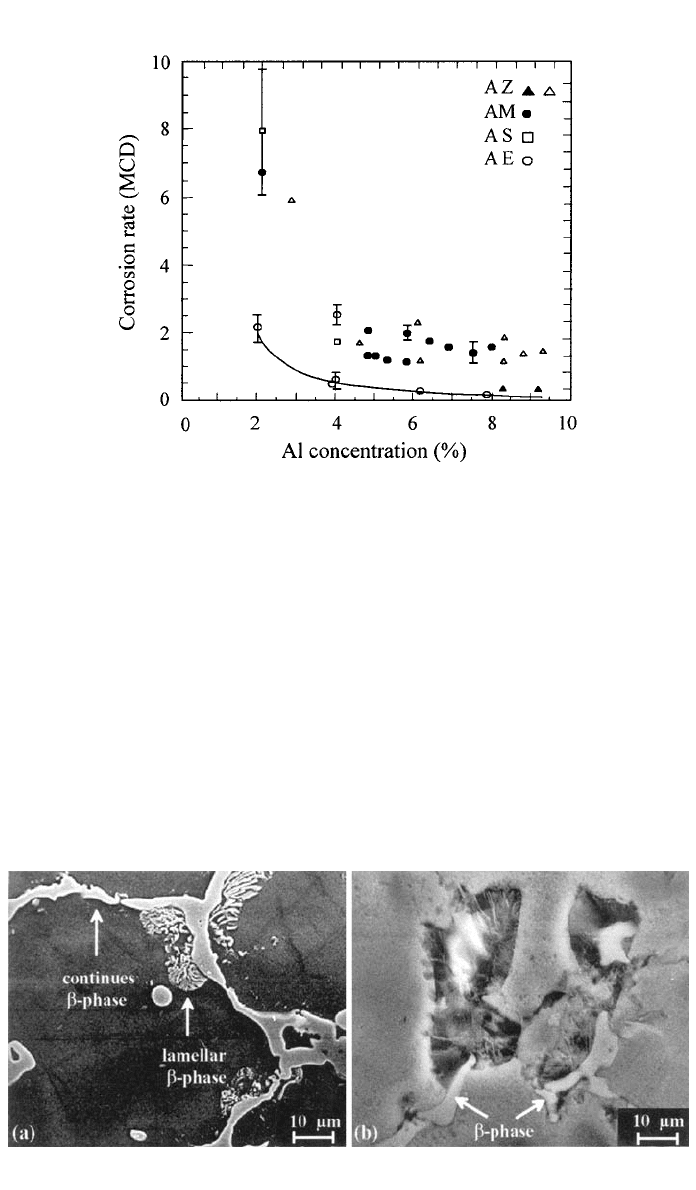
3 CORROSION 281
Fig. 9 Influence of Al concentration on the corrosion rate of die-cast Mg alloys during immer-
sion in 5% NaCl.
51
(Reprinted with the permission from SAE paper number 930755
䉷 1993 Society of Automotive Engineers, Inc.)
Fig. 10 AZ91 ingots: (a) typical microstructure and (b) preferential dissolution of the matrix and
stability of the

phase after 24 h immersion in 5% NaCl solution.
53
Aluminum additionally improves corrosion resistance by improving the prop-
erties of the surface film. With increasing Al content all layers of the film formed
on Mg–Al alloys become continuously enriched in Al
2
O
3
and dehydrated, and
layer thickness decreases.
12,13
Such stabilization of the film is especially advan-
tageous for the inner layer, which is responsible for the passivity of the surface
in the presence of water. The beneficial changes are significant up to 4% Al
in the alloy and cause the sudden decrease in the corrosion rate observed in
Fig. 9. Further alloying results in only minor changes in the oxide properties as
well as in corrosion resistance. This transition relates with the alumina compo-
nent in the film. Alloying up to 4% causes the oxide to enrich gradually in Al

282 CORROSION AND OXIDATION OF MAGNESIUM ALLOYS
and the film properties to improve. At around 4% of alloyed Al a threshold of
35% Al in the oxide film is achieved and further alloying does not enhance
corrosion resistance significantly because the amount of Al in the oxide remains
unchanged. Improvements at this composition range have to be correlated with
other factors such as an increase of

-phase. The Al threshold agrees well with
the composition of oxides grown on the same type of alloys in ultrahigh vacuum
and dry air shown in Fig. 1.
13
Alloying with Al is an effective way to develop corrosion-resistant Mg alloys
having surface films with reduced propensity for hydration and ionic conductiv-
ity. However, alloying with at least 4% Al is necessary to obtain an oxide with
optimum corrosion properties. Only at this threshold the Al
2
O
3
component forms
a continuous passivating network, which could be a skeletal structure in the
amorphous mixture of aluminum and magnesium (hydr-)oxides.
12,55
It was also found that aluminum can have a detrimental effect on corrosion.
Al was claimed to decrease the tolerance limit for Fe in an almost linear way
37,56
and in small amounts (below 8%) to produce an anodically more active Mg solid
solution.
33
The negative trend in the
␣
phase, however, reverses for high Al
contents.
Rare Earth (RE) Elements. Further improvement in the corrosion resistance
of Mg–Al alloys can be achieved by alloying with RE elements. RE elements
further reduce the hydration of the oxide film, being a possibility to diminish
the Al threshold for passivity.
57
The presence of RE promotes the enrichment of
the inner oxide layer uniformly with Al
2
O
3,
also for Al contents in the alloy
below the critical value of 4%. The formation of an oxide that is more uniformly
enriched in Al agrees with the relatively small effect of RE on the oxide prop-
erties in the absence of Al.
57
An enrichment of trace amounts of RE in the oxide
film was suggested by several authors but could not be definitely verified.
Another advantage of RE elements is their electrochemical potential, which
being very close to that of Mg does not promote galvanic processes. Although
RE show a small equilibrium solubility in the Mg matrix in the presence of Al
and precipitate as Al–RE particles, these phases are electrochemically passive.
Precipitation of Al
4
RE was even suggested to enhance corrosion resistance due
to the formation of Al coring along the grain boundaries.
51
RE elements also
assist in removing Fe from the melt by settling Al–Mn–Fe–RE phases and
reduce their cathodic activity.
55,58
Some RE-based intermetallics exhibit high
melting temperatures and enhance creep resistance at high temperatures.
Mg–Al–Zn alloys with RE and Mn additions exhibit attractive combination
of corrosion resistance, strength, and ductility. These alloys were successfully
developed by Allied-Signal’s Metals, both by conventionally casting and RS.
59,60
Another standard Mg alloy containing RE is the WE43 [approx. 4% Y, 3.3%
Nd
⫹ hydrogen reaction equilibrium (HRE)] developed by Magnesium Elektron
Ltd., which is a high-strength and corrosion-resistant alloy with long-term sta-
bility at higher temperatures. In chloride solutions AE alloys exhibit lower cor-
rosion rates than AS, AM, and AZ alloys with similar Al content (see Fig. 9).
Small additions of RE, typically 1–2%, were reported to reduce the dissolution
rate of conventional AM alloys by a factor of six.
24

3 CORROSION 283
Zinc. The presence of Zn improves corrosion behavior, although this effect
is not very remarkable. Zinc has an appreciable solubility in the Mg matrix and
the

phase. It is reported to increase the tolerance limits, although to a lesser
extent than Mn,
32,44
and to make the Mg(Al) matrix more noble.
33
In alloys
containing Al it was suggested to improve the stability of the oxide in the same
manner as RE.
13,57
However, a related improvement in the corrosion rate of AZ
alloys is not observed probably because Zn also increases the cathodic reaction
rate.
54
Silicon. Silicon is added to AS alloys where it increases the strength due to
precipitation of Mg
2
Si particles. Mg
2
Si exhibits a corrosion potential close to
that of Mg being relatively innocuous to corrosion behavior.
51
A small fraction
of the alloyed Si is present in the form of Al–Mn–Fe–Si compounds and plays
a similar role to that of Mn in reducing their cathodic activity.
58
Zirconium. Mg alloys containing Zr generally exhibit good corrosion per-
formance and are relatively insensible to Fe and Ni. Zr combines with Fe to
form insoluble particles that precipitate before casting.
37
Since Zr also forms
stable compounds with Al or Mn and is removed from the melt, it cannot be
added to alloys containing these elements.
Calcium. The effect of Ca on the corrosion resistance of Mg is yet to be
understood. Regarding the electrochemical potential, addition of Ca should result
in low microgalvanic effects and reduce the anodically active area upon pre-
cipitation. This was reported for Mg–Ca–Cu splats where precipitation of
Mg
2
Ca neutralizes the galvanic effect from the Mg
2
Cu phase. In the case of
RS AZ91 alloy an addition of about 2% Ca improved the corrosion rate from
0.8 to 0.2 mm/yr.
61
However, a detrimental influence of Ca was reported for
both conventional and RS binary Mg alloys.
44,62
Alloying conventional AM
alloys with small amounts of Ca slightly increases the corrosion rate in NaCl.
24
Films formed on AM50⫹1%Ca are extremely fine nanocrystalline [identified as
Mg
6
Al
2
(OH)
18
䡠 4.5H
2
O and Mg(OH)
2
] and consist of at least two layers (see
Fig. 11): A hydrated inner layer, which under electron irradiation develops a
cellular structure and transforms into MgO, and a thin, more stable outer layer.
Similar morphology is observed in films formed on Mg and Mg–Al alloys in
water-containing environments (see Fig. 7). Sometimes an additional crystalline
layer is observed at the interface alloy/inner layer.
Ca is very unstable in aqueous solutions and Ca(OH)
2
forms at pH even higher
than Mg(OH)
2
. The presence of Ca(OH)
2
is not necessarily expected to stabilize
the Mg film due to its greater tendency to dissociate chemically in the presence
of water.
31
The question whether a combination of Ca and RE is a more prom-
ising approach is now under investigation. Mechanical properties and thermal
stability also benefit from the presence of Ca and RE.
Processing
For a given alloy composition corrosion properties are further influenced by the
microstructure, which obviously depends on the production process. It is gen-
erally accepted that processes resulting in fine-grained, pore-free, and heat-

284 CORROSION AND OXIDATION OF MAGNESIUM ALLOYS
Fig. 11 TEM cross section of the film formed on AM50⫹1%Ca during immersion in NaCl
(after Ref. 24).
treatable microstructures enhance corrosion resistance. In this connection, high
cooling rates as achieved by new techniques such as RS are very useful.
Conventional Methods. Pressure die casting typically takes place at lower
temperatures and solidifies in a fraction of time as compared to gravity casting,
thus leading to finer microstructures. Die-cast alloys, e.g., AZ91, are generally
more corrosion resistant than their mold-cast counterparts.
47
If the matrix grains
are fine, a more continuous

phase forms along their boundaries, and the barrier
area per material volume is larger.
58
The casting process also affects the size
and distribution of cathodic phases formed when impurities are present. In AZ91
alloys the Ni tolerance was reported to be significantly lower for the gravity-
cast application than for die-cast application.
47
The Fe tolerance was essentially
unaffected by the cast process, being in both cases determined by the amount
of Mn present. Heat treatments seem to have no influence on size and distri-
bution of primary phases in the Al–Mn–Fe series.
33
The corrosion resistance of Mg alloys can be further improved by heat treat-
ments which promote the precipitation of the

phase.
33,54
In fact, the corrosion
resistance of AZ91 in the artificially aged condition (T6) is superior than in the
as-cast (F) or homogenized (T4) states. A reduction on the maximum pit depth
is also observed in artificially aged specimens. Aging causes precipitation of
secondary

phase along the grain boundaries and improves the barrier effect
against corrosion. On the other hand, solution heat treatment dissolves the

phase and often leads to slightly increased corrosion rates relative to the as-cast
condition.
Thixomolding is the high-speed injection molding of thixotropic, semisolid
alloys. In the recent years the thixomolding process is becoming popular for
production of Mg parts. In this technique fluxes are eliminated because no ex-
ternal melting is required and oxide formation is precluded by the use of an
argon atmosphere. Thixomolded materials exhibit less porosity and nonmetallic
inclusions. The corrosion rate of thixotropic-molded Mg alloys containing Al is

3 CORROSION 285
reduced by 50% or more as compared to die-cast counterparts.
1
This reduction
is attributed to an Al enrichment at the surface, due to shear thinning that results
in few if any
␣
particles at the surface, and a reduction in pinhole porosity. Die
castings are more prone to porosity (due to the high velocity turbulence of
molten metal entering the die) and oxide and flux inclusions.
Micropores should have a detrimental effect on the corrosion resistance of
Mg parts due to the following reasons:
27
(1) Autocatalytic corrosion cells can
form when electrochemical reactions inside the pore are obstructed by the cor-
rosion products. (2) Micropores result normally from defects in the alloy, which
are more active points for corrosion. (3) By increasing the real exposed surface,
they increase the corrosion rate (i.e., amount of corrosion per unit area of ap-
parent surface).
Rapid Solidification (RS). Rapid solidification introduces important micro-
structural changes, e.g., significant refinement of matrix and intermetallics, more
homogeneous distribution of impurities, metastable extension of the solid solu-
bility, and formation of amorphous phases, which contribute toward the forma-
tion of more protective films and the elimination of microgalvanic effects. RS
can be successfully applied to Mg alloys.
63
However, one should keep in mind
that the industrial feasibility of RS Mg alloys is restricted due to the limited
geometry of these materials necessary to achieve high cooling rates. A common
method to achieve high cooling rates during solidification is melt spinning. The
melt-spun ribbons can be mechanically grounded to powders, sealed in cans,
and extruded to produce bars. Another way to overcome the problem of ge-
ometry might be the application of the corrosion-resistant RS material at the
surface, e.g., by laser melting/alloying.
As in conventional alloys, Al suppresses the corrosion rate of binary RS Mg
alloys, in particular for large additions; however, addition of Zn, Si, and Ca
enhances it.
62,64
Zinc shows a somewhat anomalous effect, causing the corrosion
rate of RS magnesium to increase to a maximum at 18.6% Zn and then decrease
with further additions.
RS intensifies the benefits of Al and RE in the stabilization of the passive
film in two ways: It increases their solid solubility limits and provides a more
homogeneous substrate where it is easier to form the film. The maximum solid
solubility of Al in Mg was extended up to 24.5 or 23.4% by splat cooling or
melt spinning, respectively.
65,66
In 0.001 M NaCl an anodic plateau develops at
low currents, the pitting potential shifts to more noble values, and the corrosion
rate decreases by two orders of magnitude when the Al in solid solution is
extended from 9.6 to 23.4%.
66
Homogeneous Mg(Al) solid solutions are advan-
tageous because of the formation of Al-stabilized film over the entire surface.
Improvements in the passivation behavior in 0.01 M NaCl were also observed
in several RS alloys containing RE, e.g., Mg–Zn–Zr–RE (RE
⫽ Nd, Ce, or Y),
Mg–Nd, and Mg–Y.
67,68
As mentioned above, it is not clear whether RE elements
become enriched in the surface film; in RS alloys both enrichment
68,69
and de-
pletion
70
were reported.
If the films become more stable along with a more homogeneous alloy mi-
crostructure, susceptibility to pitting should be reduced. This was observed for
AZ61, where RS increases the breakdown potential by about 200 mV in Cl
⫺
-
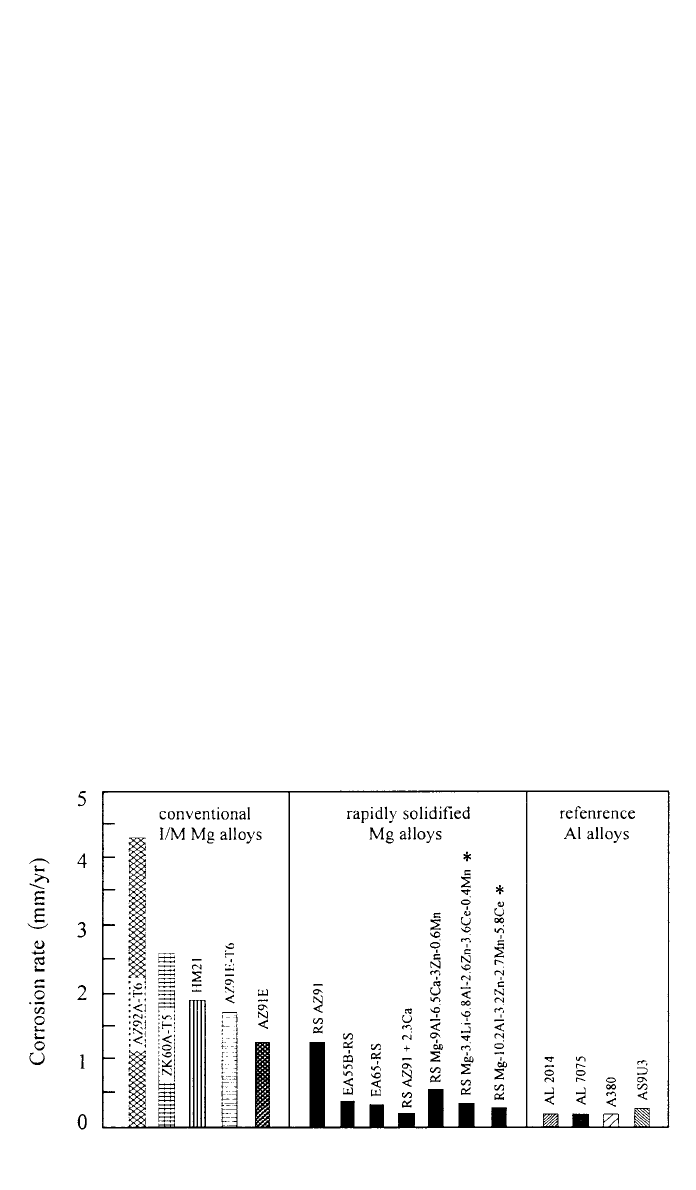
286 CORROSION AND OXIDATION OF MAGNESIUM ALLOYS
Fig. 12 Corrosion rate of light alloys obtained by weight loss measurements in 5% NaCl
solution. * Data from electrochemical impedance spectroscopy. (Reprinted, by permission
from Ref. 61. Copyright 䉷 1996 by Wiley-VCH.)
containing solutions.
62
Since pitting is assumed as a necessary precursor to SCC,
RS might help to produce SCC-resistant Mg alloys.
RS was also suggested to increase the tolerance limit for Fe.
71
The ennoble-
ment of the matrix with Al and the reduced rate of proton discharge around the
refined Fe inclusions were considered to be the origin of this improvement. RS
does not necessarily eliminate microgalvanic reaction between Mg matrix and
heavy metal precipitates as shown by the higher corrosion rates of RS Mg–Mn
alloys containing Ni in comparison with RS pure Mg.
72
However, if RS leads
to a sufficiently fine partially crystallized or fully amorphous structure (where
heavy metals and Mn are dispersed or even solved), the microgalvanic reaction
is suppressed and the corrosion moves to a more general kind.
73,74
RS Mg–Al–Zn alloys (around 9–21% Al and 3–7% Zn) exhibit up to one
order of magnitude lower corrosion rates and better anodic polarization behavior
in NaCl than the conventionally cast counterparts.
75
A several years development
program on extruded RS Mg–Al–Zn alloys with, e.g., Mn and RE additions,
resulted in the evaluation of alloys, e.g., EA55RS (4.8–5.2% Al
⫹ 4.7–5.0% Zn
⫹ 5.0–5.5% Nd), with better performance than the corrosion resistant AZ91.
76
Figure 12 shows that corrosion rates of RS alloys in NaCl are approaching to
those of Al alloys.
Amorphous materials are well known for their improved corrosion resistance,
arising from the chemically homogeneous single-phase nature without compo-
sitional fluctuations and crystal defects. Glass-forming ability can be found in
many Mg systems, e.g., in Mg–Ni, Mg–Cu, Mg–Ln (Ln
⫽ lanthanide metal),
Mg–Y and Mg–Ca systems often with ternary additions
63
but allows only thin
glassy ribbons. Many of these new partially or fully amorphous alloys are at-
tractive, since they attain high corrosion resistance. For example, amorphous
and/or nanocrystalline Mg–Y (20–26 at.% Y) films produced by magnetron
cosputter deposition perform better in 0.1 M NaCl than pure Mg and even the

3 CORROSION 287
WE43 alloy.
69
Recently, new perspectives were open with bulk amorphous alloys
in the system Mg–TM–Ln (where TM is Ni or Cu and Ln is a lanthanide
metal),
77–79
which could be used as structural materials. These glasses display
an exceptionally large glass-forming ability and high thermal stability. The
supercooled liquid region before crystallization is very wide (
⬎60⬚C for
Mg
65
Cu
25
Y
10
and ⬎40⬚C for Mg
50
Ni
30
Y
20
) and the temperature of crystallization
increases with the solute content.
77,80
The most promising bulk amorphous alloy
is Mg
65
Cu
25
Y
10,
which can be produced with a thickness of 4 mm by metallic
mold casting and 7 mm by high-pressure die casting
77,78
and exhibits good cor-
rosion resistance.
81,82
Bulk amorphous Mg–Ni–Nd alloys were obtained with a
thickness of 3.5 mm and show higher corrosion resistance than AZ91E and
EA55RS alloys in 3.5% NaCl solutions.
83
Surface Protection
As already pointed out, the films that form on Mg, although quite protective in
the absence of humidity and contaminants, do not serve perfectly in all environ-
ments. Coatings are used to supplement or replace the natural film, or as better
foundation for subsequently applied paint. Direct application of a paint on Mg
alloys is difficult due to the basic character of the naturally formed film, typically
with a pH of 10.5. Coating can be done by chemical conversion, metal plating,
anodization, or application of an organic coat.
84
Effective protection against cor-
rosion usually requires finishing schemes that combine several of these tech-
niques.
Traditional chemical treatments for Mg are generally based on chromate so-
lutions, which are under increasing environmental regulation. With high-purity
alloys, selected phosphate treatments can be as effective as chromates. Chemi-
cal treatments increase paintability and retard the natural alkali that forms
at any point of damage on a painted Mg surface. Used alone they do not
provide enough corrosion protection (among other reasons due to their solubility
in water).
Any metal that can be electrodeposited can be applied successfully to Mg,
but caution should be taken when using electroplating to protect components
exposed to corrosive environments. This process is used to attain bright, tarnish-
resistant surfaces and to improve wear resistance. In general, plating of Mg
consists of a zinc immersion plating and a copper strike, followed by electro-
plating in standard plating baths. Copper–nickel–chromium plating systems
provide good protection, even though galvanic corrosion problems would be
expected when the plate is damaged.
Anodic coatings combine reasonable corrosion resistance with excellent paint
base qualities and abrasion resistance. These hard ceramiclike coatings exhibit
different degrees of inherent porosity. The pores must be sealed to enhance
corrosion resistance. The coatings can be filled with polymers such as polytet-
rafluoroethylene (PTFE) to obtain special properties, e.g., lubricity. Typical an-
odic coating processes are HAE and Dow 17.
1
Both processes give rise to
coatings with thickness in the range of 5–30
m, consisting mainly of mixed
oxides. Anodic coatings represent a special type of chemical conversion coating
because some Mg is used to build up the protecting film. The Dow 17 process
utilizes a chromate-based formulation. Permanganate is the key ingredient in the

288 CORROSION AND OXIDATION OF MAGNESIUM ALLOYS
HAE process, however, a chromate sealant is necessary to obtain acceptable
corrosion resistance.
Tagnite
85
and Magoxid-Coat
86
are anodic spark processes carried out at higher
voltages (final voltage up to about 400 V AC). These anodic coatings are more
protective and not based on chromates. The Tagnite process uses a hydroxide–
silicate–fluorid electrolyte and the thickness of the coating can be varied be-
tween 2 and 30
m depending on current density and time. Magoxid-Coat is
formed in a slightly alkaline electrolyte and results in a partially amorphous
oxidic layer based on Mg, Al, and P. The coating consists of a thin barrier layer,
a virtually nonporous intermediate ceramic layer responsible for the good cor-
rosion resistance and a very porous outermost ceramic layer. The coating builds
up to an optimal thickness of 15–25
m. Probably, the amorphous nature of the
coating contributes toward its improved corrosion behavior. Codeposition of
transition metal oxides and organic materials is used to produce additional char-
acteristics, e.g., colored coatings.
Painting of Mg parts may be used to apply a decorative finish or as a mean
of protection against corrosion and tarnish. Organic coating can vary from simple
oils or waxes to multipaint coatings. If the environment is severe, primers should
be based in alkali-resistant vehicles as, e.g., vinyl, epoxy, polyvinyl butyral,
acrylic resins, or baked phenolic resins, and contain inhibitive pigments with a
slightly soluble chromate.
Following the recent developments of surface engineering, a variety of new
surface modification technologies, including ion implantation, chemical vapor
deposition/physical vapor deposition (CVD/PVD), and laser surface alloying/
melting, can be used to develop more effective and environmental friendly coat-
ings.
87
Hydride coatings were recently proposed as an alternative to chromate
coatings.
88
A magnesium hydride or hydrogen-rich layer with thickness of 1–2
m is created on the Mg surface by cathodic electric charging in aqueous so-
lution. The hydride coating promotes pseudo-passivation of Mg. It can be used
either as a stand-alone protective coating or as an effective paint base. Implan-
tation of N
2
⫹
ions on AZ91D surfaces enhances corrosion resistance; an optimal
dose of 5
⫻ 10
16
ions/cm
2
results in a corrosion rate approximately 15% of that
for the unimplanted alloy.
89
4 OUTLOOK
Whereas oxidation resistance of Mg alloys is generally considered to be suffi-
cient, at least in the temperature range of typical application, corrosion can still
be a problem. Further trends to improve corrosion properties of Mg alloys in-
clude: (1) Control of heavy metal content, (2) alloying with elements that sta-
bilize the surface oxide, e.g., RE, and with Mn that improves corrosion behavior
due to iron control effect, (3) use of RS techniques, and (4) coating and painting.
Heavy metal impurity control is the key action in the design of highly cor-
rosion resistant Mg alloys. These impurities enhance corrosion by orders of
magnitude and only their accurate control gives the indispensable basis for a
successful application of all other corrosion protection measures.
In marked contrast to Al and its alloys, Mg does not form a dense protective
oxide. The films naturally formed on Mg, although stable in dry environments,
are rapidly undermined in the presence of water and contaminants. The useful-
ness of Mg alloys can be successfully increased by improving the oxide layer
