Jan Lindhe. Clinical Periodontology
Подождите немного. Документ загружается.

ALVEOLAR BONE FORMATION •
877
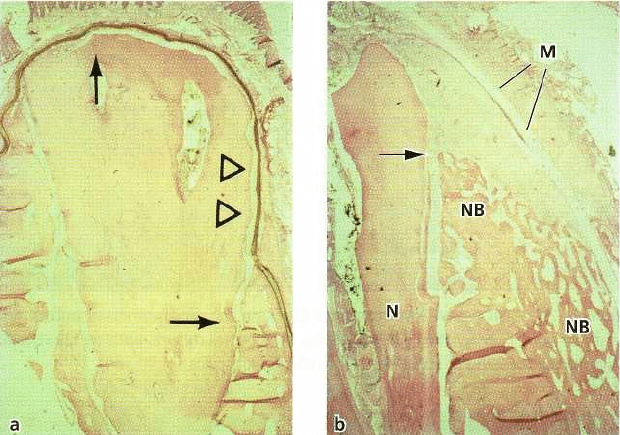
Fig. 38-14. Microphotographs showing submerged membrane-covered roots. (a) Membrane has collapsed, leaving
a narrow space adjacent to the root surface (arrowheads). The formation of bone underneath the membrane is negli
gible, but a thin layer of new cementum covers the root surface between the arrows. (b) Root covered with a
mem
brane (M) which has maintained considerable space adjacent to the root surface. New cementum is seen on
the root
surface from the notch (N) to the arrow, and considerable amounts of new bone (NB) have formed
underneath the
membrane. Note that in the area below the notch (N), new bone (NB) has also formed on top of the
original buccal
bone plate where bone has not existed before.
healing in osseous defects or to augment atrophic
alveolar ridges has been evaluated in a number of
experimental and clinical studies (Boyne 1970,
Thompson & Casson 1970, Steinhauser & Hardt 1977,
Fazili et al. 1978, Baker et al. 1979, Mulliken &
Glowacki 1980, Curtis & William 1983, Swart & Allard
1985, Block et al. 1987, Cullum et al. 1988, Hupp &
McKenna 1988). However, there are several reports
indicating that this type of treatment fails to predict-
ably produce bone fill and augment alveolar ridges (
Korlof et al. 1973, Curtis & Ware 1977, Steinhauser &
Hardt 1977, Taylor 1983, Davis et al. 1984, Jackson et
al. 1986, Hupp & McKenna 1988). Often the bone
grafts do not attach to the graft site through bony
attachment and there is bone resorption and bone loss
associated with grafting procedures. As a conse-
quence, much of the intended volume is lost, and
frequently the defects heal with a fibrous connective
tissue instead of bone.
CONCEPT OF GUIDED TISSUE
REGENERATION (GTR)
The principles of guided tissue regeneration (GTR)
have been developed on the basis of a number of
experimental animal studies on periodontal regenera-
tion (see Chapter 33). In one of these studies (Gottlow
et al. 1984), barrier membranes were placed over
crown-resected roots in monkeys. The membrane-
covered roots were then submerged. Following 3
months of healing, it was noticed that, in situations
where the membranes were collapsed, leaving a nar-
row space adjacent to the root surface, new cementum
had formed on the root surface, but the amount of
newly formed alveolar bone was negligible (Fig. 38-
14a). On the other hand, in situations where the mem
branes had not collapsed, leaving a wider space
adja
cent to the root surface, considerable amounts of
new
bone had formed in addition to the new
connective
tissue attachment to the root surface,
even in areas
where bone had not existed before (Fig.
38-14b). This
observation suggested that the GTR
principle may be applied successfully in bone
regeneration as well by creating a secluded space
which can only be invaded
by cells with bone-forming
capacity from existing
bone.
Animal studies
Alveolar bone defects
The application of the GTR principle for bone regen-
eration (guided bone regeneration (GBR)) was first
investigated by Dahlin et al. (1988) in an experimental
study in rats. Transmandibular defects 5 mm in di-
ameter were surgically created bilaterally, while the
test sites were covered on either side of the defect with
a barrier membrane to allow the exclusive ingrowth
of tissue from the mandibular bone, at the same time
excluding the fibrous tissue of the area from prolifer-
ating into the defect. The control sites were left with-
out the placement of a membrane. On the test side,
almost complete bone healing was demonstrated both
on defleshed mandibles and in histologic prepara-
878 • CHAPTER 38
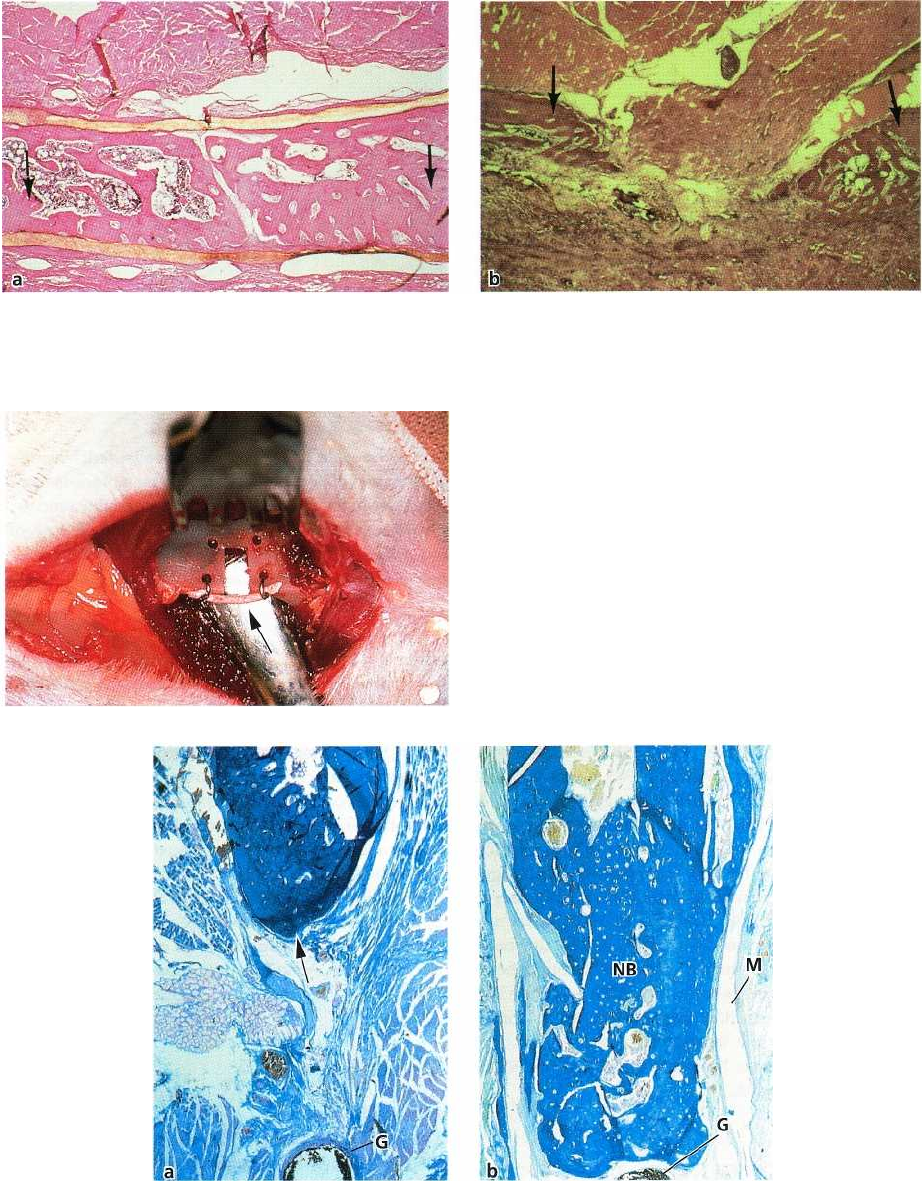
Fig. 38-15. (a) Test defects of critical size with membranes blocking the proliferation of undesired cells into the de
-
fect, resulting in complete bone regeneration after 6 weeks. (b) Control defects with residual transmandibular de
-
fects after 9 weeks due to the invasion of soft tissues into the defect space.
Fig. 38-16. 2 x 3 mm standardized defect produced at
the inferior border of the mandible of a rat. A gutta-per-
cha point (arrow) is placed to indicate the original level
of the inferior border.
Fig. 38-17. Microphotographs of control (a) and test (b) jaw bone defects 6 months following surgery. Some new
bone has formed in the bottom of the control defect (arrow) while the remaining part contains muscle and connec
-
tive tissue. The gutta-percha point (G) indicates the original inferior border of the mandible. In the test defect (b),
covered with a membrane (M), the newly formed bone (NB) is filling out the defect and has reached the gutta-per-
cha point (G), indicating the original level of the inferior border of the mandible.
tions after 6 weeks (Fig. 38-15a). Control sites, on the
tissues were invading the defects, hindering the bone-
other hand, demonstrated residual transmandibular regenerating cells from occupying the wound space
defects, although somewhat diminished in diameter
(Fig. 38-15b).
after 9 weeks due to the fact that surrounding soft
Similar results have been reported in an experimen-
ALVEOLAR BONE FORMATION •
8
79
tal model in rats where standardized mandibular de-
fects were covered with a bio-absorbable membrane
(
Kostopoulos & Karring 1994a). The mandibular ra-
mus of the rats was exposed on both sides and a 2 x 3
mm defect was created at its lower border (Fig. 38-16).
A gutta-percha point was placed to indicate the origi-
nal level of the border. On one side the defects were
covered with a resorbable membrane, while the con-
tralateral sides remained uncovered. The jaws were
subjected to histologic analysis. In addition, defleshed
specimens were prepared. These specimens revealed
minimal bone fill in the control defects (Fig. 38-17a),
while all test defects healed to or close to the gutta-
percha point, indicating the original inferior border of
the jaw (Fig. 38-17b). Likewise, the histologic analysis
showed that bone regeneration in the experimental
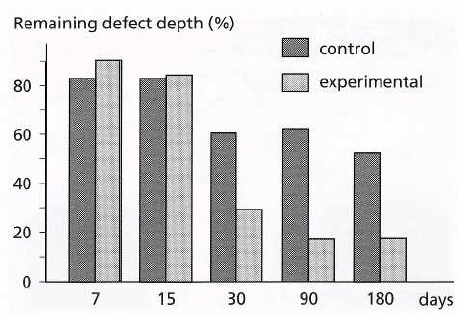
specimens occurred gradually over 7-180 days,
amounting to 85% of the initial defect depth at 180
days (Fig. 38-18). In the control defects only some bone
regeneration occurred within the first month follow-
ing surgery. Bone regeneration in the control spec-
imens amounted to 48% of the defect at 180 days. The
rest of the control defect was filled with muscular,
glandular and connective tissue (Fig. 38-17a).
Bone regeneration adjacent to implants
Titanium dental implants were inserted into the tibial
bone in rabbits in such a way that three to four coronal
threads were exposed on one side of each implant
(
Dahlin et al. 1989). At the test sites the implants were
covered with a Teflon membrane, whereas at the con-
trol sites the implants remained uncovered. The over-
lying soft tissues were then sutured to obtain complete
closure. Histologic analysis after 6 weeks revealed
that, in the test sites, new bone was completely cover
-
ing the exposed threads of the implants, while the
threads of the implants at the control sites were cov-
ered by connective tissue. In a similar study in dogs (
Becker et al. 1990), titanium implants were inserted
in such
a way
that some of their threads remained
exposed. Again, before closure of the wounds with a
mucoperiosteal flap, the test sites were covered with a
Teflon membrane, while the control sites remained
uncovered. Following a healing period of 18 weeks,
the specimens were subjected to clinical, radiographic
and histologic examination. For the majority of the test
implants, new bone was covering the previously ex-
posed implant threads. The average gain in bone
height was 1.37 mm for the test and 0.23 mm for the
control implants. In the control sites, loosely adherent
connective tissue was covering the exposed threads.
Immediate implant placement
The effect of GTR on osseointegration of titanium
implants inserted into fresh extraction sockets was
investigated by Warrer et al. (1991) in an experimental
study in monkeys. The experimental sites were cov-
ered with a Teflon membrane, while the controls re-
mained uncovered at the time of complete wound
closure. Following 3 months of healing, histologic
Fig. 38-18. Diagram showing the regeneration of bone
in experimental and control defects. The columns indi-
cate the remaining defect area, not filled with bone at
various observation times. It can be seen that bone for-
mation in the control defects is arrested after 1 month,
while new bone continues to fill out the experimental
defects.
analysis of the experimental sites revealed complete
bone regeneration to the top of the implants and com-
plete osseointegration. However, when exposure of
the
membrane had occurred during healing, less bone
regeneration was observed and the coronal part of the
fixtures was not osseointegrated. The controls also
exhibited incomplete osseointegration. The authors
concluded that osseointegration may be achieved pre-
dictably on dental implants placed into extraction
sockets and covered with a membrane, provided the
membrane is kept without communication to the oral
cavity during healing. These results are in agreement
with the results of other experimental studies in dogs
(
Becker et al. 1991, Gotfredsen et al. 1993), where
titanium dental implants were placed in fresh extrac-
tion sockets or where HA-coated implants were
placed in stimulated sockets before coverage with
e-
PTFE membranes (Caudill & Meffert 1991, Caudill
&
Lancaster 1993). Again, substantial amounts of new
bone formed under the membranes, while minimal
amounts were seen in control sites.
A comparison of e-PTFE membranes alone or in
combination with platelet-derived growth factor
(
PDGF) and insulin-like growth factor (IGF-I) or
demineralized freeze-dried bone (DFDB) in stimulat-
ing bone formation around immediate extraction
socket implants was investigated in dogs by Becker et
al. (1992). Following 18 weeks of healing, the his-
tologic analysis revealed that e-PTFE membranes
alone or e-PTFE membranes combined with PDGF/
IGF-I were equally effective in promoting bone
growth
around the implants. Bone regeneration in the
DFDB-
treated specimens was highly variable and did
not
improve the efficacy of the Teflon membranes. The
authors concluded that, on the basis of these results,
the clinical use of DFDB must be questioned.
A study in monkeys has indicated that bony defects
similar to those occurring around failed implants can

88o •
CHAPTER
38
Fig. 38-19. Microphotographs of bone defects around implants covered with membranes (M). The defect seen in (b)
was grafted with hydroxylapatite (arrows). Both non-grafted (a) and grafted (b) defects are filled with bone which
extends coronally to the implant, to the level of the membrane (M).
Fig. 38-20. Microphotographs of
bone defects around implants
treated without membranes. (b)
Defect grafted with hydroxylapa-
tite (arrows). (a) Non-grafted de-
fect shows incomplete bone fill,
while new bone has formed in the
grafted defect (b) to the top of the
implant. Some hydroxylapatite
particles (arrows) are seen in the
soft tissue coronal to the implant.
Fig. 38-21. Microphotograph showing that the space be
tween the membrane (arrowheads) and the original
border of the mandible (arrows) is filled with new
bone,
and the naturally existing curvature is eliminated.
also be successfully treated with membranes (Gotfred-
sen et al. 1991). Standardized bony defects were pre-
pared in the alveolar ridge of edentulous areas in
monkeys. A titanium dental implant was then placed
in the middle of these defects. In each monkey one
defect was covered with a membrane. Another was
grafted with hydroxylapatite particles before cover-
age
with a membrane. A third defect was grafted with
hydroxylapatite only and a fourth defect, serving as
control, was covered only by the raised tissue flaps.
Histologic analysis after 3 months of healing showed
that all bony defects treated only with a membrane
(
Fig. 38-19a) and the majority of those grafted with
hydroxylapatite and subsequently covered with a
membrane were completely filled with bone (Fig. 38-
19b). The defects treated with hydroxylapatite alone
(
Fig. 38-20b) and the control defects (Fig. 38-20a) often
demonstrated incomplete bone fill in the defects.
Thus, the placement of hydroxylapatite in the defects
in addition to membrane coverage did not improve
the
healing results.
Localized ridge augmentation
The principle of GBR was applied in localized ridge
augmentation in dogs (Seibert & Nyman 1990). Surgi-
cally created alveolar ridge defects in the mandibles
of
dogs were covered with e-PTFE membrane alone,
ALVEOLAR BONE FORMATION • 881
Fig. 38-22 a,b. Microphotographs showing new bone formation (NB) between the membrane (arrows) and the
mandibular border. A rupture in the membrane, seen at high magnification in (b), has allowed soft connective tis
-
sue to invade the space underneath the membrane, thereby preventing osseointegration between the newly formed
bone and the surface of the micro-titanium implant.
Fig.
38-23.
After elevation of a musculo-periosteal flap exposing the mandibular ramus (a), a non-porous Teflon
capsule is placed with its opening facing the mandibular ramus (b).
or treated with a combination of membrane placement
and porous hydroxylapatite (HA) blocks, functioning
as
a space keeper, or with a combination of membrane
and
tissue growth matrix (porous PTFE). Tissue
growth
matrix as well as porous HA blocks were also
used
without membranes. In the control specimens,
neither
membranes nor implants were placed. His
tologic
analysis after 55-90 days of healing revealed
that in the
specimens covered only with membranes,
bone had
filled out the entire space underneath the
membrane.
At the sites where a membrane and HA
were used,
bone had also filled out the entire space.
However, the
pores of the HA adjacent to the subsur
face of the
membrane were only partially filled with
bone. Bone
deposition was mainly observed in the
apical and
middle part of the implant. The control specimens
without membranes showed no new bone
formation.
Vertical augmentation of the mandible at its inferior
border using a bioresorbable membrane adapted to
create a secluded space for ingrowth of bone tissue
was evaluated in a study in rats (Kostopoulos & Kar-
ring 1994b). Following exposure of the mandibular
ramus, a standardized titanium microimplant serving
as a space maker and a fixed reference point was
inserted in the naturally existing curvature at the in-
ferior border of the mandible. The implant consisted
of
two parts, a threaded and a non-threaded part,
separated by a stopper ring. One side of the mandible
was covered with a bioabsorbable membrane, in such
a
way that a space was created in the curvature be-
tween the membrane and the inferior border of the
mandible. The contralateral sides remained uncov
ered
and served as controls. After 6 months of healing the
specimens were defleshed and prepared for his
tologic
analysis. The control specimens revealed mini
mal
bone formation and the naturally existing curva
ture at
the inferior border of the mandible had persist-
ed. In
contrast, the test specimens revealed consider-
able
bone formation and the non-threaded part of the
titanium microimplants was osseointegrated (Fig. 38-
21). However, in specimens where soft tissue from the
environment had escaped underneath the membrane,
bone formation was reduced and osseointegration of
the implants prevented (Fig.
38-22).
In order to evaluate the potential to produce bone
with GTR in a space where bone has not existed before,
an experimental model was developed by Kostopou
los
et al. (1994). Non-porous, rigid, Teflon capsules of
identical size were placed on the lateral surface of the
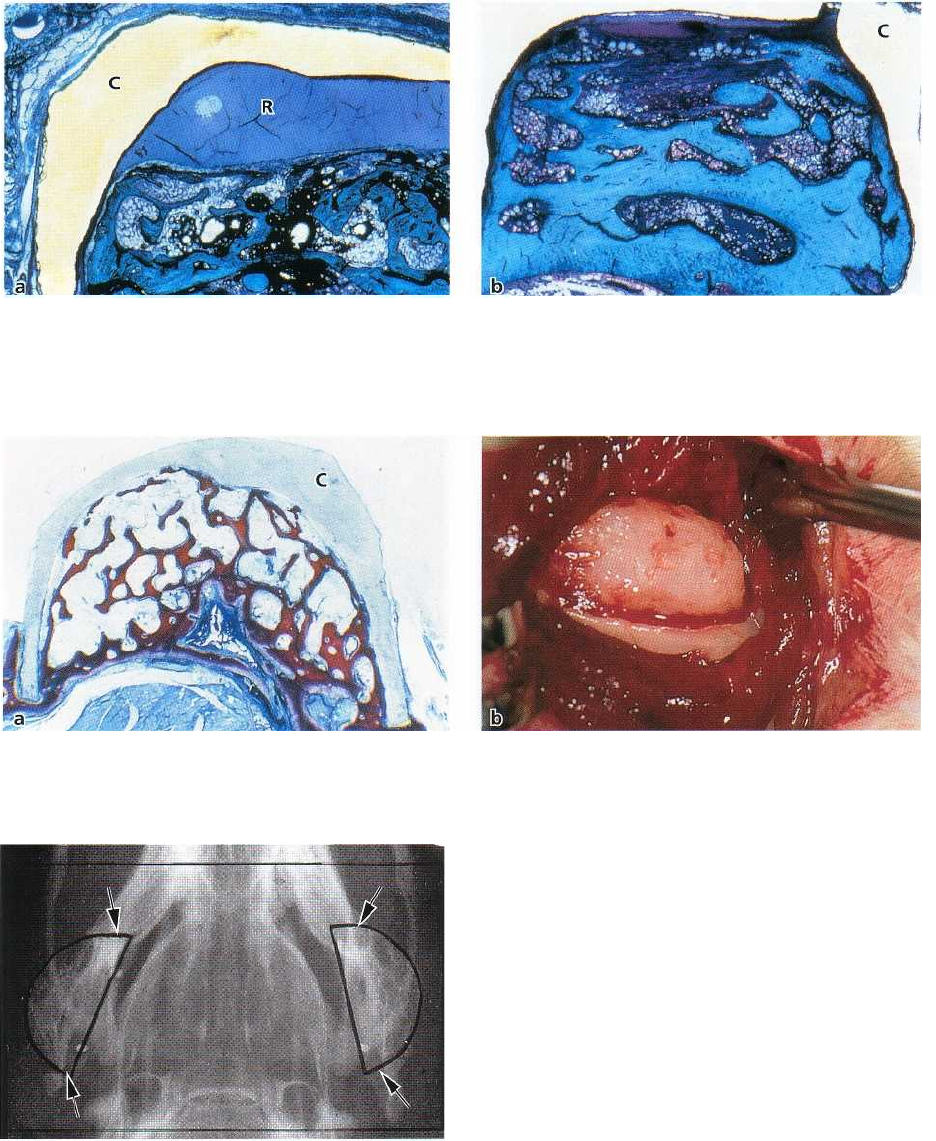
Fig. 38-24. Microphotographs of Teflon capsules 2 months (a) and 4 months (b) after placement on the mandibular
ramus. After 2 months (a) the capsule (C) is about half filled with bone, while after 4 months (b) new bone is filling
out
the entire capsule. R: Remaining capsule space.
Fig. 38-25. Microphotograph of Teflon capsule (C) prior to removal after 6 months (a). The capsule is completely
filled with bone. (b) Bone tuberosity present at the mandibular ramus after removal of the capsule.
Fig. 38-26. Radiograph showing the bone tuberosities
(arrows) formed on the lateral aspect of the ramus at
both sides of the mandible.
mandibular ramus of rats in such a way that the open
part of the capsules was adjoining the lateral surface
of the mandibular ramus which was either covered
with periosteum or denuded (Fig. 38-23). The his-
tologic analysis revealed that new bone formation
gradually occurred in the capsules (Fig. 38-24a). At 120
days, the capsules were filled out or almost filled out
with bone amounting to five to six times the original
width of the mandibular ramus at both the pe-
riosteum-covered and the denuded sides (Fig. 38-24b).
However, up to 60 days following surgery the amount
of generated bone was significantly greater at the
periosteum-covered sites than at the denuded sites.
The authors suggested that a secluded space created
adjacent to an existing bone surface, covered or not
with periosteum, will inevitably be filled with newly
formed bone.
The stability of such bone tuberosities produced by
GTR in areas where bone has not existed before was
evaluated by Lioubavina et al. (1997). Non-porous
Teflon capsules were placed on the lateral surfaces of
the mandibular ramus of rats. At one side the pe-
riosteum was preserved, while the other side was
denuded. Histologic analysis of some of the animals
after 6 months showed that the capsules were com-
pletely filled with bone (Fig. 38-25). The capsules in
the remaining animals were removed by a second
operation at 6 months and the structure and size of the
bone tuberosities were evaluated histologically and
radiographically until 1 year following removal of the
capsules. The bone tuberosities diminished slightly in
size immediately after capsule removal, after which
882 . CHAPTER 38
ALVEOLAR BONE FORMATION • 883
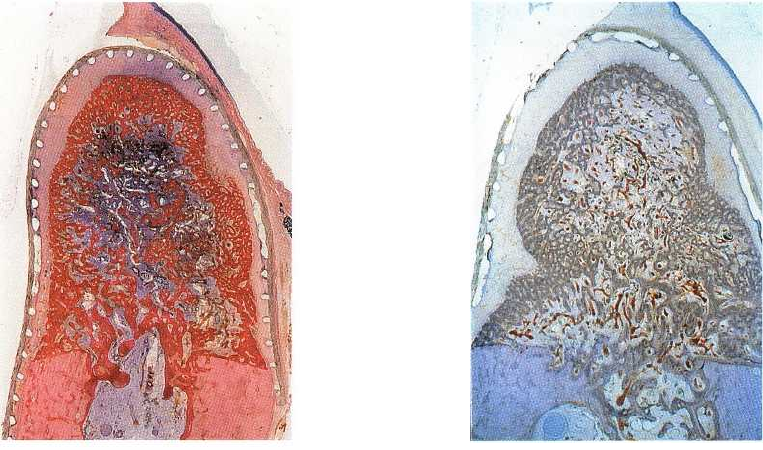
Fig. 38-27. Buccolingual section of a defect covered by a
barrier membrane after 2 months. Complete bone fill in
the secluded space beneath the membrane yields
pri-
mary spongiosa.
(From Schenk et al. (1994))
Fig. 38-28. Transformation of the primary spongework
into cortical and cancellous bone. After 2 months, the
peripheral spongiosa is somewhat denser than the cen
-
ter. (From Schenk et al. (1994))
time no further resorption was observed (Fig. 38-26).
This observation indicates that bone formed by GTR
is
stable over the long term.
Healing of GTR-treated bone defects
An experimental study in a canine model (Schenk et al.
1994) provided detailed information about the se-
quence and pattern of bone regeneration in surgically
created, membrane-protected defects in the alveolar
ridge. This histologic documentation confirmed that
bone regeneration in membrane-protected defects fol-
lowed closely the pattern of normal intramembrane-
ous bone growth in extraction sites and a development
through a similar sequence of maturation steps. Fol-
lowing initial organization of the blood clot, protected
by the membrane, regeneration was initiated by depo-
sition of woven bone along
new vascular structures
origi-
nating from the three surgically created bony walls that
defined the defect margins. This
primary spongiosa
was
characterized by blood vessels originating from marrow
spaces (Fig. 38-27).
Secondarily, the network of woven bone was rein-
forced by concentrically deposited parallel-fibered
la-
mellar bone,
which resulted in the development of a
new cortical structure at the periphery of the defects
(
Fig. 38-28). Finally, the onset of bone remodeling with
the formation of
secondary osteons
could be observed
in
the newly formed bone close to the defect margins
(
Fig. 38-29). The duration of the maturation process
obviously exceeded 4 months in the large defects cre-
ated, since small remaining defects were still found in
the midcrestal portion of the defect after 4 months
(
Fig. 38-30).
Subsequently, a similar second study was per-
formed in dogs as well (Schenk et al. 1994) to address
questions about osseointegration of titanium implants
into regenerated bone and the remodeling process of
regenerated bone under functional loading conditions.
In this study, complete bone fill was demon
strated in
the defects after 6 months of healing, which
histologically represented regular compact and can-
cellous bone perfectly suitable for the osseointegra
tion
of implants. Hence, all the implants inserted
yielded
primary stability. An intimate viable regener
ated bone
to implant contact was demonstrated in the
histologic
preparations. Secondary osteon formation
and signs of
ongoing remodeling were found in close
proximity to
the implant interface. Formation of bone
trabeculae to
"support" the implant was also identi
fied in the more
spongy region of the regenerated
alveolar bone. The
bone remodeling process, however,
was not influenced
by functional loading of the im
plants. On the other
hand, regenerated control sites
with no implant
placement exhibited a rarefied bone
structure
characterized by a thin cortical layer and
sparse bone
trabeculae. Hence, it may be stated that
the
incorporation of titanium implants into newly
regenerated bone may provide the necessary stimulus
to activate bone maturation and remodeling.
Human experimental studies
At present, most of the information regarding the
biologic events which lead to new bone formation is
derived from animal studies. Results regarding bone
formation collected in animal studies have to be ap-
plied with proper caution in humans. In particular, the
time sequence of the various steps ultimately leading
to the formation of mineralized mature bone in man
884 • CHAPTER 38
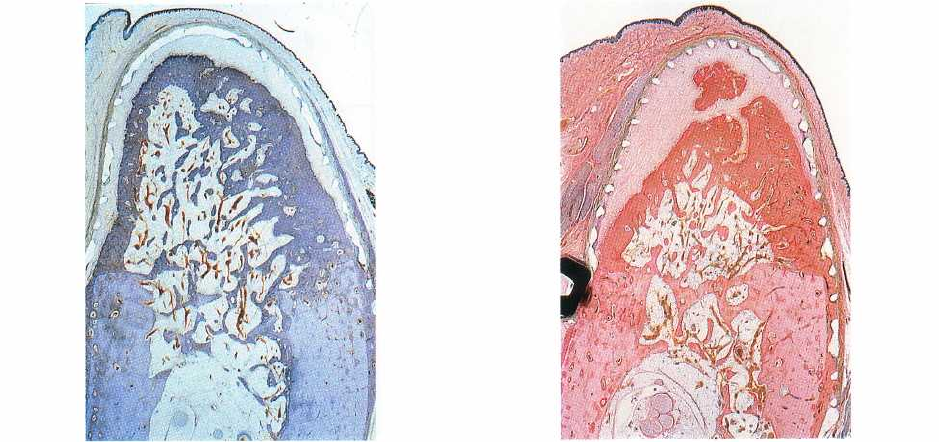
Fig. 38-29. Cortical bone formation and
secondary
spongiosa
after 4 months. A compact bone layer in the
periphery including haversian remodeling confines a
cancellous bone in the center with well-defined trabecu
lae and bone marrow. (From Schenk et al. (1994))
is different from that in all experimental animal sys-
tems known. A few human specimens, often har-
vested under poorly controlled conditions, contribute
relatively little to the understanding of the biologic
events of bone regeneration in humans.
A model system was designed to obtain human
specimens of regenerated and also newly generated
alveolar bone for the study of the biologic events
under a variety of conditions (Hammerle et al. 1996).
A mucoperiosteal flap was raised in the retromolar
area of the mandible of nine healthy volunteers. Fol-
lowing flap reflection, a standardized hole was drilled
through the cortical bone into the bone marrow. Con
-
gruent test cylinders were firmly placed into the pre-
pared bony bed, yielding primary stability;
1
1
/2-2 mm
of the test device were submerged below the level of
the surrounding bone, leaving 2-3 mm above the bone
surface. The bone-facing end of the cylinder was left
open, while the coronal soft tissue-facing end was
closed by an ePTFE-membrane. The flap was sutured
to obtain primary wound closure. In order to prevent
infection, penicillin was prescribed systemically and
oral rinses of chlorhexidine were administered. After
2, 7 and 12 weeks, one test device, including the regen
erated tissue, was surgically harvested, while after
16,
24 and 36 weeks, respectively, two devices were
har
vested and processed for soft or hard tissue
histology
or histochemistry The tissue generated
after 2 and 7
weeks (Fig. 38-31) presented with a
cylindrical shape,
whereas the specimens harvested
at 12 weeks and
thereafter resembled the form of an
hourglass. Speci
mens of 12 weeks and less
regeneration time were
Fig. 38-30. The barrier membrane separates an outer
gingival compartment from the compartment that is
mainly accessible from the marrow space. In the latter,
a well-vascularized connective tissue derived from
bone marrow forms a periosteal envelope along the
bone surface. (From Schenk et al. (1994).)
almost entirely composed of soft tissue, while speci-
mens with a regeneration time of 4 months and more
were composed of both soft and increasing amounts
of mineralized tissue (Fig. 38-32). It was concluded
that the model system is suitable for studying tempo-
ral dynamics and tissue physiology of bone regenera-
tion in humans with minimal risk of complications or
adverse effects for the volunteers.
In a retrospective re-entry study (Lang et al. 1994a),
the bone volume regenerated using non-bioresorbable
membrane barriers was assessed. Nineteen patients
with jaw bone defects of various sizes and configura-
tions were included. Combined split-thickness/full-
thickness mucosal flaps were elevated in the area of
missing bone. The size of the defects was assessed
geometrically. Following the placement of Gore-Tex
®
augmentation material as a barrier, the maximum pos
sible volume for bone regeneration was calculated. At
the time of membrane removal (3-8 months later), the
same measurements were performed and the percent
-
ages of regenerated bone in relation to the possible
volume for regeneration determined. In six patients in
whom the membranes had to be removed early, be-
tween 3 and 5 months, due to an increased risk of
infection, bone regeneration varied between 0 and
60%. In 13 patients in whom the membranes were left
for 6-8 months, regenerated bone filled 90-100% of the
possible volume. It was concluded that successful
bone regeneration consistently occurred with an un-
disturbed healing period of at least 6 months.
ALVEOLAR BONE FORMATION •
885
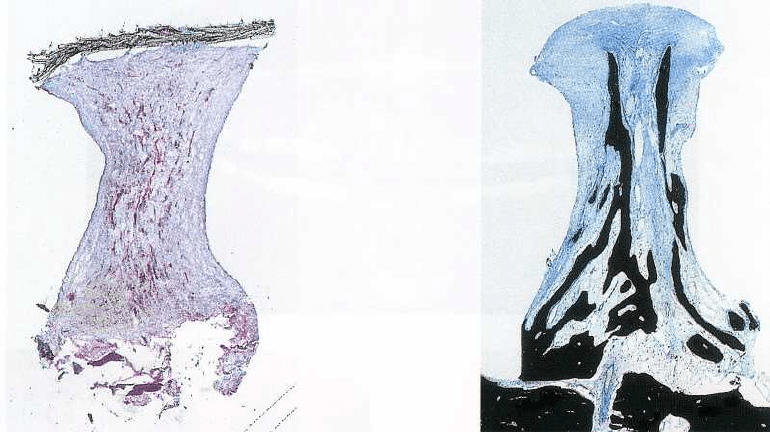
Fig. 38-31. Histologic section of a 7-week specimen,
comprising non-mineralized connective tissue in the
shape of an hourglass. Note the covering e-PTFE mem
brane.
Fig. 38-32. Histologic section of a 9-month specimen.
The height of the mineralized tissue has reached the
top 20% of the cylinder space area.
CLINICAL APPLICATIONS
As a result of the animal studies elaborated above,
several clinical applications of the principle of
"guided
tissue regeneration", in conjunction with the
treatment of oral defects prior to or concomitantly
with the placement of oral implants, have been devel-
oped to produce predictable treatment outcomes.
These include:
•
Alveolar bone defect closure
•
Enlargement or augmentation of alveolar ridges
•
Alveolar bone dehiscences or fenestrations in
asso
ciation with oral implants
•
Immediate implant placement following tooth ex-
traction.
Alveolar bone defect closure
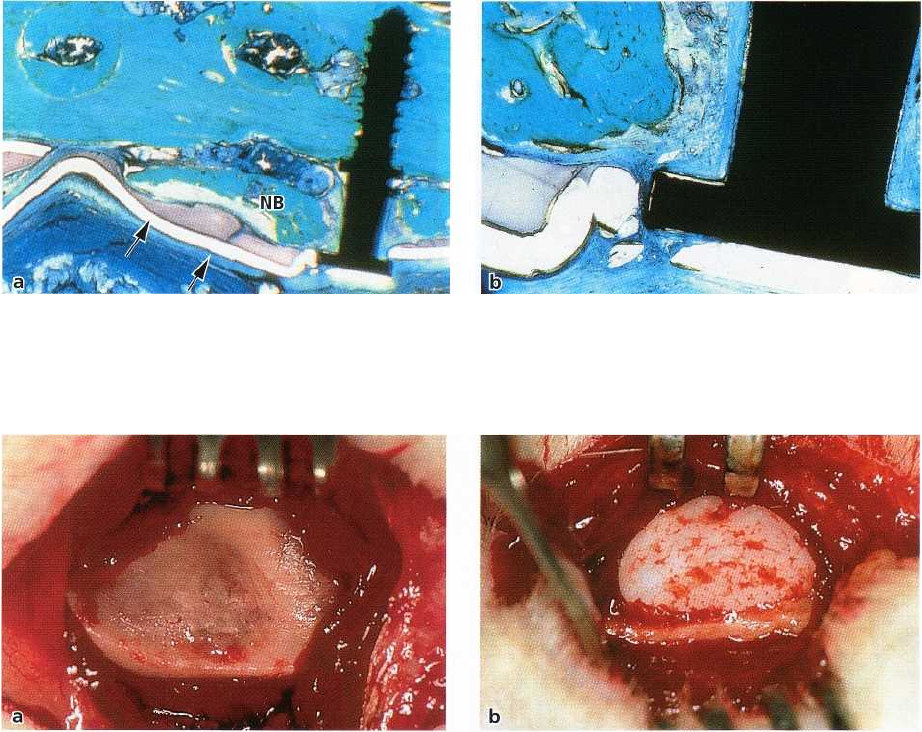
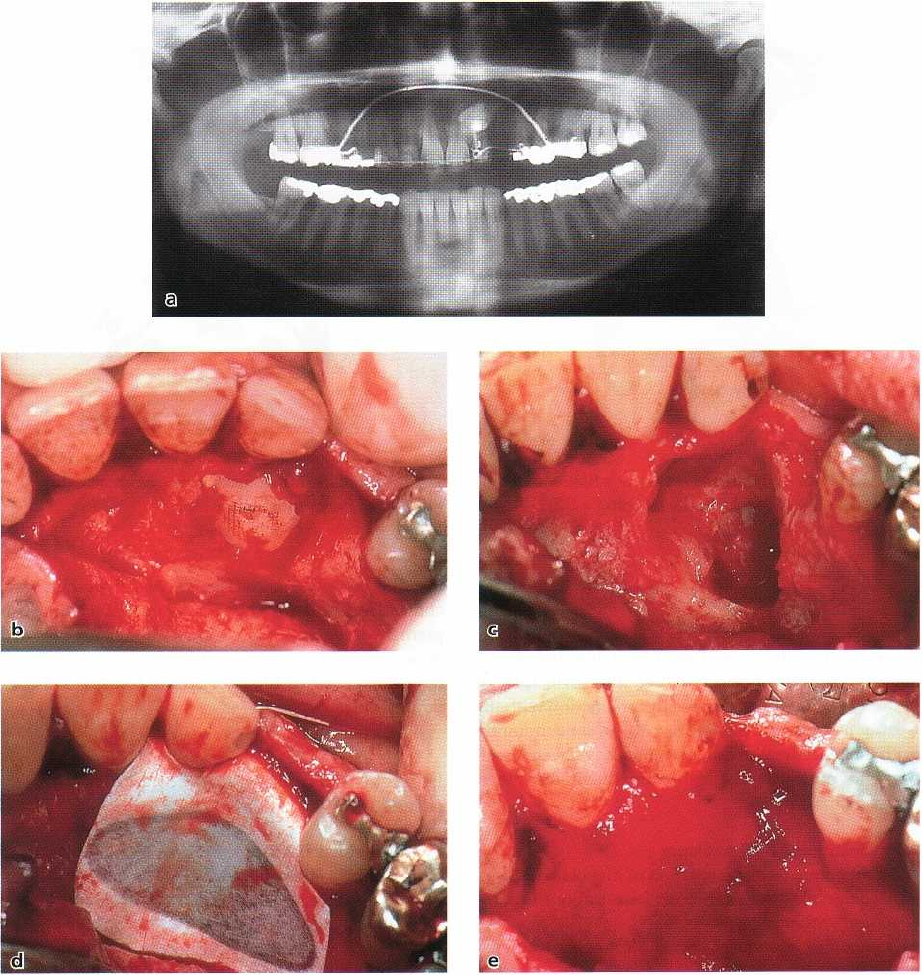
A clinical example is described in Figs. 38-33a-i. The
defect is self-limited and presents with well-defined
bony borders.
An ankylosed retained canine (Fig. 38-33a) had to
be removed (Fig. 38-33b), leaving a cavity the size of
a
cherry which extended into the edentulous ridge of
tooth 23 (Fig. 38-33c). An e-PTFE membrane was
tightly adapted to cover the palatal defect completely (
Fig. 38-33d). After 6 months, the membrane was re-
moved (Fig. 38-33e), and a bed for a one-stage trans-
mucosal implant was prepared (Fig. 38-33f). The cen-
tral bone core of the ITI hollow screw implant was
processed histologically (Fig. 38-33g). Regular intra-
membraneous bone formation with woven bone and
remodeling processes evidenced by the apposition of
lamellar bone was seen. Fig. 38-33h-i documents the
clinical and radiographic appearance of a stable im-
plant 8 years after the placement into the regenerated
bone. It should be mentioned that no bone substitutes
have been used as fillers or scaffold in this self-con-
taining defect.
Enlargement or augmentation of alveolar
ridges
In areas with a partially resorbed alveolar ridge, the
bone volume is often insufficient to contain an im-
plant. Therefore, enlargement of the alveolar ridge is
frequently necessary prior to the placement of an
implant.
As opposed to a self-contained jaw bone defect
guaranteeing the maintenance of the secluded space
into which exclusively osteogenic cells may prolifer-
ate, such a space will first have to be created for the
enlargement of atrophic jaw bone crests.
A series of case reports and clinical studies (Nyman
et al. 1990, Buser et al. 1991, 1993, 1995a,b, 1996a,b)
were initiated to develop a surgical protocol resulting
in predictable treatment outcomes of localized ridge
augmentation and, later on, to validate the technique
(
Buser et al. 1994). In all these trials, ePTFE membranes
were closely adapted to the bone surfaces and fixed
with fixation screws or pins, while the space under the
membrane was provided by means of specially de-
signed supporting screws (Memfix0, Straumann In-
stitute, Waldenburg, Switzerland) (Buser et al. 1994).
In order to prevent membrane collapse, autogenous
bone grafts were also used as support under the mem
-
branes. The clinical experience regarding optimal
treatment outcomes was presented in a methodologic
886 • CHAPTER 38
Fig. 38-33. Alveolar bone defect closure. (a) Orthopantomogram of an ankylosed retained maxillary canine. (b) Fol-
lowing flap elevation the crown of the ankylosed canine (23) is prepared free from its bony coverage. (c) A large de-
fect extending into the edentulous ridge of tooth 23 is visible. (d) The self-containing defect is covered with a mem-
brane barrier (e-PTFE). (e) At removal of the membrane after 6 months the defect has been filled with new bone. (f)
Implantation of a hollow cylinder implant (ITI) is now possible. (g) Histologic preparation of the central bone core
of
the implant bed showed woven bone undergoing remodeling. (h) Clinical appearance of the crowned implant in
position of tooth 23, 8 years after implant placement. (i) Radiographic documentation after 8 years showing im-
plant stability
report (Buser et al. 1995a,b). The essential criteria for
success were:
1.
Achievement of primary soft tissue healing to
avoid
membrane exposure by utilizing a lateral
incision
technique
2.
Creation and maintenance of a secluded space un-
der the membrane to avoid collapse of the mem-
brane by utilizing appropriate membrane support
with or without autogenous bone grafts or osteo-
conductive substitutes
3.
Stabilization and close adaptation of the membrane
to the supporting bone to prevent the ingrowth of
competing non-osteogenic cells into the defect area
by utilizing fixation screws or pins
4.
Allowance of an adequate healing period of at least
6-
7 months to obtain complete bone regeneration
and
bone maturation.
