Kutz M. Handbook of materials selection
Подождите немного. Документ загружается.


2 OXIDATION 269
environment (humid air or aqueous environments) degrades the film by the for-
mation of a less stable hydrated oxide.
An important criterion to predict the formation of a protective oxide is the
Pilling–Bedworth ratio,
4
defined as the ratio of the molecular volume of the
oxide to the atomic volume of the metal from which the oxide is formed. If this
ratio is less than one as in the case of magnesium (V
MgO
/V
Mg
⫽ 0.81), the oxide
grows under internal stress leading to a discontinuous, porous film possessing
low protective properties. To explain the good stability of magnesium at low
temperatures, it was suggested that initially an unstable structural modification
of MgO grows oriented with the metal substrate, building a very thin compact
layer. Above a critical oxide thickness, however, the normal cubic lattice forms
and the arising stresses lead to cracking of the film.
Generally, it is recognized that the initial oxidation of Mg proceeds by: (1)
oxygen chemiesorption, (2) formation of the oxide layer (nucleation and lateral
growth), and (3) oxide thickening
5–8
; in some cases a ‘‘precursor’’ chemisorption
stage before oxide formation was claimed. Initially oxygen is adsorbed on the
clean metal surface and forms a chemisorbed monolayer. A rearrangement of
valence electrons of the metal and the gas is then necessary for the formation
of chemical bonds. In the second stage additional layers of oxide build up form-
ing oxide islands; place exchange and surface diffusion are the important factors.
The island growth was reported to be fast and linearly dependent on the oxygen
exposure; the oxide thickening stage following coalescence of the oxide islands
is slow and diffusion controlled.
9
The film was reported to grow by a field-
assisted cation transport mechanism described by Cabrera–Mott kinetics.
10
A recent study
11
showed that the oxide film naturally formed on pure Mg as
a result of exposure to air is thin (20–50 nm) and dense. It exhibits an amorphous
structure, which is probably the reason for the protective behavior. In low-
humidity atmospheres, no further increase in film thickness is observed. X-ray
photoelectron spectroscopy (XPS) data indicated a mixture of elemental Mg,
MgO, and 50–60 wt. % Mg(OH)
2
.
Mg–Al alloys form similar films,
12,13
which are largely enriched in Al, es-
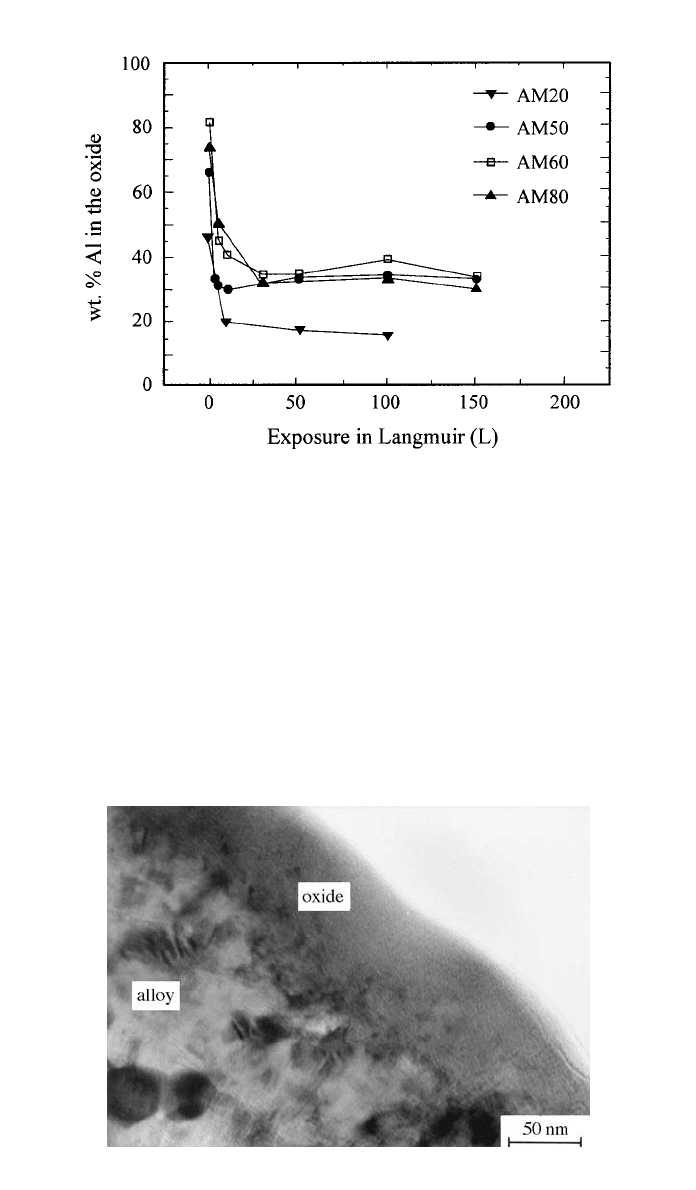
pecially for alloys containing more than 4% Al. Figure 1 shows that there is a
terminal value of about 35% Al that can be incorporated in the oxide: All alloys
containing more than 4% Al produce an oxide containing 35% Al; however, if
the alloy contains less than 4% Al, the Al level in the oxide never attains 35%
and becomes a function of the alloyed Al. The presence of Al in the oxide film
causes no chemical shift of the XPS signal for MgO, indicating that the oxide
is a mixture of MgO and Al
2
O
3
.
The initial air-formed film on commercial Mg alloys is also dense and pro-
tective.
14
The thin film can be visualized by transmission electron microscopy
(TEM) analysis on ultramicrotomed cross sections (see Fig. 2); electron diffrac-
tion reveals its amorphous structure. Since there is no need for accommodation
between the lattice of matrix and oxide, less internal stresses will develop. In
addition, amorphous films are in general regarded to have better protective prop-
erties than the crystalline ones.
15
In extended atmospheric exposures of Mg and Mg alloys, the presence of
humidity and acid gases as CO
2
and SO
2
alters the composition of the oxide
270 CORROSION AND OXIDATION OF MAGNESIUM ALLOYS
Fig. 1 Al concentration in the oxide on homogenized AM alloys (in order to dissolve the

phase) as a function of oxygen exposure.
13
Fig. 2 TEM cross section of an oxide film formed naturally on AZ91 alloys exposed
to the atmosphere.
14
film. Hydroxides, carbonates, sulfites, or sulfates will form preferentially, thus
reducing the film stability.
2.2 High Temperature
Most of the work dealing with oxidation of Mg at high temperatures is rather
old.
16–20
The rate of oxidation of Mg alloys increases with the temperature obey-
ing an Arrhenius equation. Magnesium hydroxide is not stable at high temper-
atures and begins to decompose above 350
⬚C.
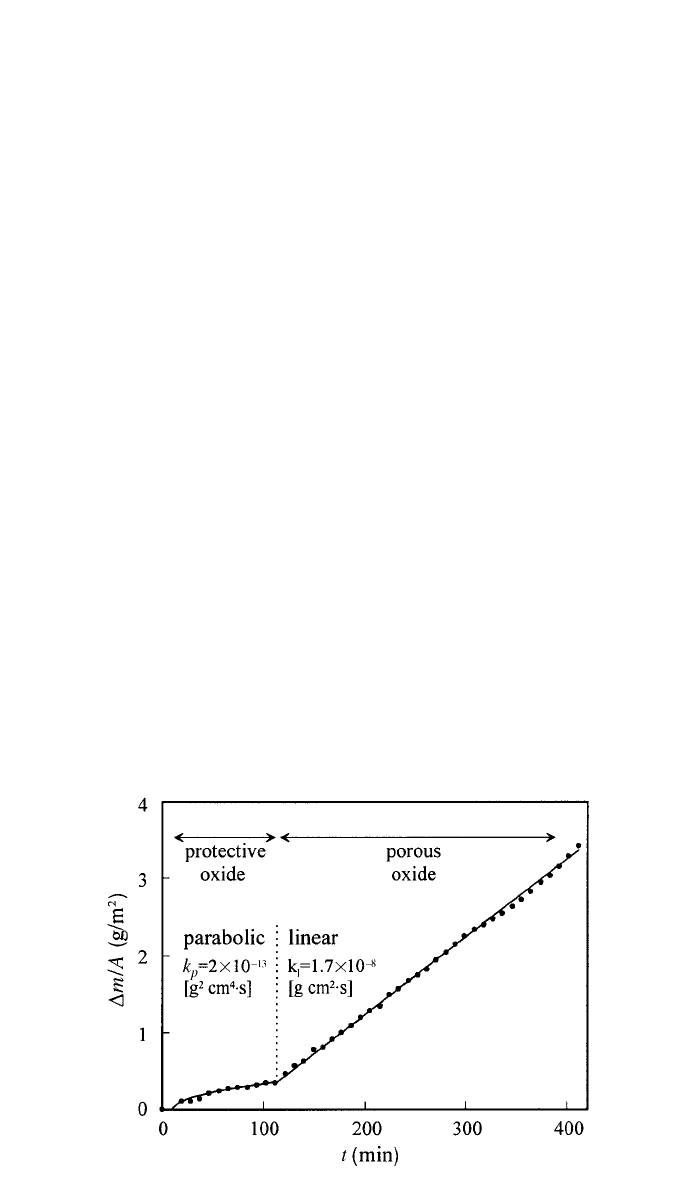
2 OXIDATION 271
Fig. 3 Oxidation kinetics of AM50 ingots at 450⬚C in natural air (after Ref. 21).
At temperatures below 450⬚C in dry oxygen or at 380⬚C in moist oxygen, the
oxide film formed on magnesium is protective for considerable lengths of time;
16,18
the weight law governing the oxidation is parabolic. However, the MgO
film that forms above 450
⬚C is not protective,
16,17,19
and a linear oxidation law
is observed. During this degradation of the film, a porous, white oxide grows
over the surface, producing a constant oxidation rate. The micromechanism in-
volved in this reaction is yet to be investigated. The activation energy for oxi-
dation between 475 and 575
⬚C was found to be about 211 kJ/mol;
17
this value
may represent the true rate of the chemical reaction since in the linear regime
oxygen has free access to the reaction surface. Above 600
⬚C oxidation is ex-
tremely fast leading to ignition.
A transition in the mechanism of oxidation is also observed in commercial
Mg alloys, as exemplified in Fig. 3 for high-purity AM50.
21
Two different
regimes were observed in the oxidation curve of AM50 ingots at 450
⬚C: Ini-
tially a protective oxide is formed with a parabolic kinetics (k
p
⫽ 2 ⫻ 10
⫺
13
g
2
/cm
4
䡠 s) and after about 100 min a porous and less protective oxide forms
with a linear kinetics (k
l
⫽ 1.7 ⫻ 10
⫺
8
g/cm
2
䡠 s). Only thin MgO films as
formed at the first stages of oxidation are dense and act as a diffusion barrier
for both, oxygen and metal cations. Above a certain thickness tensile stresses
caused by the unfavorable Pilling–Bedworth ratio are assumed to be too high,
and partial cracking of the film is likely to occur, permitting the access of oxygen
to the metal surface. Additionally, if cracks allow the metal to become exposed,
Mg might, in view of its appreciable vapor pressure, volatilize and react in the
vapor phase with the oxygen. The initial protective period gradually shortens
with the temperature since the critical thickness is more rapidly attained at higher
temperature.
The effect of alloying with various metals nobler than Mg on oxidation
was studied by Leontis and Rhines in the temperature range between 400 and
575
⬚C;
17
their results are represented in Fig. 4. Most alloying elements, e.g., Al
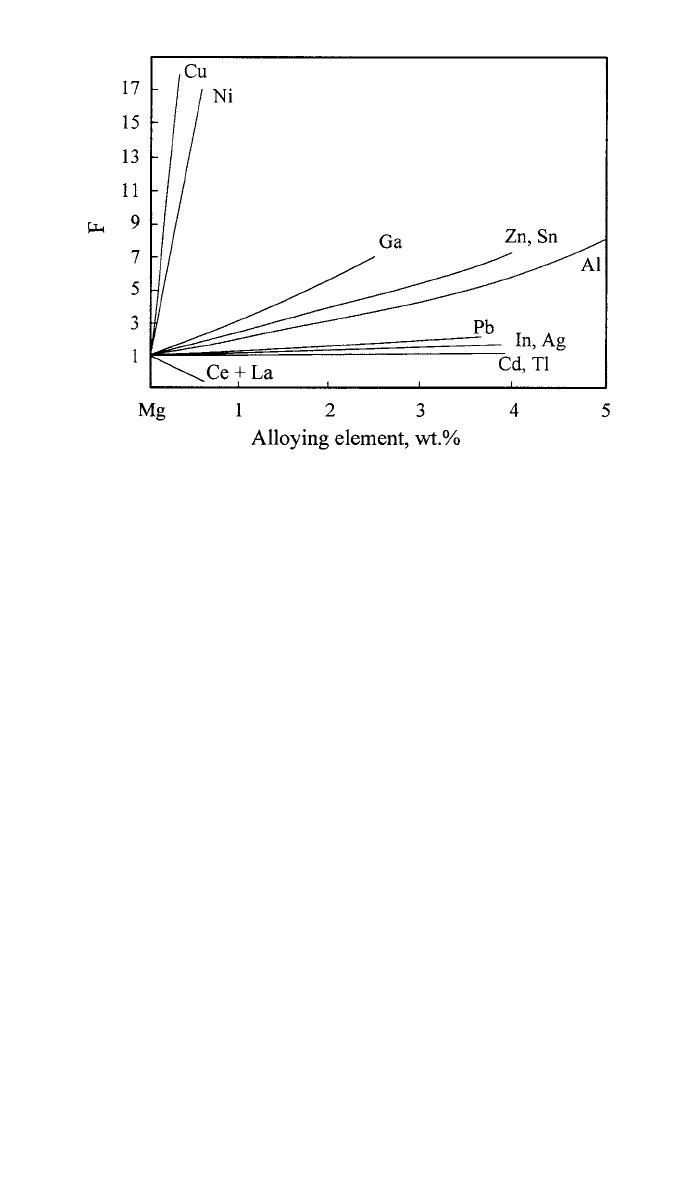
272 CORROSION AND OXIDATION OF MAGNESIUM ALLOYS
Fig. 4 Effect of alloying elements on the oxidation of Mg at ⬃475⬚C
(after Ref. 22, values from Ref. 17).
and Zn, increase the rate of oxidation; only Ce and La improve the resistance
of Mg to oxidation. The authors suggested that the increase in oxidation rate
results from a lowering of the melting temperature by the various additions. The
reactions were considered to take place at the metal/oxide interface. In contrast
to Ce and La, which are enriched in the surface layers, all other alloying ele-
ments get slightly depleted in the oxide.
Ca and Be are two of the few elements that are more reactive to oxygen than
Mg. They are known to have a remarkable effect on the oxidation resistance of
Mg,
23
in particular when added together with Al. The addition of as little as
0.001% Be will increase the ignition temperature of Mg by as much as 200
⬚C.
Thus, small additions of Be during melting help to control Mg loss and minimize
the use of fluxes. A protective BeO film forms on the surface, reducing drossing
and increasing metal yield and cleanliness. These benefits carry over casting. Be
provides the further advantage of precipitating Fe and Mg impurities from the
melt.
Recent studies
24
performed on conventional AM and AZ alloys at 450⬚C
confirmed the beneficial effect of RE elements predicted by Leontis and Rhines
17
and showed that Ca strongly improve oxidation performance; rapid solidification
(RS) was observed to further reduce the oxidation rate. The results shown in
Fig. 5 for AM50 alloys indicate that the modified alloys are very resistant to
oxidation. As little as 1% of Ca is enough to reduce the oxidation rate to ex-
tremely low values, even for longer oxidation times. Rare earths (added in the
form of mischmetal, Mm) are not as effective as Ca, and slightly higher contents
are necessary to achieve similar performance (see Fig. 5a).
The oxide formed on alloys with conventional composition is thick and po-
rous, whereas on Ca-containing alloys it is very thin so that metallic glance
essentially remains after testing. The film consists of nanocrystalline MgO as
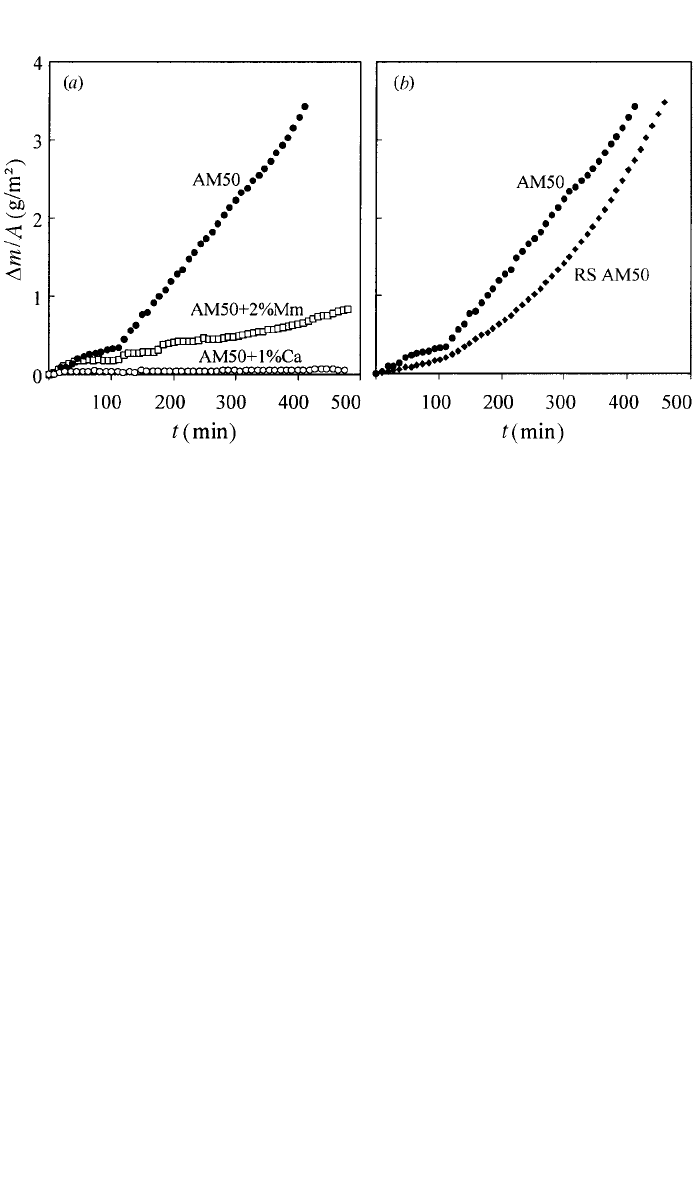
2 OXIDATION 273
Fig. 5 Oxidation kinetics of AM50 ingots at 450⬚C in natural air: (a) effect of Ca and Mm addi-
tions and (b) effect of rapid solidification (after Ref. 21).
showed by X-ray defraction (XRD) and TEM cross sections (for AM50 the grain
size
⬍40 nm). In alloys containing Ca an enrichment of Ca in the MgO film in
particular in its outermost part was detected by means of atomic emission spec-
troscopy (AES). Similar Ca enrichment was found in binary Mg–Ca alloys ox-
idized at 500
⬚C, being identified as CaO.
25
Therefore, the suppression of the
oxidation rate of Mg at high temperatures can be attributed to the formation of
CaO, which replaces MgO near the surface and acts as a protective layer; similar
explanation was given for the protection of the Mg melt by Ca additions.
26
The
role of RE in the film formation is still under investigation. There is some
evidence that the effect of Ca becomes detrimental at lower temperatures. For
example, a recent study on AM50 alloys (same compositions as in Fig. 5) in-
dicated that the presence of 1% Ca increases the oxidation rate at 300
⬚C, while
both unalloyed and RE-containing AM50 alloys undergo almost no oxidation at
this temperature.
14
Precipitation of Al–Ca intermetallics might be responsible
for this deterioration. Measurements at even lower temperatures, within the limit
for application of Mg alloys, were not performed yet. Since at lower temperature
kinetics becomes very sluggish, extreme long-term measurements would be nec-
essary.
As shown in Fig. 5b, an additional beneficial effect can be achieved by means
of RS; the improvement due to Ca and RE remains in the RS material. Until
now the influence of RS on the high-temperature oxidation deserved very little
attention. It seems that the advantages of RS, e.g., microstructural homogeneity,
contribute toward the formation of more stable films as in the case of aqueous
corrosion.
In sulfur-containing atmospheres the oxide film produced in the high-
temperature range was observed to be more protective.
17
Therefore, addition of
SO
2
into the atmosphere retards the oxidation of Mg at high temperatures as

274 CORROSION AND OXIDATION OF MAGNESIUM ALLOYS
well as during casting. In gases containing H
2
SorSO
2,
magnesium alloys are
among the most oxidation-resistant materials.
22
Addition of CO
2
also suppresses
oxidation of solid Mg.
17
In summary, the interaction of oxygen with Mg leads to the formation of a
thin, protective oxide layer. However, temperature as well as moisture accelerate
the transition to less protective films. At elevated temperatures the MgO film
rapidly attains a critical thickness and starts to crack, thus allowing further ox-
idation. Ca and RE improve the protective properties of the MgO film and retard
the transition into a porous film. Water, if present in the environment, rapidly
undermines the protective film leading to a less stable, hydrated oxide and elec-
trochemical dissolution of the metal. This process of aqueous corrosion is the
subject of the next section.
3 CORROSION
In spite of the great advantages of Mg alloys, their application as engineering
material is still restricted by their high susceptibility to corrosion in aqueous
environments, especially in the presence of chlorides or when they contain noble
metals as impurities. In this section the poor corrosion behavior of Mg alloys
and the presently available methods to improve it are discussed. Over the past
30 years important improvements, which include modifications of the bulk alloy
and surface, were achieved and nowadays most limitations can be successfully
overcome by the correct use of Mg alloys. For further information other recent
reviews are recommended.
27,28
3.1 Electrochemical Properties
In aqueous environments magnesium dissolves electrochemically according to
reaction (2) with the formation of magnesium hydroxide and hydrogen, a mech-
anism that is highly insensitive to the oxygen concentration.
29
If sites for easy
hydrogen discharge are available, corrosion can proceed rapidly.
↑
Mg ⫹ 2H O → Mg(OH) ⫹ H (2)
222
The hydroxide film has a hexagonal structure with alternating layers of Mg and
hydroxide ions, thus facilitating easy basal cleavage. Cracking and curling of
the film was observed, but it is not clear whether this is caused by the properties
of the film or by the evolution of hydrogen gas. The Pilling–Bedworth ratio is
1.77, indicating that the film is under compression. Thus, a combination of
internal stresses and the easy basal cleavage may account at least partly for the
cracking and curling of the film.
30
The standard reduction potential of Mg is ⫺2.4 V; at higher potentials Mg
corrodes rapidly. Despite the anodic polarization effect arising from the forma-
tion of Mg(OH)
2,
the open-circuit potential in neutral aqueous solutions still falls
below
⫺1.5 V. Only at pH values higher than about 11 can a stable layer of
Mg(OH)
2
form and provide protection against further corrosion (see Fig. 6).
This protection is highly dependent on the environmental conditions, and Mg
turns out to be opposite in character to Al. Mg is resistant to alkalies and poorly
buffered environments where the surface pH may increase due to the initial
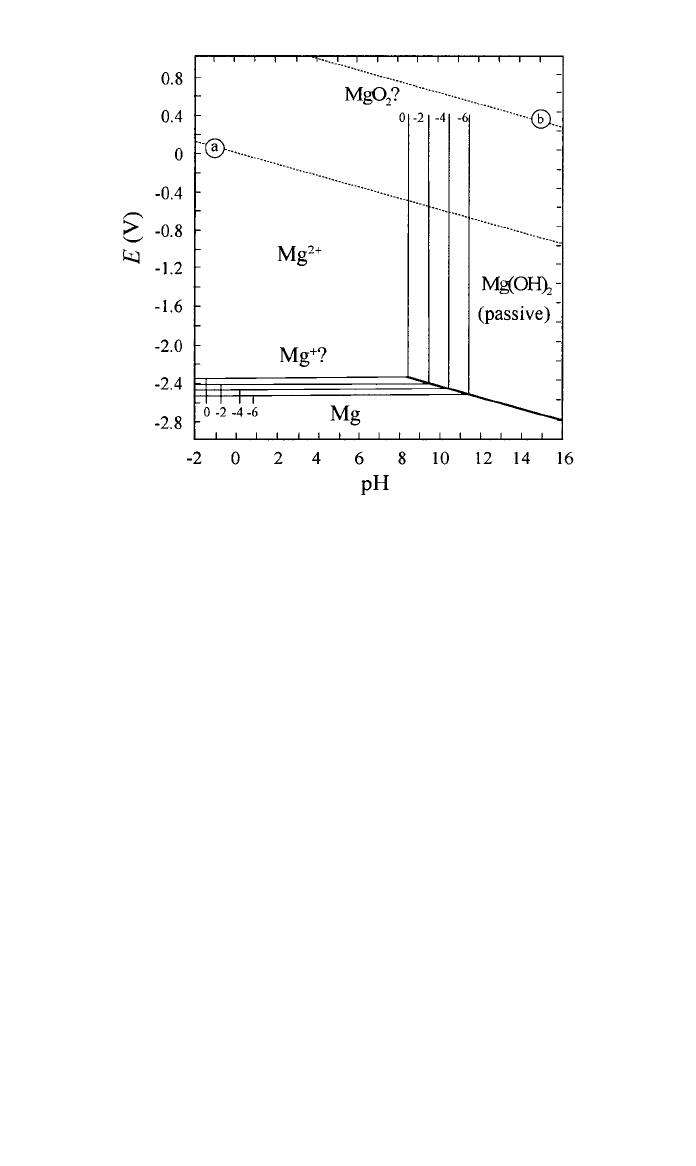
3 CORROSION 275
Fig. 6 Pourbaix diagram showing the equilibria for Mg–H
2
O system at 25⬚C.
31
Lines (a) and (b)
identify the hydrogen and oxygen evolution reaction, respectively.
Mg(OH)
2
formation but not to acids. In contrast Al is resistant to weak acids
but attacked by strong alkalies.
3.2 Types of Corrosion
Mg alloys undergo both general and localized corrosion. The very electronega-
tive potential of Mg makes these alloys very prone to galvanic corrosion. The
galvanic attack can be internal due to microstructural components with more
noble potential (e.g., impurities or second phases) or external if Mg is coupled
with other metals. While metals with low hydrogen overvoltage (e.g., Ni, Fe,
and Cu) constitute efficient cathodes for Mg and cause severe galvanic corrosion,
metals that combine an active corrosion potential with a high hydrogen over-
voltage (e.g., Al and Zn) are much less damaging. Internal attack can be mini-
mized by selecting high-purity alloys. To avoid external galvanic corrosion, one
of the following measures is recommended:
32
(1) Selection of galvanic compat-
ible dissimilar metals (e.g., Al alloys 5052, 5056, and 6061; or tin, cadmium,
or zinc-plated ferrous alloys). (2) Protection of Mg and dissimilar metal by
suitable surface treatments; painting of the dissimilar metal or even better of
both metals is recommended to avoid area effects. (3) Use of insulating washer
or gasket between Mg and dissimilar metals to prevent the completion of an
electrical circuit. (4) Inhibition of the galvanic cell by using chromates in the
sealing compounds or primers. (5) Proper design to avoid electrolyte entrapment.
Intergranular and crevice corrosion are not likely to occur in Mg alloys be-
cause the phases at the grain boundaries are always cathodic to the grain interior
and the corrosion reaction is insensitive to oxygen concentration differences.
15

276 CORROSION AND OXIDATION OF MAGNESIUM ALLOYS
Usually, corrosion concentrates at regions adjacent to second-phase particles and
boundaries. Some grains or particles might be undermined and fall out, resulting
in higher mass losses than solely due to electrochemical dissolution; this partly
explains the so-called negative difference effect observed during anodic polari-
zation of Mg.
27
As a naturally passive metal, Mg may suffer pitting corrosion. AZ91 in
chloride-containing environments exhibits a variety of localized forms of cor-
rosion, including pitting at the outset (initiation sites are few and associated with
intermetallic particles), filiform corrosion at early stages of propagation, and a
cellular type of attack in the terminal stage.
33–35
The occurrence of filiform cor-
rosion on uncoated surfaces of AZ91, which is not the case for unalloyed, bare
Mg,
36
suggests the presence of a more resistant air-formed oxide on the alloy.
Pure Mg is resistant to stress–corrosion cracking (SCC). However, alloying
with Al and Zn promotes SCC, which increases with increasing Al content.
1
Thus, Mg–Al–Zn alloys have the greatest susceptibility to SCC. Alloys con-
taining Zr are essentially free from the phenomenon. SCC in Mg alloys
15,37,38
is
usually transgranular occurring along twin boundaries or various crystallographic
planes. There is general agreement that hydrogen embrittlement is the dominant
mechanism. Since hydrogen cannot penetrate the passive film unless it is already
damaged, pitting is likely to be the first step on SCC. Therefore, solutions that
are nonactive to Mg (such as diluted alkalies, concentrated HF, and chromic
acid) or in which general corrosion predominates do not induce SCC. High-
humidity content, dissolved oxygen, and seawater accelerate SCC; heavy metal
content does not seem to influence it. SCC was studied mainly in Mg–Al systems
and information is still lacking for other Mg alloys, in particular for new emerg-
ing alloys, e.g., glassy alloys or those containing RE.
3.3 Environment and Surface Film
Owing to the very active electrochemical character of Mg, the corrosion behavior
of its alloys reflects the protective qualities of the surface film in various envi-
ronments. As discussed in Section 2.1, in natural environments with low hu-
midity a stable MgO film [or a mixture of MgO and Mg(OH)
2
] forms on
magnesium and protection is essentially perfect. Atmospheric corrosion is a
threat only in the presence of humidity that leads to condensed water. The cor-
rosion rate of magnesium during exposure to humid air is less than 10
⫺
5
mm/
yr in the absence of condensation, while it becomes greater than 1.5
⫻ 10
⫺
2
mm/yr when water condenses on the surface.
39
Hence, for indoor service Mg
alloys serve well without any further protection.
Although stable and dense, the air-formed film is permeable to water and
soluble Mg species. Exposure to humid air rapidly causes hydration of the oxide
and formation of thicker films (e.g., 100–150 nm after 4 days) with a duplex
structure.
11,12
The outer layer of the film (20–40 nm) is amorphous and similar
to the film formed in dry air (see Fig. 2), but an additional hydrated layer is
formed adjacent to the metal. The hydrated layer reduces the passivity of the
metal surface by allowing Mg dissolution. This layer is quite sensitive to electron
irradiation and develops a cellular structure in the microscope; continued ex-
posure to the electron beam causes release of water and formation of nanocrys-
talline fcc MgO. Immersion in water leads to the formation at the outermost
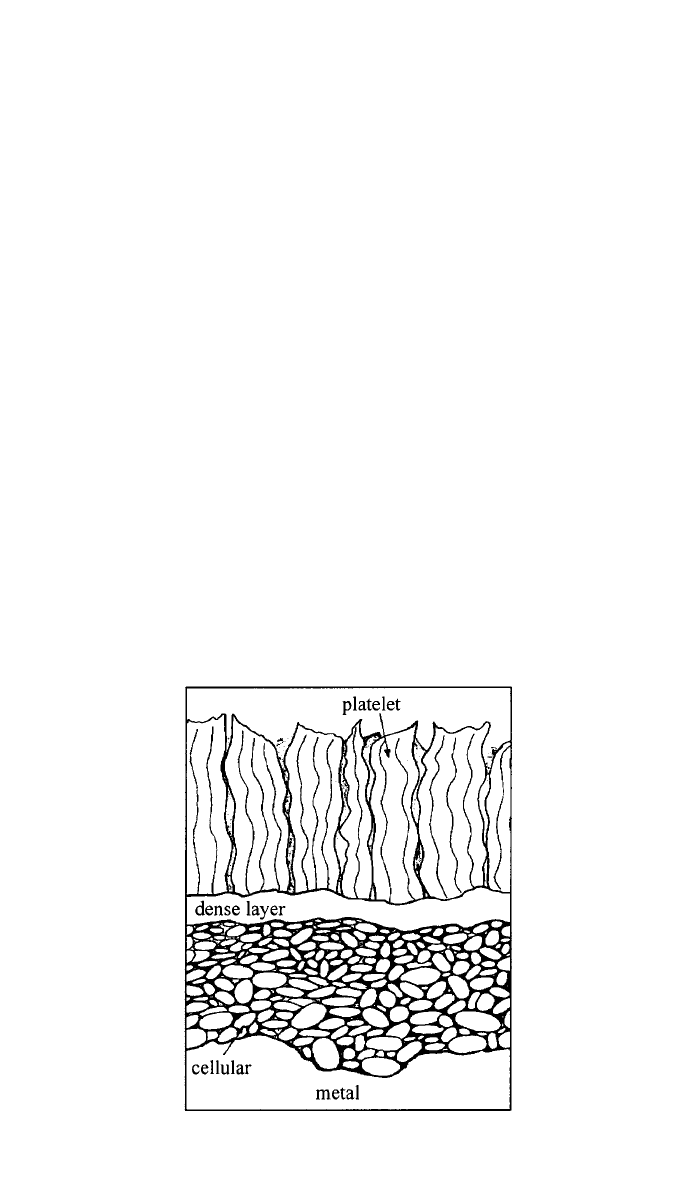
3 CORROSION 277
Fig. 7 Schema of the three layered oxide formed on Mg and Mg–Al alloys in water-containing
environments. (Reprinted from Corrosion Science, 39, J. H. Nordlien, S. Ono, N. Masuko, and
K. Nisancioglu, ‘‘A TEM Investigation of Naturally Formed Oxide Films on Pure Magnesium,’’
pp. 1397 –1414. Copyright 䉷 1997, with permission from Elsevier Science.)
surface of a third layer with plateletlike morphology, which probably grows by
precipitation of Mg
2
⫹
or other soluble Mg species that migrate outward from
the innermost layer. Figure 7 shows schematically the morphology of the layered
film formed in the presence of water.
Films formed in water on Mg alloys are sensitive to temperature, agitation,
and contaminants.
32
The corrosion rate in water may increase by one or two
orders of magnitude when the temperature rises from room temperature to
around 100
⬚C.
Immersion in a small volume of stagnant water allows rapid formation of the
protective hydroxide film. Owing to the initial dissolution of hydroxide, the pH
of the system rises until the point is reached where further dissolution is inhibited
and the metal undergoes passivation. Agitation can prevent the pH to rise so
that the solubility limit of Mg(OH)
2
is never reached and corrosion continues at
the initial high rate. For example, AZ31B shows little attack in stagnant distilled
water, but in constantly replenished water the corrosion rate is significant (0.18
mm/yr).
The presence of small amounts of dissolved salts, in particular chlorides and
heavy metal salts, breaks down locally the protective film formed in aqueous
environments. All solutions containing salts of heavy metals such as Fe, Ni, and
Cu are extremely aggressive because the heavy metal and/or heavy metal basic
salt can precipitate to form active cathodes on the anodic Mg surface.
Anions such as chloride, sulfate, and nitrate destroy the passivity of magne-
sium and cause severe damage. Saline environments such as marine atmospheres,
salty road splash, etc. are always harsh environments for Mg alloys, even when
protected. For this reason, the majority of corrosion data refer to salt spray test

278 CORROSION AND OXIDATION OF MAGNESIUM ALLOYS
and immersion in NaCl solutions operated according to the ASTM Standards
B117-90 and G31-72, respectively.
40
These data must be interpreted with caution
because salt based accelerate tests usually lead to higher corrosion rates than
marine atmosphere exposure and show very poor correlation with rural, urban
or industrial atmosphere exposure.
32
The CO
2
in the atmosphere dissolves in water to form carbonic acid, which
reacts with the Mg(OH)
2
film to form carbonates.
41
While these films are quite
protective, they are slightly soluble in water and do not provide complete pro-
tection over extended periods of time. The same is true for other acid-producing
compounds, such as SO
2
in industrial atmospheres. Exposure to wet SO
2
was
suggested to accelerate the corrosion of magnesium
42
through the conversion of
the more protective hydroxide and carbonate compounds to the highly soluble
sulfate and sulfite, which are then eroded. If Al is present in the alloy, the effect
of CO
2
is smoother due to the formation of hydrotalcite, MgCO
3
䡠 5Mg(OH)
2
䡠
2Al(OH)
3
䡠 4H
2
O, acting as a sealer for the hydroxide film. In the presence of
chlorides, the accelerating effect of CO
2
on the atmospheric corrosion rate is
rather weak; it becomes more important only for a low degree of salt contami-
nation.
43
In the presence of fluoride, the film changes from magnesium hydroxide to a
very protective magnesium fluoride and protection is greatly increased, even at
high temperatures. Mg is even used for constructing handling equipment for
concentrated HF. Chromate also forms highly protective films on Mg, and chro-
mic acid is the basis for many protective surface treatments.
A good rule of thumb is that environments that are basic, neutral, or contain
fluorine cause little or no corrosion, while environments that are acid do cause
corrosion of Mg and its alloys.
3.4 Improving Corrosion Resistance
Apart from the influence of the environment, the corrosion behavior of Mg alloys
depends (similar to other metallic materials) on the presence of impurities, com-
position (e.g., type and amount of alloying elements) and processing methods
(e.g., type of casting and heat treatment). However, the relative importance of
these factors is greatly amplified for all Mg alloys because of the very negative
corrosion potential of the matrix combined with the unstable nature of the sur-
face film.
There are four main approaches that can be used to improve the corrosion
behavior of Mg alloys: (1) The first approach is the production of high-purity
alloys with very low impurity levels; high-purity alloys appeared in the 1980s
and presently their use is standard practice. (2) Further important improvements
on corrosion can be achieved by alloying with beneficial elements. This is a
rather old method, however, in recent years new compositions are been studied.
(3) Selective processing that optimizes the microstructure also enhances corro-
sion resistance; new processing techniques such as RS were shown to be very
promising. (4) A further approach, which is complementary to those influencing
the alloy itself, is the application of protective films or coatings.
Control of Impurities
In general, the factor with the far strongest influence on the corrosion of Mg
alloys is the amount of cathodic impurities, particularly of those with low-
