Jan Lindhe. Clinical Periodontology
Подождите немного. Документ загружается.

REGENERATIVE PERIODONTAL THERAPY • 665
failed to confirm the regenerative potential of DFDBA
grafting (Sonis et al. 1985, Caplanis et al. 1998).
The controversial results regarding the effect of
DFDBA on the regeneration of periodontal intraos-
seous defects along with great differences in the
osteoinductive potential (ranging from high to no
osteoinductive effect) of commercially available
DFDBA (Becker et al. 1994,1995, Shigeyama et al. 1995,
Schwartz et al. 1996, Garraway et al. 1998), and the (
although minute) risk for disease transmission have
raised concern about the clinical applicability of
DFDBA. In EU countries, the commercially available
DFDBA is not granted a CE mark permitting distribu
-
tion of the material within the community.
Xenogeneic grafts
The use of xenogeneic bone grafts (xenografts) in
regenerative periodontal surgery was examined sev-
eral years ago. Nielsen et al. (1981) treated 46 in-
trabony defects with Kielbone
®
(i.e. defatted and de-
proteinized ox bone) and another 46 defects with in-
traoral autogenous bone grafts. The results, which
were evaluated by periodontal probing and radiog-
raphically, showed no difference between the amount
of clinical gain of attachment and bone fill obtained in
the two categories of defect. A study in monkeys also
demonstrated that the two types of bone graft dis-
played similar histologic features and were frequently
seen in the connective tissue of the healed defects as
isolated bone particles surrounded by a cementum-
like substance (Nielsen et al. 1980).
Recently, new processing and purification methods
have been utilized which make it possible to remove
all organic components from a bovine bone source and
leave a non-organic bone matrix in an unchanged
inorganic form (e.g. Bio-Oss
"
, Geistlich AG, Wol-
husen, Switzerland; Endobone ", Merck Biomateri-
alen, Darmstadt, Germany; Laddec
®
, Ost Develop-
ment, Clermont-Ferrand, France; Bon-Apatite
®
, Bio-
Interfaces Inc., San Diego, US). However, differences
in the purification and manipulation methods of the
bovine bone exist, leading to commercially available
products with different chemical properties and pos-
sibly different biological behavior. These materials are
available in different particle sizes or as block grafts.
To date, no controlled human study has compared
the effect of such graft materials in periodontal defects
with flap surgery alone, but a recent clinical study
demonstrated that implantation of Bio-Oss
®
resulted
in pocket reduction, gain of attachment and bone fill
in periodontal defects to the same extent as that of
DFDBA (Richardson et al. 1999). Human histology
(
Camelo et al. 1998) and animal experiments (Cler-
geau et al. 1996) have also suggested a beneficial effect
of placing bovine bone-derived biomaterials in peri-
odontal bone defects.
The use of coral skeleton as a bone graft substitute
was proposed some decades ago (Holmes 1979,
Guillemin et al. 1987). Depending on the pre-treat-
ment procedure, the natural coral turns into non-re-
sorbable porous hydroxyapatite (e.g. Interpore 200,
Interpore International, Irvine, US) or to a resorbable
calcium carbonate (e.g. Biocoral, Inoteb, St Gonnery,
France) skeleton (Nasr et al. 1999). Implantation of
coralline porous hydroxyapatite in intrabony peri-
odontal defects in humans produced more probing
pocket depth reduction, clinical attachment gain and
defect fill than non-grafting (Kenney et al. 1985, Krejci
et al. 1987, Yukna 1994, Mora & Ouhayoun 1995,
Yukna & Yukna 1998), and similar results were found
when compared with grafting of FDBA (Barnett et al.
1989). When porous hydroxyapatite was compared
with DFDBA for the treatment of intraosseous defects,
similar results were also obtained (Bowen et al. 1989),
but another study reported clinical results in favor of
this material (Oreamuno et al. 1990). However, both
animal (West & Brustein 1985, Ettel et al. 1989) and
human studies (Carranza et al. 1987, Stahl & Froum
1987) have provided only vague histologic evidence
that grafting of natural coral may enhance the forma-
tion of true new attachment. In most cases, the graft
particles were embedded in connective tissue with
minimal bone formation.
Alloplastic materials
Alloplastic materials are synthetic, inorganic, biocom-
patible and/or bioactive bone graft substitutes which
are claimed to promote bone healing through osteo-
conduction. There are four kinds of alloplastic mate-
rials, which are frequently used in regenerative peri-
odontal surgery: hydroxyapatite (HA), beta tricalcium
phosphate (13-TCP), polymers, and bio-active glasses
(
bio-glasses).
Hydroxyapatite (HA)
The HA products used in periodontology are of two
forms: a particulate non-resorbable ceramic form (e.g.
Periograf
®
, Miter Inc., Warsaw, IN, US; Calcitite
"
, Cal-
citek Inc., San Diego, US) and a particulate, resorbable
non-ceramic form (e.g. OsteoGraf/LD
®
, CeraMed
Dental, Lakewood, CO, US). In controlled clinical
studies, grafting of intrabony periodontal lesions with
HA resulted in a PAL-gain of 1.1-3.3 mm and also in a
greater bone defect fill as compared with non-grafted
surgically debrided controls (Meffert et al. 1985,
Yukna et al. 1985, 1986, 1989, Galgut et al. 1992). In
these studies, improvement of clinical parameters (i.e.
PPD reduction and PAL gain) was more evident in the
grafted sites than in the sites treated only with de-
bridement, especially for initially deep defects. How-
ever, animal studies (Barney et al. 1986, Minabe et al.
1988, Wilson & Low 1992), and human histologic data
(Froum et al. 1982, Moskow & Lubarr 1983, Ganeles et
al. 1986, Sapkos 1986) showed that bone formation
was limited and that a true new attachment was not
formed consistently after grafting of intrabony peri-
odontal defects with HA. The majority of the HA
particles were embedded in connective tissue and new
bone was only observed occasionally around particles
666 • CHAPTER
28
in close proximity to host bone. A junctional epithe-
lium was lining the major part of the roots.
Beta-tricalcium phosphate ((3-TCP)
b-TCP (Ca
3
(PO
4
)
2
) (e.g. Synthograft
®
, Johnson and
Johnson, New Brunswick, NJ, US) has been used in a
series of case reports for the treatment of periodontal
osseous lesions (Nery & Lynch 1978, Strub et al. 1979,
Snyder et al. 1984, Baldock et al. 1985). After variable
time intervals, a significant gain of bone was observed
by means of re-entry or radiographs. However, there
is
no controlled study comparing the result of 13-TCP
grafting with that of open flap debridement, and his-
tologic data from animal (Levin et al. 1974, Barney et
al. 1986) and human studies (Dragoo & Kaldahl 1983,
Baldock et al. 1985, Bowers et al. 1986, Stahl & Froum
1986, Froum & Stahl 1987, Saffar et al. 1990) showed
that 13-TCP is rapidly resorbed or encapsulated by
connective tissue, with minimal bone formation and
no periodontal regeneration.
Polymers
There are two polymer materials that have been used
as bone graft substitutes in the treatment of periodon
-
tal defects: a non-resorbable, calcium hydroxide coat-
ed co-polymer of poly-methyl-methacrylate (PMMA)
and poly-hydroxylethyl-methacrylate (PHEMA)
which
is often referred to as HTR (hard tissue replace
ment) (
e.g. HTR
TM
, Bioplant Inc., New York, NY, US),
and a
resorbable polylactic acid (PLA) polymer
(Driloc ®,
Osmed Corp., Costa Mesa, CA, US).
In controlled clinical studies, implantation of HTR
polymer grafts in intrabony defects resulted in a defect
fill of approximately 2 mm, representing about 60% of
the initial defect depth, but the improved clinical re-
sponse with grafting was not significantly better than
that following solely flap operation (Yukna 1990,
Shahmiri et al. 1992). Human histologic data from an
experimental study (Plotzke et al. 1993), and from two
case reports (Stahl et al. 1990b, Froum 1996) also re-
vealed that grafting of osseous periodontal defects
with HTR does not promote periodontal regeneration.
The HTR particles were most frequently encapsulated
by connective tissue with only scarce evidence of bone
formation. Healing resulted in a long junctional epi-
thelium along the root surface, and true new attach-
ment formation was not observed.
When PLA particles were implanted into intrabony
defects in humans and compared with DFDBA or
surgically debrided controls, it was found that the
healing results were less favorable than after flap
operation alone, both in terms of clinical parameters
(
PPD and PAL gain), and in terms of bone fill (Mead-
ows et al. 1993).
Bioactive glasses (Bio-glasses)
Bio-glasses are composed of SiO
2
, Na
2
O, P
2
O
5
and are
resorbable or not resorbable depending on the relative
proportion of these components. When bio-glasses are
exposed to tissue fluids, a double layer of silica gel and
calcium phosphate is formed on their surface.
Through this layer the material promotes absorption
and concentration of proteins used by osteoblasts to
form extracellular bone matrix which theoretically
may promote bone formation (Hench et al. 1972).
Commercially available bio-glasses in particulate
form, and theoretically resorbable, have been pro-
posed for periodontal treatment (e.g. PerioGlass'j, US
Biomaterials Corp., Alachua, FL, US; BioGran
®
, Or-
thovita, Malvern, PA, US).
A human case report demonstrated that implanta
-
tion of bio-glass in periodontal osseous defects re-
sulted in a gain of clinical attachment of 2.0-5.3 mm
and a radiographic bone fill of 3.5 mm, and in a
controlled study, the treatment with bio-glass in in-
trabony defects also resulted in greater clinical im-
provements than surgical debridement alone (Froum
et al. 1998). However, other controlled studies (Zamet
et al. 1997) and split-mouth studies on grafting of
intrabony defects with bio-glass (Ong et al. 1998)
failed to demonstrate statistically significant better
clinical results than surgery alone or DFDBA grafting
(
Lovelace et al. 1998). Although experimental studies
in monkeys have suggested that bio-glass grafting of
periodontal intrabony defects (Karatzas et al. 1999)
may favor new cementum formation and inhibit epi-
thelial down-growth, there is no histological evidence
in humans that bio-glass may promote true periodon
-
tal regeneration. In a histologic evaluation of bio-glass
implanted in intrabony defects in humans it was ob-
served that although clinically satisfactory results
were produced, healing had most frequently occurred
with a junctional epithelium along the previously dis
-
eased part of the root, and new cementum with insert
ing collagen fibers was found in only one out of five
treated teeth. Bone formation was limited in all speci
-
mens (Nevins et al. 2000).
Evaluation of alloplastic materials
There are no controlled clinical studies demonstrating
that grafting with tricalcium phosphate or polymers
results in significant clinical improvements beyond
that of flap surgery, whereas several reports have
indicated that grafting with hydroxyapatite or bio-ac-
tive glasses may produce more gain of attachment
than open flap debridement (Galgut et al. 1992, Zamet
et al. 1997, Froum et al. 1998) or a gain similar to that
obtained following grafting with DFDBA (Lovelace et
al. 1998). Histologic evidence that the use of alloplastic
or synthetic graft materials may lead to periodontal
regeneration in humans is lacking, and animal experi
ments have failed to demonstrate regeneration of a
functional periodontium following implantation of
hydroxyapatite, tricalcium phosphate or polymers in
periodontal lesions (Barney et al. 1986, Shahmiri et al.
1992). It was reported, however, that treatment with
bio-active glasses in experimental animals produced
significantly more bone fill and new attachment com
-
pared with that in non-grafted controls (Fetner et al.
1994, Karatzas et al. 1999) or in sites grafted with
REGENERATIVE PERIODONTAL THERAPY •
667
hydroxyapatite or tricalcium phosphate (Wilson &
Low 1992). Although some bone formation has been
reported following the use of alloplastic materials,
there is no evidence that these materials may stimulate
the formation of new cementum with inserting colla-
gen fibers. At the 1996 American Academy of Perio-
dontology World Workshop, it was concluded that
synthetic graft materials function primarily as defect
fillers. If regeneration is the desired treatment out-
come, other materials are recommended.
Biologic concept of using bone grafts or alloplastic
materials
The biologic rationale behind the use of bone grafts or
alloplastic materials in periodontal regenerative sur-
gery is the assumption that these materials may either
(1) contain bone forming cells (osteogenesis), (2) serve
as a scaffold for bone formation (osteoconduction), or
that (3) the matrix of the grafting material contains
bone inductive substances (osteoinduction). It is be-
lieved that the use of such materials may stimulate not
only the regrowth of alveolar bone but also the forma-
tion of new attachment. However, such complete re-
generation of the periodontal attachment apparatus
following such grafting procedures would imply that
cells derived from bone would possess the ability to
form new cementum with inserting collagen fibers on
a previously periodontitis-affected root surface. This
assumption is in conflict with current knowledge
about the biology of periodontal wound healing, that
repopulation of the detached root surface with cells
from the periodontal ligament is the prerequisite for
new attachment formation. This means that all thera
-
peutic procedures involving the placement of bone
grafts or bone substitute implants are based on a
biologic concept which cannot explain how such treat
-
ment should result in regeneration of the periodon-
tium.
Root surface biomodification
Much research has been directed to altering the peri-
odontitis-involved root surface in a manner that will
promote the formation of a new connective tissue
attachment. Removal of bacterial deposits, calculus
and endotoxins in the cementum is generally consid-
ered essential for the formation of a new connective
attachment (Garrett 1977). However, it was suggested
by Stahl et al. (1972) that demineralization of the root
surface, exposing the collagen of the dentin, would
facilitate the deposition of cementum by inducing
mesenchymal cells in the adjacent tissue to differenti
-
ate into cementoblasts. The biologic concept is that
exposure of collagen fibers of the dentin matrix may
facilitate adhesion of the blood clot to the root surface
and thereby favor migration of the fibroblasts.
Several studies using various animal models dem-
onstrated an improved healing response histologi-
cally following citric acid and tetracycline root surface
demineralization (Register & Burdick 1976, Crigger et
al. 1978, Polson & Proye 1982, Claffey et al. 1987).
However, in a study in dogs where naturally occur-
ring furcations were treated with citric acid, several
specimens demonstrated ankylosis and root resorp-
tion (Bogle et al. 1981). This finding corroborates that
of Magnusson et al. (1985a) in monkeys where citric
acid conditioning was evaluated in combination with
coronally displaced tissue flaps after 6 months. These
investigators found root resorption on 28 out of 40
surfaces examined and 21 of these also presented
ankylosis.
New connective tissue attachment following citric
acid demineralization of root surfaces has been dem-
onstrated histologically in humans (Cole et al. 1980,
Frank et al. 1983, Stahl et al. 1983, Stahl & Froum
1991a). Cole et al. (1980) showed histologic evidence
of a new connective tissue attachment and bone for-
mation coronal to reference notches placed in the api
-
cal extent of calculus, identified on the root surface at
the time of surgery. However, despite histologic evi-
dence of regeneration following root surface biomodi-
fication with citric acid, results of controlled clinical
trials failed to show any improvements in clinical
conditions compared to non-acid treated controls
(
Moore et al. 1987, Fuentes et al. 1993).
In recent years, biomodification of the root surface
with enamel matrix proteins (Emdogain
®
) during sur-
gery and following demineralization with EDTA has
been introduced to encourage periodontal regenera-
tion. The biologic concept is that the application of
enamel matrix (amelogenins) proteins may promote
periodontal regeneration because it mimics events
that took place during the development of the peri-
odontal tissues (Hammarstrom 1997, Gestrelius et al.
2000). This view is based on the finding that the cells
of the Hertwigs epithelial root sheath deposit enamel
matrix proteins on the root surface prior to cementum
formation and that such proteins are the initiating
factor for the formation of cementum. The commer-
cially available product, Emdogain ", a purified acid
extract of porcine origin contains enamel matrix de-
rivatives (EMD), supposed to be able to promote peri-
odontal regeneration. In case series reports, 4-4.5 mm
gain of clinical attachment, and about 70% bone fill in
intrabony defects were reported following treatment
with EMD (Heden et al. 1999, Heden 2000).
In a multicenter clinical study involving 33 subjects
with 34 paired intrabony defects, application of EMD
resulted in larger amounts of PAL-gain (2.2 mm) and
statistically significantly more bone gain (2.6 mm)
than open flap debridement after 36 months, evalu-
ated clinically and radiographically (Heijl et al. 1997).
Similar results were reported in another split-mouth
clinical trial (23 patients) published more recently (
Froum et al. 2001). In that study a PPD reduction of
4.9 mm, a PAL gain of 4.3, and a bone gain of 3.8 mm
(evaluated by re-entry surgery) were observed after
EMD application in 53 intrabony defects. These values
were statistically significantly larger than those ob-
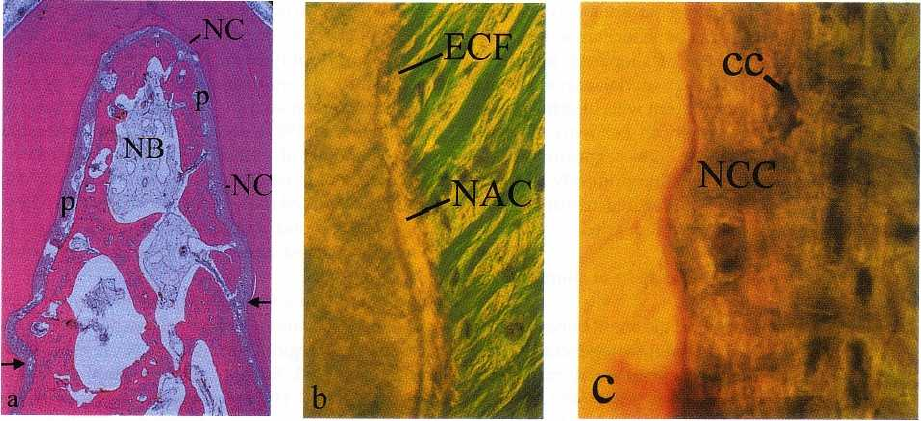
668 • CHAPTER 28
Fig. 28-20. Photomicrograph (a) of a degree III furcation defect in a dog following root surface biomodification with
enamel matrix proteins and subsequently covered with a resorbable membrane. The defect has healed completely
with bone (NB), a periodontal ligament (p) and new cementum (NC). The arrows indicate the apical extension of
the lesion. (b) The cementum (NAC) formed on the root surface in the apical portion of the defect was acellular
with inserting extrinsic collagen fibers (ECF) while (c) that (NCC) formed in the coronal portion had a cellular (cc)
character.
tained by flap surgery (2.2 mm, 2.7 mm and 1.5 mm,
respectively, in 31 defects).
In a more recent prospective multicenter random-
ized controlled clinical trial, the clinical outcomes of
papilla preservation flap surgery (simplified papilla
preservation flap, SPPF) with or without the applica-
tion of enamel matrix proteins, were compared (Ton-
etti et al. 2002). A total of 83 test and 83 control patients
with similar baseline periodontal conditions and de-
fect characteristics were treated with either SPPF and
Emdogain" or with SPPF alone. The test defects exhib
-
ited significantly more CAL gain than the controls (3.1
± 1.5 mm and 2.5 ± 1.5 mm, respectively). In addition,
this study demonstrated that the treatment outcomes
of the test group were significantly affected by the
smoking status of the patient and the number of walls
of the intrabony defect. Smokers gained less attach-
ment than non-smokers. Defects with very dense cor-
tical or very porous and bleeding walls gained less
CAL than normal corticalized defects, and intrabony
defects with three walls had a higher predictability in
CAL gains than those with one-wall.
Similar results were also reported in other control-
led studies (Pontoriero et al. 1999, Silvestri et al. 2000).
When application of EMD was compared with GTR
treatment, it was found that similar clinical improve-
ments were obtained. In a randomized controlled
clinical study, Pontoriero et al. (1999) compared EMD
application with GTR with resorbable (two kinds:
Guidor and Resolut) and non-resorbable (e-PTFE)
membranes in intrabony defects (10 patients per
group). After 12 months, there were no significant
differences among the groups, and EMD application
resulted in a PPD reduction of 4.4 mm and a PAL gain
of 2.9 mm, while the corresponding values from the
membrane-treated sites (all GTR groups combined)
were 4.5 mm and 3.1 mm, respectively (Pontorierio et
al. 1999). Silvestri et al. (2000) reported a PPD reduc
-
tion of 4.8 mm and a PAL gain of 4.5 mm after EMD
application in intrabony defects versus 5.9 mm and 4.8
mm, respectively, after GTR with non-resorbable
membranes (randomized controlled study, 10 patients
per group). Similar results were reported by Sculean
et al. (1999a,b).
Histologic evidence of new cementum formation
with inserting collagen fibers on a previously perio-
dontitis-affected root surface and the formation of
new alveolar bone in human specimens have been
demonstrated following EMD treatment (Mellonig
1999, Sculean et al. 1999b). However, while in the
study of Mellonig (1999) healing had occurred with
acellular cementum on the root surface, the newly
formed cementum in the study of Sculean et al. (1999b)
displayed a predominantly cellular character. The
ability of EMD to produce regeneration has been con
-
firmed in controlled animal experiments (Fig. 28-20),
following the treatment of intrabony, furcation and
dehiscence defects (Hammarstrom et al. 1997, Araujo
& Lindhe 1998, Sculean et al. 2000).
Further research is needed to elucidate the function
of EMD and the predictability of this regenerative
periodontal procedure.
Growth regulatory factors for periodontal
regeneration
Growth factor is a general term to denote a class of
polypeptide hormones that stimulate a wide variety
of cellular events such as proliferation, chemotaxis,
REGENERATIVE PERIODONTAL THERAPY •
669
differentiation and production of extracellular matrix
proteins (Terranova & Wikesjo 1987). Proliferation
and migration of periodontal ligament cells and syn-
thesis of extracellular matrix as well as differentiation
of cementoblasts and osteoblasts is a prerequisite for
obtaining periodontal regeneration. Therefore, it is
conceivable that growth factors may represent a po-
tential aid in attempts to regenerate the periodontium.
The effects of various growth factors were studied in
vitro, and a significant regeneration potential of
growth factors was also demonstrated in animal mod
-
els. Lynch et al. (1989, 1991) examined the effect of
placing a combination of platelet derived growth fac-
tors (PDGF) and insulin-like growth factors (IGF) in
naturally occurring periodontal defects in dogs. The
control sites treated without growth factors healed
with a long junctional epithelium and no new cemen-
tum or bone formation, while regeneration of a peri-
odontal attachment apparatus occurred at the sites
treated with growth factors. Similar results were re-
ported by other investigators following application of a
combination of PDGF and IGF in experimentally-in-
duced periodontal lesions in monkeys (Rutherford et
al. 1992, Giannobile et al. 1994, 1996). One study ex
-
amined the effect of PDGF and IGF in periodontal
intrabony defects and degree II furcations in humans
(
Howell et al. 1997). At re-entry after 9 months, signifi
-
cantly increased bone fill was only observed at the
furcation sites treated with growth factors. It can be
concluded that growth factors seem to have a positive
effect on periodontal regeneration, but several impor-
tant questions need to be resolved before this type of
regenerative treatment can be used in humans (Graves
& Cochran 1994).
Bone morphogenetic proteins (BMPs) are osteoin-
ductive factors that may have the potential to stimu-
late mesenchymal cells to differentiate into bone-
forming cells (Wozney et al. 1988). Sigurdsson et al.
(
1995) evaluated bone and cementum formation fol-
lowing regenerative periodontal surgery using re-
combinant human BMP in surgically-created supra-
alveolar defects in dogs. Following application of
BMP the flaps were advanced to submerge the teeth
and sutured. Histologic analysis showed significantly
more cementum formation and regrowth of alveolar
bone on BMP treated sites as compared to the controls.
Ripamonti et al. (1994) also reported about the efficacy
of bovine BMPs to induce periodontal regeneration in
degree II furcations in baboons. On one side BMP was
implanted with a collagenous matrix, while at the
control sites only the collagen matrix was used. Con-
siderable regeneration of cementum, periodontal liga-
ment and bone was observed in the BMP treated
furcations as compared to the control furcations
treated without BMP. Further experimentation is
needed to evaluate a possible role of BMP in periodon
-
tal regeneration.
GUIDED TISSUE REGENERATION
(GTR)
The experimental studies (Karring et al. 1980, Nyman
et al. 1980, Buser et al. 1990a,b, Warrer et al. 1993)
described previously have documented that the pro-
genitor cells for the formation of a new connective
tissue attachment are residing in the periodontal liga
-
ment. Consequently, it should be expected that a new
connective tissue attachment would be predictably
achieved if such cells populate the root surface during
healing. This view was confirmed in a study in mon-
keys in which both gingival connective tissue and
gingival epithelium were prevented from contacting
the root surface during healing by the use of a barrier
membrane (Gottlow et al. 1984). After reduction of the
supporting tissues around selected experimental
teeth, the root surfaces were exposed to plaque accu-
mulation for 6 months. Soft tissue flaps were then
raised and the exposed root surfaces were curetted.
The crowns of the teeth were resected and the roots
were submerged. However, prior to complete closure
of the wound, a membrane was placed over the curet
-
ted root surfaces on one side of the jaws in order (1) to
prevent gingival connective tissue contacting the root
surface during healing, and (2) to provide a space for
ingrowth of periodontal ligament tissue. No mem-
branes were placed over the contralateral roots. The
histologic analysis after 3 months of healing demon-
strated that the roots covered with membranes exhib
-
ited considerably more new attachment than the non
-
covered roots (Fig. 28-21). In four of the nine test roots,
new cementum covered the entire length of the root.
In all control specimens the surface coronal to the
newly formed cementum presented multinucleated
cells and resorption cavities. In one control specimen
virtually half the root was resorbed. Coronal regrowth
of alveolar bone had occurred to a varying extent in
test and control roots, and no relationship was found
between the amount of new cementum formation and
the degree of bone regrowth. The results of this study
strongly suggested that the exclusion of epithelial and
gingival connective tissue cells from the healing area
by the use of a physical barrier may allow (guide)
periodontal ligament cells to repopulate the detached
root surface. This observation provided the basis for
the clinical application of the treatment principle
termed "guided tissue regeneration" (GTR).
Clinical application of GTR
Clinical application of guided tissue regeneration
(
GTR) in periodontal therapy involves the placement
of
a physical barrier to ensure that the previous peri-
odontitis-affected root surface becomes repopulated
with cells from the periodontal ligament (Fig. 28-22).
Treatment of the first human tooth with GTR was
reported by Nyman et al. in 1982. Due to extensive
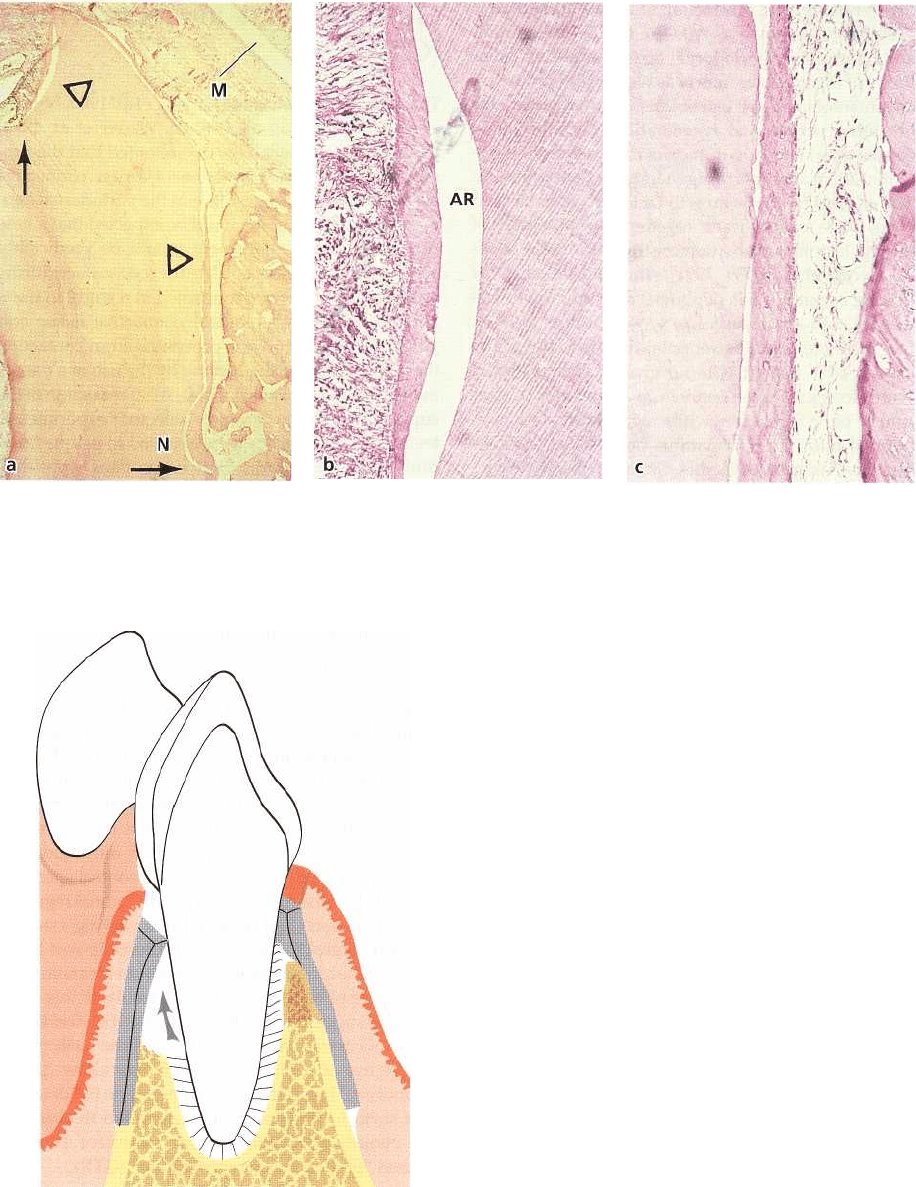
670 • CHAPTER 28
Fig. 28-21. (a) Microphotograph of membrane (M) covered root. Newly formed cementum is visible on the entire
length of the buccal root surface coronal to the notch (N) and also on part of the coronal cut surface (arrow). (b, c)
Higher magnifications of the areas at the upper and lower triangles in (a), showing that collagen fibers are inserted
into the newly formed cementum. AR: artifact.
Fig. 28-22. Drawing illustrating the placement of the
physical barrier which prevents the epithelium and gin
-
gival connective tissue from contacting the root surface
during healing. At the same time the membrane allows
cells from the periodontal ligament (arrow) to repopu-
late the previously periodontitis-involved root surface.
periodontal destruction, the tooth was scheduled for
extraction. This offered the possibility of obtaining
histologic documentation of the result of the treat
ment.
Following elevation of full thickness flaps, scal
ing of
the root surface and removal of all granulation
tissue, an
11 mm deep periodontal lesion was ascertained. Prior
to flap closure, a membrane was adjusted to cover parts
of the detached root surfaces, the osse
ous defect and
parts of the surrounding bone. His-
tologic analysis after 3 months of healing revealed that
new cementum with inserting collagen fibers had
formed on the previously exposed root surface (Fig.
28-
23). In a later study (Gottlow et al. 1986), 12 cases
treated with GTR were evaluated clinically, and in five
of
these cases histologic documentation was also pre-
sented. The results showed that considerable but
varying amounts of new connective tissue attachment
had formed on the treated teeth. Frequently, however,
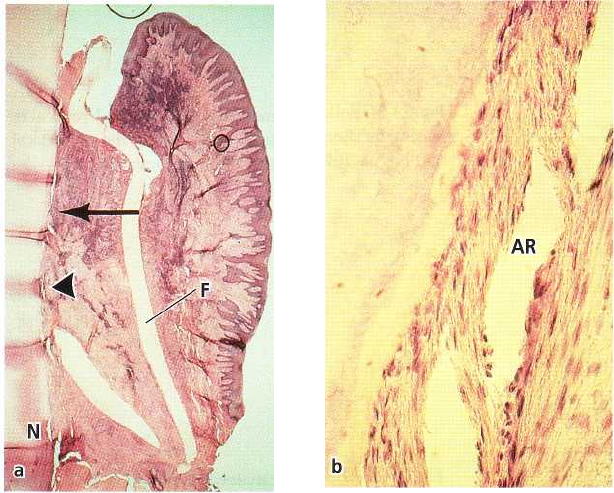
REGENERATIVE PERIODONTAL THERAPY • 671
Fig. 28-23. Microphotograph of a
human tooth (a) 3 months follow-
ing GTR treatment using a Mil-
lipore filter (F). New cementum
with inserting collagen fibers
(
about 5 mm) has formed from the
notch (N) to the level of the arrow.
Bone formation underneath the fil
ter is lacking, probably due to the
inflammatory infiltrate seen in the
tissues adjacent to the filter. (b)
Higher magnification of the area
indicated by the arrowhead in (a)
showing newly formed cementum
with inserting collagen fibers. AR:
artifact.
bone formation was incomplete. The varying results
were ascribed to factors such as the amount of remain
ing periodontal ligament, the morphology of the
treated defect, technical difficulties regarding mem-
brane placement, gingival recession and bacterial con
-
tamination of the membrane and the wound during
healing.
In the last decades, GTR has been applied in a
number of clinical trials for the treatment of various
periodontal defects such as intrabony defects (for re-
view see Cortellini & Bowers 1995), furcation involve-
ments (for review see Machtei & Schallhorn 1995,
Karring & Cortellini 1999), and localized gingival re-
cession defects (Pini-Prato et al. 1996).
The clinical outcomes of GTR are most frequently
evaluated by changes in clinical attachment levels
(
CAL), bone levels (BL), probing pocket depths (PPD),
and the position of the gingival margin. In some of the
studies on degree II and III furcations, horizontal
changes in clinical attachment, bone level and pocket
depth were also measured. As previously pointed out,
evidence of true regeneration of periodontal attach-
ment can only be provided by histologic means. How-
ever, based on reports about the formation of a new
attachment apparatus in histologic specimens from
human biopsies harvested following GTR treatment (
Nyman et al. 1982, Gottlow et al. 1986, Becker et al.
1987, Stahl et al. 1990a, Cortellini et al. 1993a) and
based on the biologic concept of GTR (Karring et al.
1980, 1985, 1993, Nyman et al. 1980, Gottlow et al.
1984), it was suggested that clinical signs of probing
attachment gain and bone fill can be accepted as evi-
dence of periodontal regeneration in the evaluation of
GTR procedures (Lindhe & Echeverria 1994).
Procedural
guidelines
GTR is not a procedure for the treatment of periodon-
titis, but rather a technique for regenerating defects
which have developed as a result of periodontitis.
Therefore, appropriate periodontal treatment should
always be completed before GTR is initiated.
Surgery is initiated by sulcular or marginal inci-
sions at both the buccal and lingual aspect of the jaw,
followed by buccal and vertical releasing incisions.
The releasing incisions must be placed a minimum of
one tooth anterior and/or posterior to the tooth that
is being treated (Fig. 28-24). Care should be taken
during this procedure to preserve the interdental pa-
pillae. All pocket epithelium is excised so that a fresh
connective tissue is left on the full thickness flaps
following reflection. After elevation of the tissue flaps,
all granulation tissue is removed and thorough de-
bridement of the detached root surfaces is carried out
using curettes, burs, etc.
Various types of bioabsorbable and non-bioabsor-
bable barrier materials are available in a variety of
configurations designed for specific applications. The
configuration most suitable for covering the defect is
selected and additional tailoring of the material is
performed. The shaping of the material is carried out
in such a way that it is adapting closely to the tooth
and is completely covering the defect, extending at
least 3 mm on the bone beyond the defect margins
after placement (Fig. 28-25). This assures a good sta-
bility of the material and protects the underlying
blood clot during healing. At placement it is essential
to ensure good adaptation of the barrier material to
the alveolar bone surrounding the defect and to avoid
overlaps or folds of the material.
Although exceptions exist, the barrier materials
available are fixed to the tooth with a suture using a
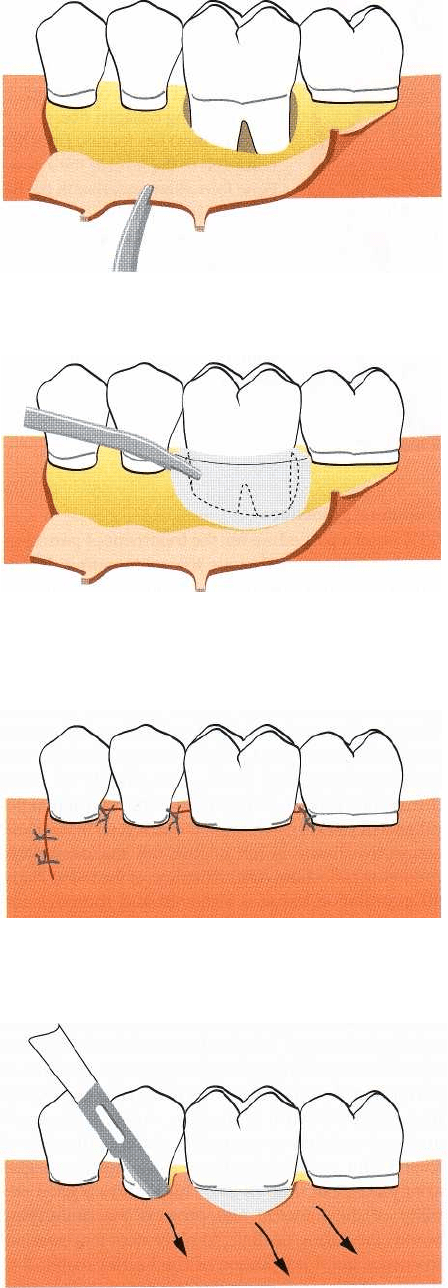
672 • CHAPTER 28
Fig. 28-24. Following marginal incisions and vertical re-
leasing incisions on the buccal aspect of the jaw, buccal
and lingual full thickness flaps are elevated.
Fig. 28-25. The barrier material is placed in such a way
that it completely covers the defect and extends at least
3
mm on the bone beyond the defect margin.
Fig. 28-26. The elevated tissue flaps are coronally dis-
placed and sutured in such
a way
that the border of
the barrier material is at least 2 mm below the flap mar-
gin.
Fig. 28-27. In order to remove the barrier material an in
-
cision is made extending one tooth mesially and dis
tally
to the border of the barrier. After reflecting the
covering
tissue flaps, the barrier can be removed with-out
compromising the newly regenerated tissue.
REGENERATIVE PERIODONTAL THERAPY •
673
sling technique. For optimal performance, the barrier
should be placed with its margin 2-3 mm apically to
the flap margin. To maximize coverage of the barrier,
a horizontal releasing incision in the periosteum may
assist in the coronal displacement of the flap at the
suturing of the wound. However, care should be taken
not to compromise the blood supply to the flap. The
interproximal space near the barrier should be closed
first. In order to achieve good closure, a vertical mat-
tress suturing technique is advocated (Fig. 28-26).
To reduce the risk of infection and to assure optimal
healing, the patient should be instructed to gently
brush the area postoperatively with a soft bristle
toothbrush and to rinse with chlorhexidine (0.2%) for
a
period of 4-6 weeks. In addition, systemic antibiotics
are frequently administered immediately prior to sur-
gery and during 1-2 weeks after surgery. When a
non-bioabsorbable barrier is used, it should be re-
moved after 4-6 weeks. However, if complications
develop it may be necessary to remove it earlier.
Removal of the material requires a minor surgical
procedure (Fig. 28-27). To obtain access to the barrier
material, a small incision is made extending one tooth
mesially and distally of the border of the barrier. The
soft tissue flap is gently reflected and the barrier ma-
terial dissected free from the flap using a sharp blade.
During this procedure it is essential not to compro-
mise the newly regenerated tissue. At removal of the
barrier material there will usually be some pocket
formation on the outer surface of the material. It is
important that this epithelium is removed so that fresh
connective tissue is in contact with the newly regen-
erated tissue after wound closure. It is essential that
the newly regenerated tissue is completely covered
after suturing. The patient is instructed to rinse with
chlorhexidine for 2-3 weeks during which period fre-
quent visits for professional tooth cleaning are recom
-
mended. After this period, brushing and interproxi-
mal cleaning can be resumed, chlorhexidine rinsing
discontinued, and the patient enrolled in a regular
periodontal maintenance program.
If the flap is excessively traumatized during sur-
gery either part or all of it may slough during healing.
Perforations may also occur, particularly in sites with
sharp bony ledges. A minor osteoplasty during place-
ment may help to allow the barrier to better follow the
contours of the ridge. Abscess formation may also
occur in the wound, probably due to severe bacterial
contamination of the barrier. Dependent on the sever
-
ity of such complications, early removal of the barrier
may be indicated.
Barrier materials for
regenerative surgery
h1
the first GTR attempts, a bacterial filter produced
from cellulose acetate (Millipore
®
) was used as an
occlusive membrane (Nyman et al. 1982, Gottlow et
al. 1984, Magnusson et al. 1985h). Although this type
of membrane served its purpose, it was not ideal for
clinical application. Later studies have utilized mem-
branes of expanded polytetrafluoroethylene (e-PTFE)
especially designed for periodontal regeneration
(Gore
Tex Periodontal Material
®
). The basic molecule
of this
material consists of a carbon-carbon bond with
four
attached fluorine atoms to form a polymer. It is
inert
and does not result in any tissue reaction when
implanted in the body. This type of membrane persists
after healing and must be removed in a second opera
-
tion. Membranes of e-PTFE have been used success-
fully in animal experiments and in several clinical
studies. It was experienced from such studies that in
order for a barrier material to function optimally, it has
to meet certain essential design criteria:
1.
To allow for good tissue acceptance it is important
that the material is biocompatible. The material
should not elicit an immune response, sensitization
or chronic inflammation which may interfere with
healing and present a hazard to the patient. Bio-
compatibility however, is a relative term since prac
-
tically no materials are completely inert.
2.
The material should act as a barrier to exclude
undesirable cell types from entering the secluded
space adjacent to the root surface. It is also consid
-
ered an advantage that the material would allow
the passage of nutrients and gases.
3.
Tissue integration is another important property of
a barrier material. Thus, tissue may grow into the
material without penetrating all the way through.
The goal of tissue integration is to prevent rapid
epithelial downgrowth on the outer surface of the
material or encapsulation of the material, and to
provide stability to the overlying flap. The impor-
tance of tissue integration was demonstrated in a
study in monkeys (Warrer et al. 1992) in which
bioabsorbable membranes of polylactic acid, a syn-
thetic polymer, were used for treatment of circum-
ferential periodontal defects. Due to the lack of
tissue integration, the membranes in this study
became surrounded by an epithelial layer and were
often encapsulated and exfoliated.
4.
It is also essential that the barrier material is
capable
of creating and maintaining a space
adjacent to the
root surface. This will allow the
ingrowth of tissue
from the periodontal ligament.
Some materials may be so soft and flexible that
they collapse into the defect. Other materials are
too stiff and may
perforate the overlying tissue.
5.
Finally, there are clinical needs in the design of a
barrier. It should be provided in configurations
which are easy to trim and to place.
Bioabsorbable materials
In recent years, natural or synthetic bioabsorbable
barrier materials for GTR have been introduced in
order to avoid a second surgery for membrane re-
moval. Barrier materials of collagen from different
species and from different anatomical sites have been
tested in animals and in humans (Blumenthal 1988,
1993, Pitaru et al. 1988, Tanner et al. 1988, Paul et al.
1992, Wang et al. 1994, Camelo et al. 1998, Mellonig
674 • CHAPTER
28
2000). Often the collagen used is a cross-linked variety
of porcine or bovine origin. When a collagen mem-
brane is implanted in the human body it is resorbed
by the enzymatic activity of macrophages and poly-
morphnuclear leucocytes (Tatakis et al. 1999). Success
ful treatment following the use of such barrier mate-
rials has been demonstrated, but the results of the
studies vary. Several complications such as early deg
-
radation, epithelial downgrowth along the material
and premature loss of the material were reported
following the use of collagen materials. The varying
results are probably due to differences in the proper-
ties of the material and the handling of the material at
the time of implantation. Although probably very
minimal, there is a risk that infectious agents from
animal products can be transmitted to humans, and
autoimmunization has also been mentioned as a risk.
Barrier materials of polylactic acid or copolymers
of polylactic acid and polyglycolic acid were evalu-
ated in animal and human studies and are commonly
used (Magnusson et al. 1988, Caffesse et al. 1994,
Caton et al. 1994, Gottlow et al. 1994, Laurell et al. 1994,
Hugoson et al. 1995, Poison et al. 1995a, Hiirzeler et
al. 1997, Sculean et al. 1999a). These materials are
biocompatible, but by definition they are not inert
since some tissue reaction may be expected during
degradation. The materials are degradated by hy-
drolysis and eliminated from the organism through
the Krebs cycle as carbon dioxide and water (Tatakis
et al. 1999).
The types of barrier materials tested in the studies
differ regarding configuration and design. It appears
that a number of bioabsorbable materials to a varying
extent meet the requirements of a good barrier listed
above. Indeed, there are several studies (Hugoson et
al. 1995, Cortellini et al. 1996b, Smith et al. 1998, Tonetti
et al. 1998, Cortellini & Tonetti 2000a) indicating that
similar satisfactory results can be obtained with bioab
sorbable barrier materials of polylactic and polygly-
colic acid as with non-bioabsorbable materials.
Intrabony defects
Early evidence that GTR treatment of deep intrabony
defects may produce clinical improvements in terms
of clinical attachment gain was presented in several
case reports (Nyman et al. 1982, Gottlow et al. 1986,
Becker et al. 1988, Schallhorn & McClain 1988, Cortel
lini et al. 1990). In recent years, a number of clinical
investigations have reported on a total of 1283 in-
trabony defects treated with GTR (Table 28-1). In these
studies, the issue of evaluating the predictability of the
clinical outcomes following application of GTR proce-
dures was addressed. The weighted mean of the re-
ported results indicates a mean gain in clinical attach
-
ment of 3.8 ± 1.7 mm, with a 95% confidence interval
ranging from 3.7 to 4.0 mm (Cortellini & Tonetti
2000a). The reported clinical attachment gains follow-
ing GTR treatment were significantly larger than the
ones obtained from conventional flap surgery. A re-
cent review (Lang 2000) on flap surgery reported a
weighted mean of 1.172 defects in 40 studies. CAL
gains were 1.8 ± 1.4 mm, with a 95% confidence inter
val ranging from 1.6 to 1.9 mm.
Different types of non-bioabsorbable (Fig. 28-28)
and bioabsorbable (Fig. 28-29) barrier materials were
used in the studies summarized in Table 28-1. A subset
analysis indicated that cases treated with non-bioab-
sorbable barrier materials (351 defects) showed a
mean gain in clinical attachment of 3.7± 1.7 mm which
did not differ from that obtained with bioabsorbable
barrier materials of 3.6 ± 1.5 mm (592 defects).
Analysis of the results reported in some of the
studies in Table 28-1 (i.e. 211 defects in 9 investiga-
tions; Proestakis et al. 1992, Cortellini et al. 1993b,
1995c, 1996b, Cortellini & Pini-Prato 1994, Laurell et
al. 1994, Mattson et al. 1995, Mellado et al. 1995, Tonetti
et al. 1996b) provides important information regard-
ing the predictability of GTR in intrabony defects.
Gains of 2-3 mm were observed in 29.2% of the defects,
gains of 4-5 mm in 35.4% of the defects, and gains of
6 mm or more in 24.9% of the defects. Only in 10.5%
of the treated defects was the gain less than 2 mm,
while no change or attachment loss was observed in
two cases.
In some of the investigations, changes in bone lev-
els were also reported (Becker et al. 1988, Handelsman
et al. 1991, Kersten et al. 1992, Cortellini et al. 1993b,c,
Selvig et al. 1993). Bone gains ranged between 1.1 and
4.3 mm and correlated with the reported gains in
clinical attachment. In a study by Tonetti et al. (1993b),
1 year after GTR the bone was found to be located 1.5
mm apically to the position of the attained clinical
attachment level.
Another important parameter related to the out-
come of regenerative procedures is the residual pocket
depth. hl the studies reported in Table 28-1, shallow
pockets were consistently found at 1 year. The
weighted mean of residual pocket depth was 3.4 ± 1.2
mm, with a 95% CI ranging from 2.3 to 3.5 mm.
The reported outcomes indicate that GTR proce-
dures predictably result in clinical improvements in
intrabony defects beyond that of flap surgery. This
was further confirmed in 11 controlled randomized
clinical trials in which guided tissue regeneration was
compared with conventional flap surgery (Table 28-2).
A total of 267 defects were treated with flap surgery
and 317 with GTR. In 9 of the 11 investigations, GTR
resulted in statistically significantly greater probing
attachment level gains when compared to flap sur-
gery. Similar results were also observed for residual
pocket depth. It should be emphasized that one of the
investigations reporting no significant differences be-
tween GTR and flap surgery was carried out in only 9
pairs of defects (18 defects) located on maxillary pre-
molars (Proestakis et al. 1992). In this study the in-
trabony component of the defects was shallow and 10
of the 18 defects had a furcation invol
v
ement. The
weighted mean of the results reported in the 11 studies
listed in Table 28-2 (Cortellini & Tonetti 2000a) indi-
cated that the gain in clinical attachment in sites
