Jones D.S.J., Pujado P.R. Handbook of Petroleum Processing
Подождите немного. Документ загружается.

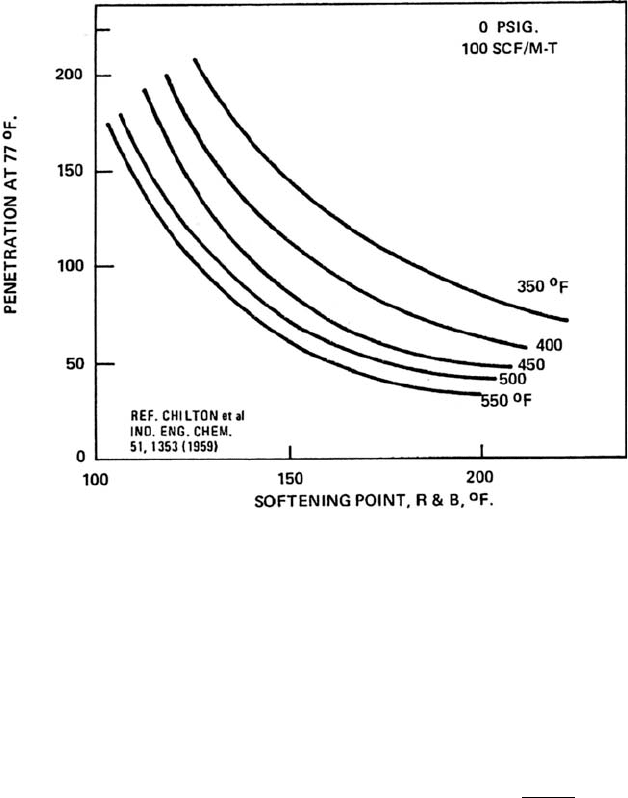
THE NON-ENERGY REFINERIES 503
Figure 12.11. Effect of temperature on softening point/penetration ratio.
increasing the volume of asphalt (depth) in the oxidizer. In the case of the batch
process this is achieved by varying the batch residence time. Figures 12.12–12.15
show the effect of the changing retention time.
The retention time or residence time required for oxidation is different in every case
depending on how much change in softening point or penetration is required. A good
rule of thumb is to calculate retention time assuming it takes one hour to increase
12
◦
F in the softening point of the asphalt. For example to increase the softening point
of 100
◦
F asphalt to a 220
◦
F product will require a residence time of
220−100
12
= 10 hr.
A second rough rule for batch processes is that it takes approximately 4–8 hr of
blowing utilizing 15 SCFM of air per ton of asphalt. That is if 25 SCFM of air is used
the residence time would be around 2.4–4.8 hr since the effect of residence time is
proportional to the oxidation rate. A continuous process usually requires less time,
about one half that of a batch process.
Increasing the contact time or the depth of the asphalt level in the oxidizer ves-
sel will increase the softening point of the asphalt if all other variables remain
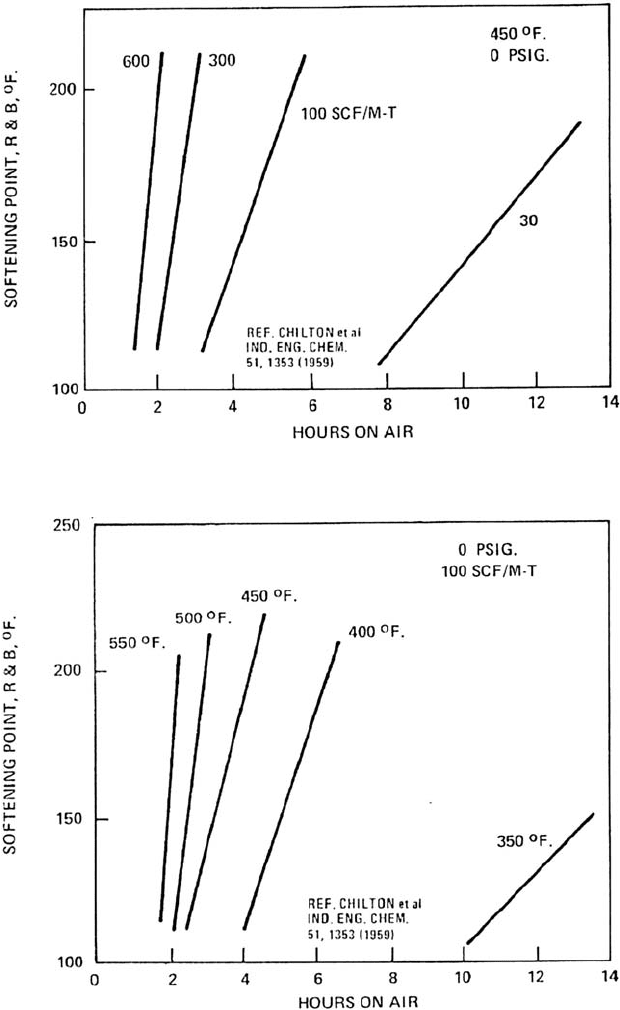
504 CHAPTER 12
Figure 12.12. Effect of retention time on softening point (or hardness) at different air rates.
Figure 12.13. Effect of retention time on softening point (or hardness) at different temperatures.
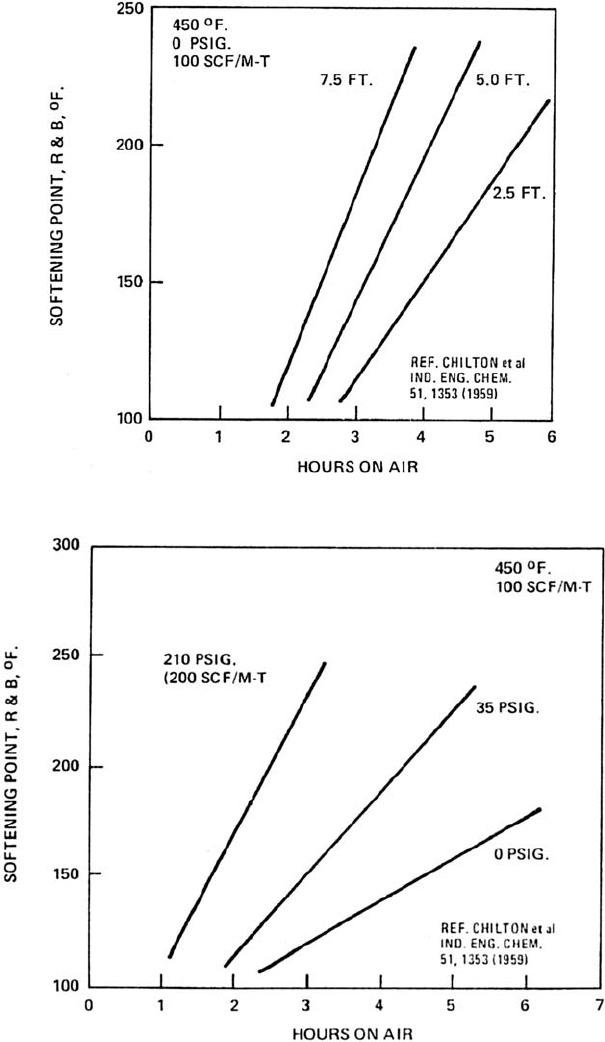
Figure 12.14. Effect of retention time on softening point (or hardness) at different liquid depth in oxidizer.
Figure 12.15. Effect of retention time on softening point (or hardness) at different oxidizer pressures.
505
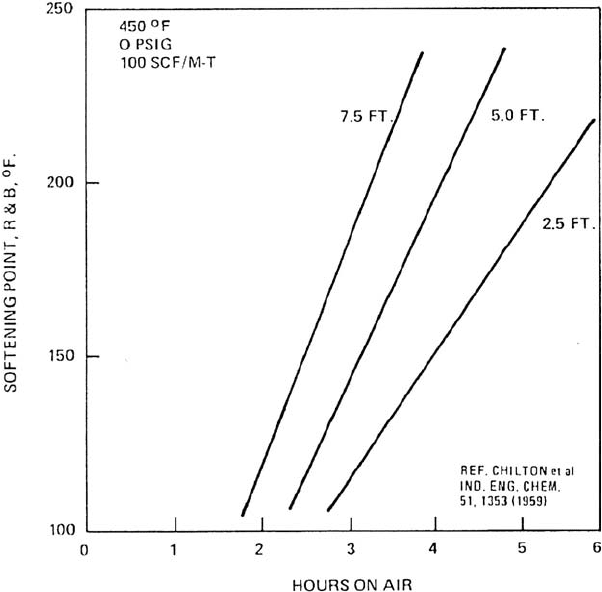
506 CHAPTER 12
Figure 12.16. Effect of oil level in oxidizer on softening point (or hardness).
constant. In this case the softening point is changed without altering the softening
point/penetration ratio. The effect of this variable is the same as increasing the resi-
dence time of the asphalt. Figure 12.16 shows this effect.
Although the height of the liquid and the residence time of the air are closely related
they don’t exactly have the same effect on the oxidizing process. The higher the liquid
level the more efficient is the oxidizing process. This is so because the same amount of
air is better utilized by remaining in contact with the oil for a longer period. Since the
air is not completely used by the time it leaves the oxidizer, asphalt can be oxidized
faster with the same air rate in a tall small diameter oxidizer than one of a larger
diameter but shorter, even though the residence time will be the same in both cases.
Normally a height to diameter ratios of between 3.5/1 and 5/1 are used in the design
of continuous process oxidizers and a somewhat lower 2.5/1 for batch processes. The
liquid level in the oxidizer is usually no more than 2/3 of the total height of the vessel.
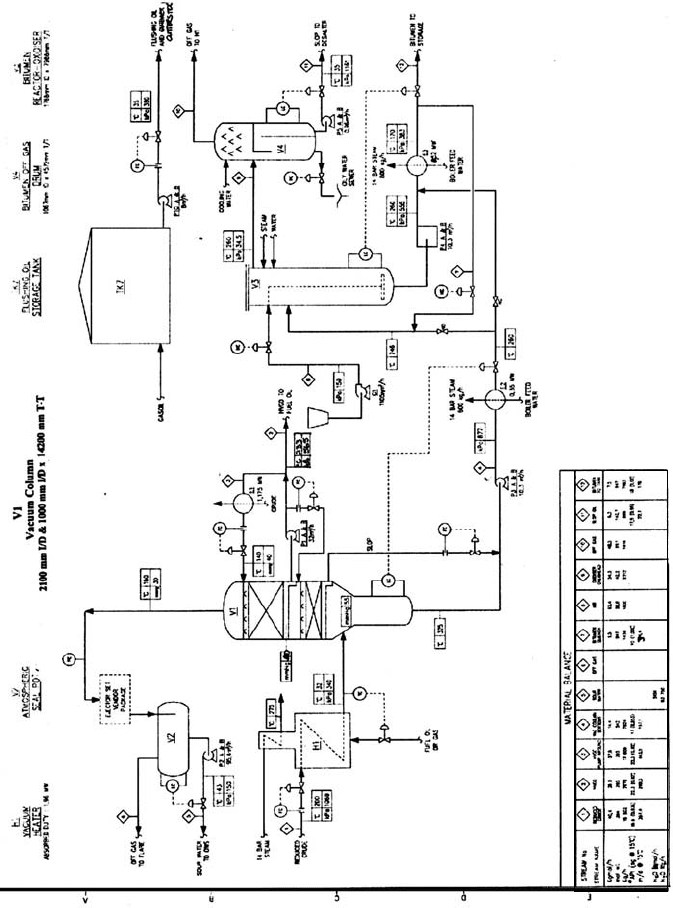
Figure 12.17. A process flow diagram of a typical bitumen manufacturing plant.
508 CHAPTER 12
At least 10 ft must remain free of liquid above the high liquid level. This is to allow
for liquid disengaging and foaming.
The system pressure
With all other variables constant, increasing the pressure of the oxidizer will increase
the asphalt softening point. The effect of increasing pressure however is not as great
as the effect of increasing any of the other variables. Perhaps the more significant
change in increasing the oxidizer pressure is in the ratio of softening point to pene-
tration of the asphalt. Usually it is not economical to operate at high pressure since
the gains on hardening rates are small relative to the effect of the other variables.
For practical purposes, pressure is not considered a design variable, and most oxidiz-
ers operate at near atmospheric pressure. A positive pressure of 5 psig is the more
usual.
A flowsheet of a typical asphalt blowing plant is given as Figure 12.17.
The petrochemical refinery
The petrochemical refinery processes crude oil to produce feedstock for chemical
plants. The two most important processes are:
r
The production of aromatics
r
The production of olefins
Both of these types of petrochemical refineries have been described briefly in Chapter
1 of this Handbook. It is not proposed to describe these type of refineries further here.
This chapter will deal with a typical example of the process configuration for the
production of aromatics only. This is probably the more common integration of the
petrochemical refinery because a normal energy refinery is more easily adapted to
aromatics with the minimum of additional processes.
The production of aromatics
The aromatics referred to here are:
r
Benzene
r
Toluene
r
Ethyl benzene
r
Para-xylene
r
Meta-xylene
r
Ortho-xylene
THE NON-ENERGY REFINERIES 509
The configuration described here begins with a mixed aromatic stream which has been
obtained by catalytic reforming of a high naphthene content naphtha. This naphtha
would probably have been a product of a hydrocracker producing energy products
from heavy waxy distillate. There are refineries that do hydrocrack heavy distillates
to extinction to produce this kind of high naphthene naphtha only. The more common
though is the energy hydrocracker producing a range of products of which the naphtha
is just one of them. The reformate from this high naphthene feed is very rich in the
aromatics listed above. To increase the aromatic content as feed to the aromatic
complex the aromatics are separated from the remaining paraffin’s by an extraction
process.
The aromatics recovery complex which takes as feed the mixed aromatic stream is
shown in Figure 12.18.
In this particular scheme the objective is to produce and maximize the benzene product
and the ortho-xylene products only. Many aromatic complexes also produce para-
xylene as product via a crystallization or adsorption step. A description of these units
and the process flow of the complex follows.
Feed fractionation
The fresh mixed aromatics is delivered from off plot to enter a 35 tray splitter tower.
Benzene and toluene are removed as overhead product while the mixed xylene streams
leave as the bottom product.
Xylene splitter and isomerization process
The mixed xylene stream leaves the splitter and is routed to a xylene splitter. This
is a super fractionating tower containing at least 135 fractionating trays. The frac-
tionation split is between the meta- and ortho-xylene components. A recycle stream
from an isomerization plant rich in ortho-xylene is also fed to this xylene splitter.
The overhead rich in ethyl benzene and the para, meta-xylenes, is routed from the
splitter to an isomerization plant. These C
8
aromatics are isomerized over a catalyst
and in the presence of a rich hydrogen stream to a product rich in ortho-xylene but
containing also benzene, toluene, and some ethyl benzene with some light hydrocar-
bons and hydrogen in equilibrium. This isomerate enters a fractionator in which the
light hydrocarbons and some ethylbenzene are removed as overheads while the bottom
product, containing mostly ortho-xylene, with the other C
8
’s in equilibrium is returned
to the xylene splitter. The light isomerate overhead product from the fractionator is
stabilized in a separate stabilizing column before being routed to the benzene recov-
ery section. The bottom product from the xylene splitter enters an ortho-xylene rerun
tower from which commercially pure ortho-xylene leaves as the overhead product. The
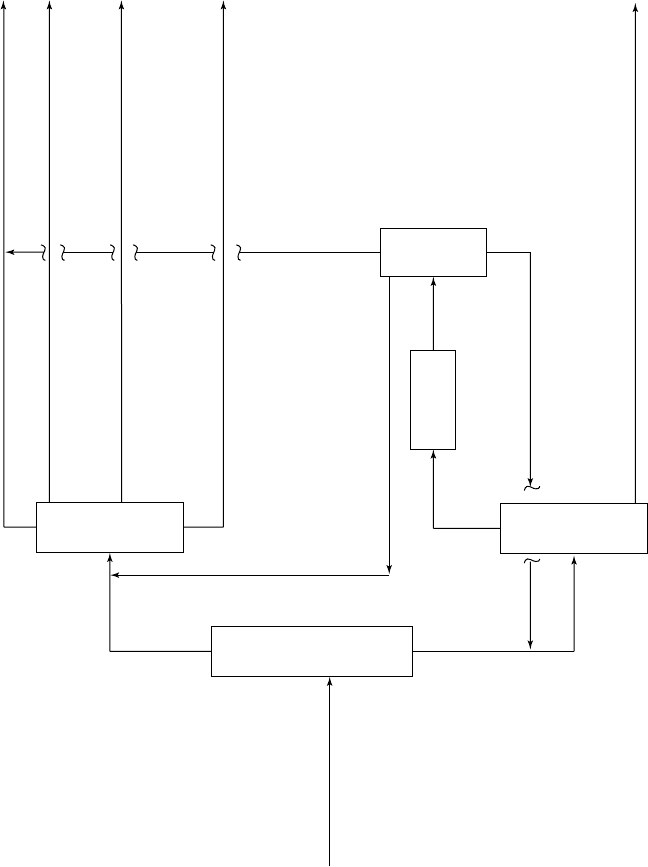
Benzene–Toluene Splitter.
Feed Splitter.
Reformate Feed
Isomerizer
Ethyl Benzente, M Xylene, P Xylene.
Isomerate Splitter
Note:
This configuration is for the production of
o-Xylene, Benzene and Toluene. The remaining
C8 aromatics may be recovered by selective distillation
and crystalization or adsorption
(in the case of P-Xylene.)
Xylene Splitter
O Xylene.
Ta r
Toluene,
Benzene
Fuel Gas
Figure 12.18. A block flow diagram of an aromatic production complex.
THE NON-ENERGY REFINERIES 511
bottom product leaving this tower contains heavy aromatics (heavier than C
8
’s) and is
routed to fuel, or, in some cases, to trans alleylation with toluene to yield more C
8
s.
Dealkylation and benzene recovery
The overheads from the feed fractionator and the bottom product from the light
isomerate stabilizer combine to form the fresh feed to a benzene fractionator. This feed
stream will contain mostly benzene and toluene. A recycle stream from the toluene
dealkylation plant, if present, containing a high proportion of benzene joins the fresh
feed to enter the benzene fractionator. Benzene is removed from this fractionator as
an overhead product, while toluene is removed as a re-boiled stripped side stream
product. A small bottom make of polymer and tar is removed as a bottom product.
The toluene stream may be routed to a dealkylation unit in which about 98 mol%
of the toluene is converted to benzene. This dealkylated product forms the recycle
stream to join the fresh feed to the benzene fractionator.
The data given in the block flow diagram. Figure 12.18 is based on processing the
following mixed aromatic feed to the units:
Benzene 20,000 long tons/year
Toluene 50,000 long tons/year
Ethyl benzene 9,000 long tons/year
Para-xylene 11,400 long tons/year
Meta-xylene 27,000 long tons/year
Ortho-xylene 12,600 long tons/year
Make-up gas to the isomerization unit and the dealkylation unit has the following
composition:
H
2
81.5 mol%
C
1
12.2 mol%
C
2
5.2 mol%
C
3
+ 1.1 mol%
The products required to be maximized are:
High purity benzene to an SG of 0.882 (Min) and 0.886 (Max) all at 60
◦
F
Ortho-xylene will contain not less than 99.0 wt% of ortho-xylene
Process discussion
Feed fractionation and xylene splitter
Good separation of the light aromatics and the xylenes can be achieved in a 35 trayed
column operating at about 35 psig in the reflux drum. At this pressure the tower
512 CHAPTER 12
overheads are at a temperature high enough to offer some preheating of the tower
feed by the overhead condensers. Splitting the xylenes however requires a tower of
about 136–140 trays. A reasonable economic overhead pressure (in the reflux drum)
is about 98 psig. Again at this pressure, as in the case of the feed fractionator, the
overhead temperatures offers some feed preheat from the overhead condensers and
to reboil the ortho-xylene rerun tower.
The isomerization unit
This is a licensed process which converts the xylene streams to approach an equilib-
rium xylene composition. Some isomerization units convert ethylbenzene to xylenes
while others essentially dealkylate the ethylbenzene. By removing one or more of
the components (ortho-xylene in this case) the remaining xylenes in the feed are iso-
merized to recover the equilibrium distribution lost by the removal. The unselected
isomers in the fresh feed are recycled to extinction by this method. The process shown
here utilizes around 1.2 mmScf/D of 81.5 mol % hydrogen.
The dealkylation unit
This unit is also a licensed process. its purpose is to de-alkylate the toluene component
in the feed to produce benzene.
This process utilizes a separate polymer reactor to increase benzene selectivity. It also
uses a hydrogenation reactor to control impurities in the benzene such as unsaturates
and sulfur products. The yield of benzene in this process is about 98.2 mole% on
toluene in the feed. Except for very small units a cryogenic step is applied to remove
the reaction products of methane and heavier. Where hydrogen from a catalytic re-
former is used as make up the C
3
’s, and heavier paraffins and any H
2
S contained in
it must be removed. An absorber operating at about 250 psig is used to remove the
hydrocarbons and a caustic wash used to remove the H
2
S. The removal of the C
3
’s
and heavier is advisable as these hydrocrack under the dealkylation reactor condi-
tions causing additional reactor temperature rise and increase in hydrogen consump-
tion.
Appendix 12.1: Sizing a bitumen oxidizer
Design specification
Product required 816 BPSD of 25 pen asphalt.
Crude source Pennington—West African offshore. (Assay data see Figures 12.19–21.)
