Kutz M. Handbook of materials selection
Подождите немного. Документ загружается.

1290 DIAMOND FILMS
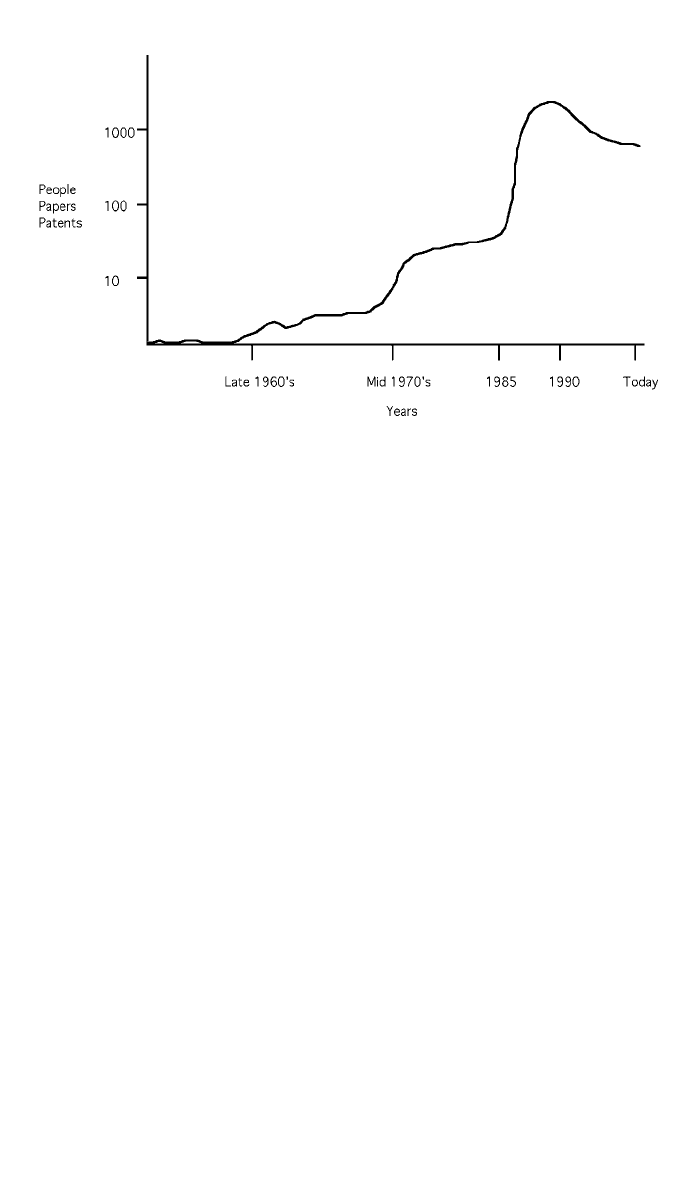
Fig. 2 Research activity in CVD diamond during the last 30 years.
material. Many of the physical, optical, chemical, and electrical properties of
CVD diamond films will be controlled or modified by their degree of deviation
from perfection. Imperfections may take on many forms. They can be structural,
such as grain boundaries or polytypes; chemical, such as variations in stoichi-
ometry; or optical, such as variations in refractive index or optical handedness.
There are also less obvious imperfections such as disordered ferroelectric do-
mains, irregularly distributed isotopes, or interfaces between discrete uniform
regions of the same or different materials (Davies, 1994).
Diamond is the hardest naturally occurring material (Field, 1991). The great
hardness of diamond makes it appropriate to consider diamond as a low-wear
material. If it is very difficult to break a bond then the chances of breaking
bonds (wear) will be low. Several other materials have higher bond strengths
than does diamond, but those strengths are anisotropic. The hardest material that
is known is a synthetically prepared type of diamond. Analysis of elastic constant
data for
13
C diamond has shown that its bond strength is greater than that of
ordinary natural diamond or commonly made CVD diamond. The reason for
this is the slightly shorter bond length that results from having an extra neutron
in the carbon atom’s nucleus. Actual hardness measurements have not been made
on the material to date, but the strength should scale with the elastic constants
(Ramdas et al., 1993). Additionally, diamond has a low coefficient of friction
(0.01) in the presence of air or hydrogen (Field, 1991). This makes the material
as slippery as Teflon but far more wear resistant. The slippery nature of the
hydrogen-saturated surface would reduce both frictional horsepower losses and
decrease surface erosion when used in wear applications (Phelps, 1993).
The thermal conductivity of diamond is a good example of the structure–
property relationship in CVD diamond. Annealed copper is the standard for
thermal conduction in the area of high-power electronic components because it
has a relatively high thermal conductivity of 4 W/cm K. Diamond is the best
thermal conductor that exists. Diamond is very attractive material for use in

3 DIAMOND FILM DEPOSITION 1291
electronic devices because a defect-free natural single-crystal diamond has a
thermal conductivity of around 20 W/cm K (Seal, 1970; Seal and van Enckevort,
1988). An average polycrystalline CVD diamond film made under typical lab-
oratory conditions has a thermal conductivity of 6–10 W/cm K (Plano and Adar,
1987). The values for polycrystalline CVD diamond are impressive until they
are compared to isotopically pure single crystal diamond. Isotopically pure sin-
gle-crystal diamond is made by taking polycrystalline isotopically pure diamond
made by CVD and using it as the feedstock in single-diamond high-pressure
synthesis. Recent measurements on that material have failed to find the upper
limit on the thermal conductivity of the samples. The value currently being
quoted is 36 W/cm K, nearly twice that of the best natural single crystal (Ban-
holzer and Anthony, 1992). Thermal stress gradients caused by rubbing are not
supported by diamond. Heat transfer in diamond is so fast that the temperature
throughout the diamond film is always uniform and the film does not suffer from
differential thermal contraction.
Diamond films are commonly considered to be excellent electrical insulators
because of the 10
15
⍀/cm resistivity measured for many natural diamonds. How-
ever, the resistivity of CVD diamond has been found to change with film com-
position. The electrical conductivity of CVD diamond has been shown to vary
with the amount of hydrogen absorbed in the structure of the film. Ravi and
Landstrass (1989) report that the resistivity of CVD diamond films can be cor-
related more with the amount of hydrogen they contain than with their Raman
spectra. An ‘‘as-grown’’ diamond film had a resistivity of about 10
6
⍀/cm. This
same film was soaked for 30 min in a 600
⬚C flowing nitrogen atmosphere, and
the resistivity was found to increase to about 10
13
⍀/cm. The process was found
to be reversible, and the hydrogen could be put back into the sample, and the
resistivity would decrease to near the as-grown value. Diamond films with sig-
nificant amounts of graphitic structural bonding have been found to be electri-
cally conductive and have physical properties that are a fraction of the natural
properties, such as thermal conductivity. Figure 3 shows how many of the special
properties of diamond lend themselves to specific applications. Several proper-
ties of diamond suggest an application in some cases such as with microwave
electronic devices.
3 DIAMOND FILM DEPOSITION
Diamond films cannot be deposited on all materials and surfaces. Substrates that
present problems to diamond deposition are the ferrous materials including iron,
nickel, cobalt, in addition to copper, zinc, tin, and the precious metals. Diamond
will grow on a substrate successfully at high temperature only if the material
has a low solubility for carbon and forms a carbide. There is nearly always an
interface that keeps diamond separated from its deposition substrate, and there
is typically no direct diamond-to-metal contact. The interface usually contains
at least a few atom layers of carbide (Srivatsa et al., 1989; Williams et al., 1990).
As an example, titanium could support diamond growth through the formation
of a titanium carbide interlayer. If, however, the carbide were too thick, then the
difference in thermal expansion between the carbide and the diamond could
fracture the interface. A diamond-like carbon film deposited on top of a substrate
can also act as an interface instead of a carbide. Figure 4 outlines the elements
1292 DIAMOND FILMS
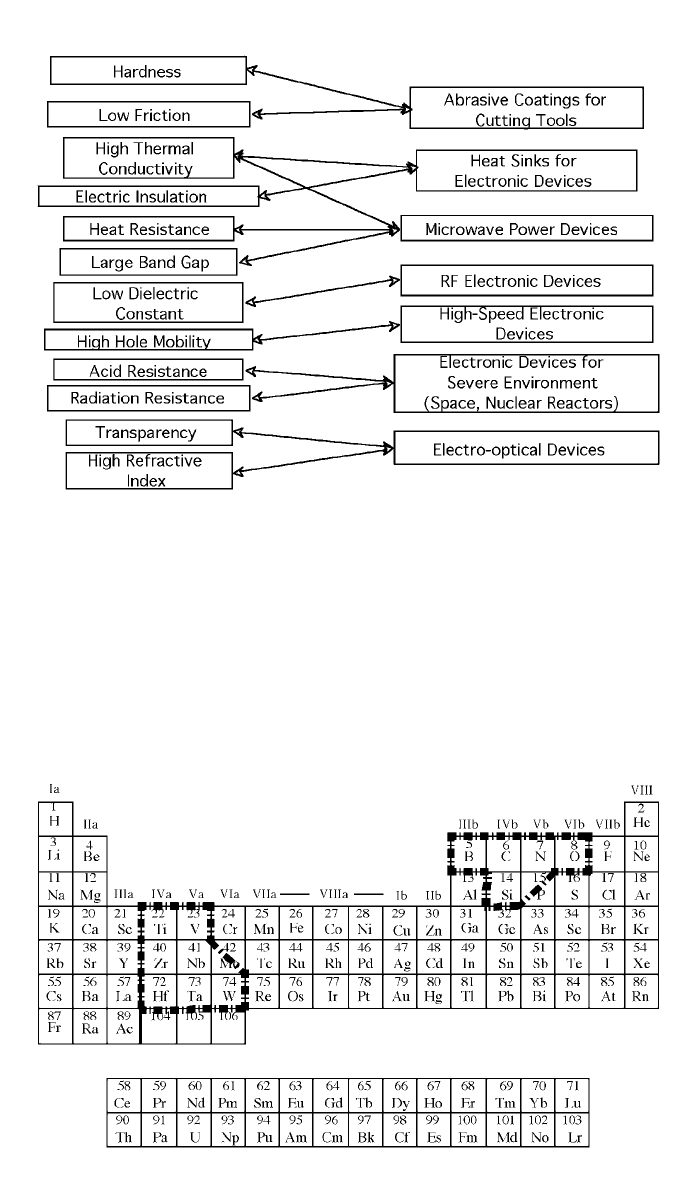
Fig. 3 Applications of diamond materials based on property criteria
as adapted from Spitsyn (1991).
Fig. 4 Regions of the periodic table that show diamond deposition compatibility where the half
boxes indicate partial compatibility.
of the periodic table that are typically compatible with diamond growth either
singly or in multiples. Phosphorus and molybdenum may only have a partial
affinity for diamond growth. The other elements are more or less compatible
with diamond growth.
Low-pressure CVD of diamond may be thought of as a process that is based
on rate variations. The deposition and etch rates of carbon in the gas phase are

3 DIAMOND FILM DEPOSITION 1293
the keystones in CVD diamond deposition. Carbon produced from the gas phase
can take the form of many carbon materials. These carbon materials include
diamond, graphite, diamondlike carbon (DLC), carbynes, fullerenes, soot, naph-
thalene, and so on. However, the rate at which each of these materials is formed
depends on the total pressure of the system, substrate composition and temper-
ature, gas temperature, flowrates, and feedstock. Most low-pressure carbon-
producing systems produce nondiamond carbons in far greater abundance than
they produce diamond. It is necessary to shift deposition parameters in a direc-
tion that favors diamond carbon growth at the expense of the growth of the other
forms of carbon. However, this needs to be done while removing nondiamond
carbon from the system. The presence of nondiamond carbons tend to poison
diamond growth and interfere with the formation of good-quality diamond crys-
tals.
The etch rate of diamond in hydrogen and oxygen appears to be far less than
all other nondiamond carbons formed. This results in a diamond-rich carbon
deposit. If diamond is being formed at 10% of the rate of the other carbon
materials but is being etched at 1% of the rate of the other materials, then after
a period of time a net deposit of diamond will form. Diamondlike carbon films
or graphite will form if the substrate temperature is below 800
⬚C. The substrate
temperature must usually be somewhere between 800 and 1000
⬚C during CVD
diamond deposition. Diamond tends not to form if the substrate temperature
rises above 1100
⬚C. CVD diamond, single-crystal natural, and high-pressure
synthetic diamond graphitize and etch above this temperature. Typically, CVD
diamond forms in a very narrow temperature window. Deposition occurs when
the methane plus hydrogen mixture is activated. Energy to activate the process
may be in the form of radio frequency (RF), thermal (hot filament), microwave
plasma, high-voltage direct current (DC), and others.
The selection of an appropriate diamond deposition process depends on the
needs of the particular development program. Concerns about deposition rate,
hydrogen concentration, substrate compatibility, and the ability to coat three-
dimensional surfaces all should be taken into account. Three-dimensional CVD
diamond films have been made by hot filament assisted CVD. Obata and Mor-
imoto (1989) demonstrated the possibility of three-dimensional coatings by
forming diamond hemispheres and sleeves. Their work used a filament-type
reactor to produce these forms. Bachmann and Lydtin (1991) present a rudi-
mentary chart to help in the selection of a deposition process presented here as
Table 1. This type of chart should be viewed as a starting point from which the
fine points of the assorted deposition techniques can then be compared for such
things as cost, scalability, and ease of use as well as being capable of producing
the particular type of diamond that is desired.
Properties that make CVD diamond films excellent for one particular appli-
cation may be completely unsuitable for other uses. For example, the highest
thermally conductive CVD diamond film has the least desirable physical prop-
erties for use as a hydrostatic bearing coating. Good thermal conductivity results
from the material having a high degree of compositional purity and no nondia-
mond carbon in its structure. Nondiamond carbon has been found to help tough-
ness and resiliency in CVD films. A low defect density is also necessary for a
high thermal conductivity. Defects in the form of grain boundaries need to be

1294
Table 1 Diamond Deposition Process Selection
Rate (
m/h) Area (cm
2
)
Quality
(Raman) Substrates Advantages Drawbacks
Flame 30–100 ⬍1 ⫹⫹⫹ Si, Mo, TiN Simple Area, stability
Heated filament 0.3–2 100
⫹⫹⫹ Si, Mo, silica, etc. Simple, large area Contamination, stability, no O
2
DC discharge (low pres-
sure)
⬍0.1 70 ⫹ Si, Mo, silica, etc. Simple, large area Quality, rate
DC discharge (medium
pressure)
20–250
⬍2 ⫹⫹⫹ Si, Mo alumina Rate, quality Area
DC plasma jet 930
⬍2 ⫹⫹⫹ Mo, Si Highest rate, quality Area, stability homogeneity
RF (low pressure)
⬍0.1 ? ⫺ / ⫹ Si, Mo, silica BN, Ni Scale-up Quality, rate, contamination
RF (thermal, 1 atm) 180 3
⫹⫹⫹ Mo Rate Area, stability, homogeneity
Microwave 1 (low pressure)
30 (high pressure)
40
⫹⫹⫹ Si, Mo, silica WC, etc. Quality, stability Rate, area
ECR Microwave 0.1
⬍40 ⫺ / ⫹ Si Area, low pressure Quality, rate, contamination
Source: From Bachmann and Lydtin (1991).

5 DIAMOND FILM ROUGHNESS 1295
reduced for thermal conductivity, and so grain sizes must be very large. Large
grains tend to have very high relief and produce rough films, also cracks are
easier to run through large crystals than smaller polycrystalline masses. Adherent
diamond films suited to hydrostatic bearings will need to be low in stress, tough,
and probably not be as thermally conductive as single crystals.
4 MODIFYING CVD DIAMOND
The physical properties of CVD diamond can be modified and controlled by
altering deposition parameters, using additives, changing substrate selection, and
varying the seeding and nucleation method. For example, a carbon-rich environ-
ment will produce a diamond film with both a larger conductivity than natural
diamond and one with a greater degree of compliance (Windischmann and Epps,
1990; Windischmann et al., 1991). Phelps and Koba (1989) showed that the
electrical conductivity and the quality of their Raman spectra of boron-doped
diamond films were found to increase and improve with increased diborane
concentration in the deposition environment. Bernholc et al. (1988, 1992) pre-
dicted that with boron as a dopant ‘‘the reduced self-diffusion activation energy
should lead to better quality material.’’ Phelps (1990) suggested that features
observed in the Raman spectra of diamond films are the result of defects in the
diamond structure, and boron doping of the material appears to substantially
affect its Raman spectra. Defects in boron-doped CVD diamond have been
shown many times to scale with the amount of boron doping in the material. A
TEM study by Wang et al. (1992) showed cross-sectional views of several doped
diamond at various dopant levels, and it is clear that the number of dislocations
in the diamond structure show a marked decrease. The oxidation resistance of
boron-doped CVD diamond is shown to increase with boron content by the
temperature measured at the onset of oxidation (Loparev et al., 1984).
5 DIAMOND FILM ROUGHNESS
The as-grown surface of CVD diamond films is typically very rough. This degree
of roughness (
⬎5
m peak to valley) would prevent the use of these materials
as bearings if there were no way of making the surface smooth. This degree of
roughness makes some diamond films very good at sanding and polishing rough
areas on other diamond films. In order for diamond films to be used for bearings,
they need to have less than a 0.4-
m peak-to-valley roughness. Surfaces with
roughness greater than this have been found to be unacceptable for bearings
made of other materials. Smooth CVD diamond films may be made by several
different methods. These include polishing, brazing the rough side down, grow-
ing the films very thin, or simply growing them very smooth at first.
It is critical that a mechanical polishing procedure be avoided for smoothing
diamond films. The reasons include the high per-part finishing cost. The only
material that is known to successfully mechanically polish diamond well is di-
amond itself (Watermeyer, 1991). This results in an expensive raw material cost
before any polishing activity even takes place. Also, a very thick film of diamond
would have to be deposited so that some film would be left after the polishing
procedure. Thickness is comparable to cost because of the slow deposition rates
at which this material is deposited and the slow rate at which diamond is re-
moved. A mechanical polishing step would also be the agent of flaw introduc-

1296 DIAMOND FILMS
tion. The diamond films made and likely to be used for bearing applications will
only be a few microns thick. A crack could very easily run through the thickness
of a diamond film during the polishing process because of diamond’s brittle
nature. Lastly, there is yet no method by which a surface coated with a diamond
film with three-dimensional reentrant surfaces can be polished to a satisfactory
smoothness while retaining size, shape, and surface quality specifications.
One technique that produces smooth diamond films is a flip-chip brazing
technique. A rough diamond film is grown on a polished substrate and removed
after deposition. The film may simply be turned over and brazed rough side
down onto a second working substrate. The smooth interfacial surface is then
the one that is outermost.
Smooth diamond films can be made by controlling the nucleation density and
deposition parameters. Making films smooth to begin with reduces the chances
of flaw introduction and makes the entire process much less expensive. Smooth
diamond films can be deposited directly on the substrate growth surface with no
postdeposition treatments. It was learned several years ago that thin, very smooth
diamond films could be grown on polished tungsten through the use of dia-
mondlike carbon films as an interlayer between the diamond and the substrate
(Phelps and Koba, 1989). The diamondlike carbon deposition method will be
covered in a later section on diamond film seeding techniques. The average size
of a CVD diamond crystallite will be small if there is no room for it grow
laterally. If crystallites are densely packed, then a thinner film can be grown that
is pinhole free. A film will be smooth if it is very thin and densely packed.
Large rough facets will not develop if crystallites can be kept small throughout
the film thickness. This can be done by poisoning the diamond growth environ-
ment with excess carbon.
6 DIAMOND FILM THICKNESS
There are several aspects to deciding what thickness of diamond film is accept-
able for a bearing surface. The working definition proposed by A. C. Gonzalez
is that for a particular system ‘‘a thick film is one that will interact with the
system as if it were bulk diamond’’ (Phelps et al., 1992).
In that same system, ‘‘a thin film will allow the properties of the substrate
material to be sampled.’’ The scale of thickness is completely dependent on the
scale of the system. A thin film might be 0.5
m thick if the maximum foreign
particle size is 0.25
m in diameter where a thick film would be 5
m thick.
Similarly, a thin film might be 70
m thick if the maximum foreign particle
size is 20
m in diameter where a thick film would be 200
m thick. A central
concern is how thick must a film be to be thick enough. The size and hardness
of foreign particulate matter arriving at the bearing surface will determine the
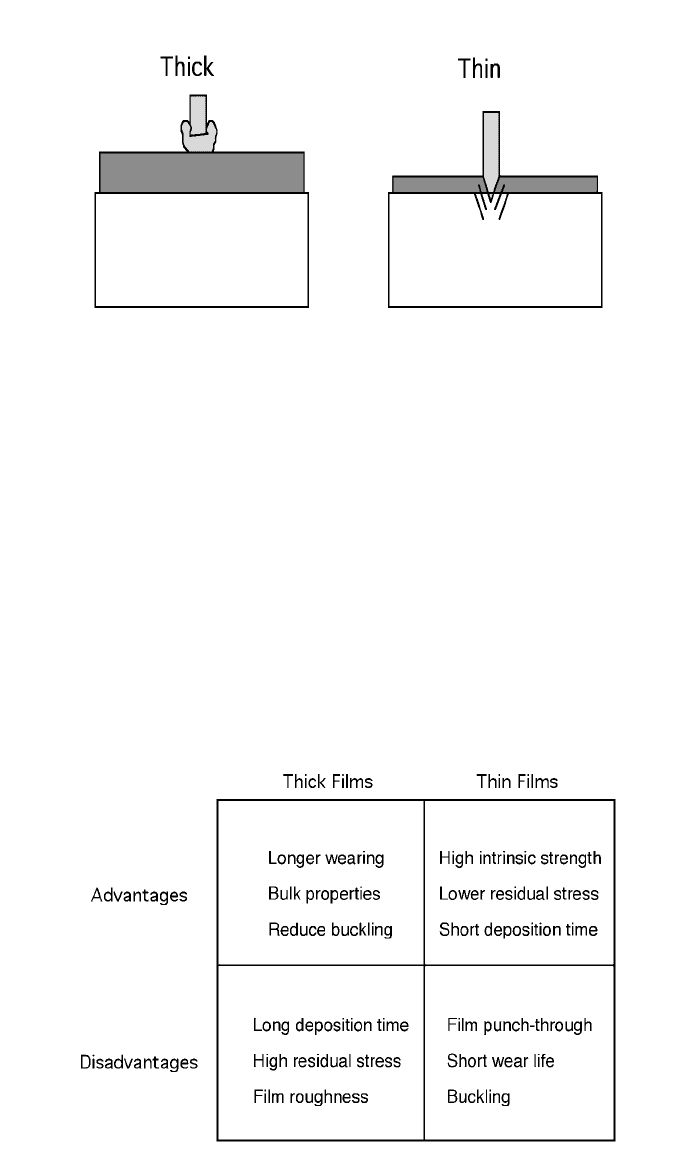
thickness of the diamond film. Figure 5 shows schematically how the definitions
of thick and thin apply to a sharp foreign object applied to the films under the
same load.
A second concern dealing with thickness takes the deposition process and the
mechanical system where the film will be used taken into account. Figure 6
shows the trades between having thick and thin films in a wear system. Currently,
there is no correct answer to ‘‘what is the proper thickness?’’
7 DIAMOND FILM ADHESION 1297
Fig. 5 Representing the difference between thick and thin coatings where a probe samples
only the bulk film properties in a thick coating and a probe samples both the coating and the
substrate in a thin coating.
Fig. 6 Decision box showing relationship between thick and thin coatings.
7 DIAMOND FILM ADHESION
Adhesion of CVD diamond films to working substrates is one of the most im-
portant aspects of diamond wear coating technology. Diamond has the particular
disadvantage that little is known about how to best attach a diamond film to a
substrate surface. Good adhesion must be considered at the system design level
in order to realize superior state-of-the-art bearings. Adequate adhesion between
the diamond film and the substrate must be present in order to use the remarkable
mechanical properties of diamond. There are many reasons why a diamond film
would not be adherent. Identifying these reasons can lend direction to solving
the adhesion problem. The first and foremost reason that diamond films delam-
inate from substrate surfaces is that the bond strength between the film and its
substrate is not sufficient to hold onto the film. The strength necessary to hold

1298 DIAMOND FILMS
onto a film will vary with the type of substrate, the film deposition conditions,
the substrate preparation, etc. Means of increasing the bond strength between
the diamond film and the substrate are chemical, physical, and mechanical. A
chemical means of enhancing adhesion is through the use of carbide forming
substrates. Molybdenum is frequently used as a substrate material for diamond
deposition, however, diamond does not form a good strong carbide with this
metal. A metal that is more suited to diamond growth is tungsten. Tungsten
forms a strong, stoichiometric carbide that supports diamond nucleation and
growth.
The thermal expansion of diamond is far less than all metals and many non-
metals. Most substrates that diamond is deposited onto have thermal expansion
coefficients that are larger than diamond such as silicon and tungsten. Diamond
films deposited on these substrates at 1000
⬚C are in compression at room tem-
perature. If the tensile stresses resulting from shear due to compression are
greater than the bond strength between the diamond and its substrate, then the
film will rupture at the substrate-to-film interface. The first means of controlling
stress and, thus, adhesion would be to reduce the thermal expansion mismatch
between the diamond film and the substrate. Pure silica glass has a lower degree
of thermal expansion from room temperature to 1000
⬚C than does diamond.
Diamond is found to be in tension at room temperature after it has been depos-
ited onto silica glass at 1000
⬚C. However, the addition of a small amount of
boron to the silica glass will raise its thermal expansion coefficient to near that
of diamond. Interlayer materials such as diamondlike carbon or carbide formers
such as tungsten or silicon could be used to control the thermal expansion dif-
ference between diamond and the substrate of choice. Likewise, a diamondlike
carbon or carbide former deposited between the diamond films and the substrate
could allow the use of substrates other than good carbide formers to be used for
diamond deposition.
It may be possible to control and manipulate the residual stress found in
deposited diamond films. Diamond films can be made more compliant by making
the film very thin or by changing the elastic constants of the diamond film. This
can be done very precisely by controlling the amount of nondiamond carbon
that is present in the film. Adding diamondlike carbon or graphite to a diamond
film reduces the stiffness of the material, and it should be possible to make a
low-stress diamond film on tungsten. This could be done through a graded in-
terface. A poor-quality diamond film would be deposited on top of the substrate,
and during the deposition process it could be possible to reduce the nondiamond
component and produce a good-quality diamond film.
The last means of enhancing the adhesion between the diamond film and its
substrate does not lower the stress at the interface but increases the rupture
strength of the interface. This is done by mechanically interlocking the film onto
the substrate. The work of a team of researchers at the University of Minnesota
(Tsai et al., 1992, 1993) has produced a method of depositing diamond films on
incompatible substrates. This is done by electroplating nickel over a partially
grown (island form) diamond film on a diamond-compatible substrate surface.
CVD diamond is further deposited on top of and across the electrodeposited
film and connecting the islands of earlier deposited diamond. The islands of
earlier diamond act as attachment anchors. Later, a continuous diamond film is
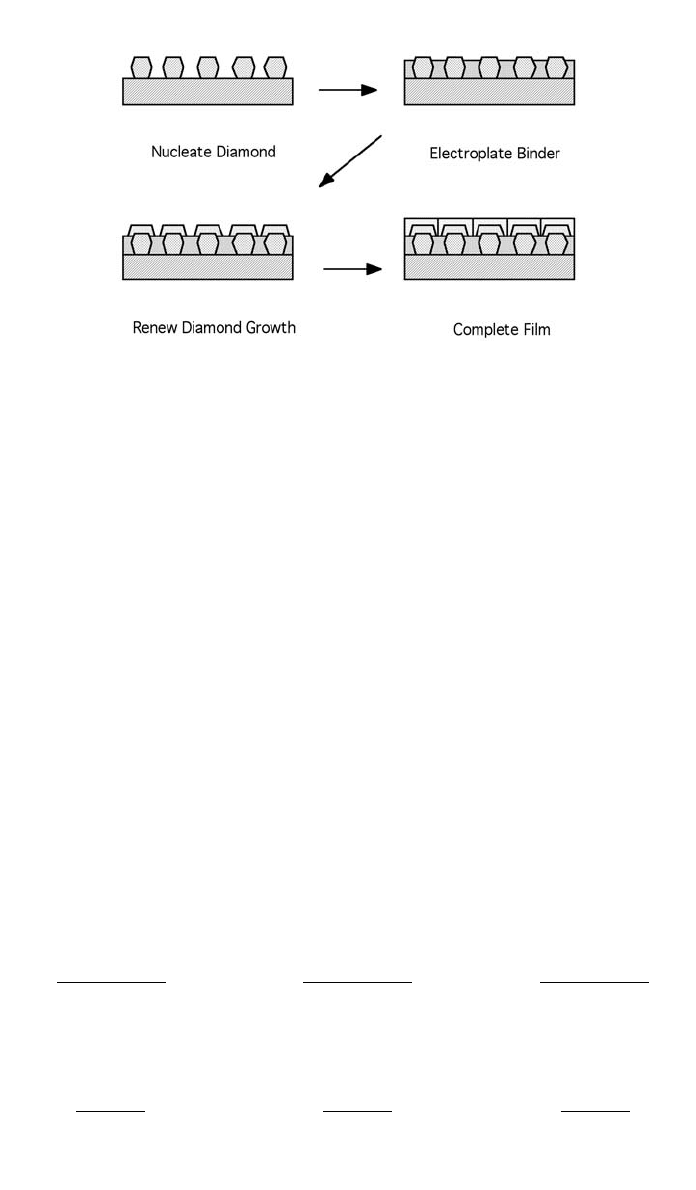
7 DIAMOND FILM ADHESION 1299
Fig. 7 Process steps used to electrodeposit a metal interlayer
between the diamond film and the substrate.
Film Adhesion
Controlled by:
Stress at Film Interface
Strength of Bond
Film Adhesion
Controls:
Maximum Film Thickness
Number of Restarts
Film Roughness
Controlled by:
Deposition
Nucleation Density
Film Roughness
Controls:
Friction Losses
Bearing Wear Rate
Film Thickness
Controlled by:
Deposition Time
Stress at Interface
Film Thickness
Controls:
Debris Punch Through
Service Life
Fig. 8 Relationships between film character, physical properties, and service behavior.
grown across these islands. The substrate does not have to be compatible with
diamond because no initial adhesion is necessary between the substrate and the
diamond seeds. The substrate simply has to be compatible with the electrode-
posited film on top and not form a eutectic composition at diamond deposition
temperatures. This method holds great promise for depositing diamond directly
on diamond-incompatible materials such as INCO 718. Adhesion is the single
most important factor currently affecting the use of diamond films as wear and
bearing surfaces.
There are several means by which an adherent diamond film may be produced
on a substrate surface.
1. Deposit the diamond wear film on top of a strong carbide-forming sub-
strate. This results in a strong diamond-to-carbide chemical bond and help
hold the film onto the substrate.
2. Deposit the diamond film on an intermediate layer that is a strong carbide
former deposited on top of the substrate. This results in a strong diamond-
to-carbide chemical bond and a strong carbide-to-substrate chemical
bond.
