James I.N. Introduction to Circulating Atmospheres
Подождите немного. Документ загружается.


5.3
Atmospheric energetics
129
-30S
0
LATITUDE
Fig.
5.10.
Cross section
of
the vertical temperature
flux,
[co'T'],
for the DJF
season.
Contour interval 0.02KPas"
1
, downward (positive) fluxes
shaded.
Based
on six
years
of
ECMWF
analyses.
simply by integrating over the entire atmosphere. Using pressure as a vertical
coordinate, we have
2
+ v
2
= -
—o—dpdxdy,
g
J
A
J Jo 2
g
J
A
J Jo 2
where fdp/g represents an integration with respect to mass; the domain A
is taken to be the entire surface of the globe, or least some fraction of that
area so extensive that there is no advection of air into or out of
it.
The result
of such an integration is generally awkwardly large, so it is conventional to
divide X by the area of the Earth's surface Ana
2
, giving a mean energy per
unit area of the Earth's surface. We will use the notation:
(5.19)
Q being any meteorological quantity, as a convenient shorthand. Having
defined the global kinetic energy, consider how it might be altered. Consistent
with the quasi-geostrophic approximation, the momentum equation may be
written:
d\ d\ v
—
+ v
•
Vv + (o— + /k x v = -V<t> (5.20)
at dp
T
D
(see Eqs. (1.65a) and (1.65b)). Note that the horizontal advection term can
130
Transient disturbances
in the
midlatitudes
be rewritten
as
V(v
•
v/2)
=
VK, where
K is the
local kinetic energy
per
unit
mass
of the
atmosphere.
The
last term
is an
idealization
of
real frictional
processes,
in the
form
of a
Rayleigh drag. Take
the
scalar product
of
this
equation with
the
horizontal components
of
the wind:
^
+ v
.
V
K + a^ =
-v.Va>-— (5.21)
dt
dp
T
D
which
is an
energy equation
for the
flow. Note that
the
Coriolis force
can
do
no
work, since
it
acts perpendicularly
to the
velocity.
The
last term states
that
the
kinetic energy
is
dissipated
at
twice
the
rate
of the
momentum.
We
are
left
to
deal with
the
vertical advection
of
kinetic energy,
and the
horizontal advection
of
the geopotential. Consider
the
latter. After using
the
continuity equation,
d
<9<t>
v
•
V<D
=
V
•
(<Dv)
+
^-(co<D)
-
co—. (5.22)
dp
dp
But
d<S>/dp
is
related
to the
potential temperature through
the
hydrostatic
relation,
Eq.
(1.68):
^
=
-Hp)d.
(5.23)
Hence, writing the advection terms
in
flux form, and incorporating Eqs. (5.22)
and (5.23)
the
kinetic energy equation becomes:
^
+ V
•
(\(K +
<D))
+
^-(co(K
+
0>))
=
-hcoO
- —.
(5.24)
dt
dp
T
D
Now integrate the kinetic energy equation over the globe
to
give
an
expression
for
the
rate
of
change
of
global kinetic energy.
By the
divergence theorem,
the integral with respect
to
area
of
the second term
can be
written:
f
f
V
•
(\(K +
<D))
dA
= I \(K +
<D)
•
dl
(5.25)
where
A
denotes
the
horizontal area
of the
atmosphere
and L
denotes
the
circuit around the edge
of
the domain. But this
is
zero, since there
is
supposed
to
be no
inflow
or
outflow across
the
boundaries
(or,
more elegantly
for a
spherical world,
it is
zero because
v and (K +
O) satisfy periodic boundary
conditions). Similarly,
the
vertical boundary conditions
are
a>
= 0 at p = p
s
and
p = 0, so
that
the
vertical integral
of the
third term
is
zero.
So
after
some algebraic labour,
the
final expression
for the
rate
of
change
of
global
kinetic energy
is
found
to be
rather straightforward:
±(K) = (-hcoe)-(2K/T
D
).
(5.26)

5.3 Atmospheric energetics 131
But this is simply the statement made in Chapter 3 on thermodynamic
grounds; kinetic energy is generated if there is a net correlation between
ascent and positive temperature anomalies, and vice versa. That is, there
must be a preponderance of thermally direct circulations. This generation of
kinetic energy may be offset by the frictional dissipation of kinetic energy.
In order to generate this kinetic energy, we must have depleted some
potential energy. But the potential energy of the atmosphere is not a very
useful concept. It would be minimized by compressing all the air into an
infinitely thin layer near the ground. But such a compression is physically
unrealistic. It would require enormous amounts of work to be done against
pressure forces, and this work would go to increase the internal energy, that
is,
the temperature, of the air. Only if this internal energy could be radiated
steadily away to space would the potential energy be reduced, and it would
have gone, not into kinetic energy, but into thermal radiation. In other
words, most of the potential energy associated with the mass distribution
in the atmosphere is unavailable for conversion into kinetic energy. That
part of the energy which is available for conversion, the 'available potential
energy', is a small fraction of the total. The available potential energy is
defined as that part of the potential energy which can be released without
any change of the internal energy of the atmosphere. From the first law of
thermodynamics, and remembering that the total mass of the atmosphere
does not change, it must be released by adiabatic mixing of air parcels.
Formally, the available potential energy is defined as the maximum amount
of potential energy which could be destroyed by adiabatic mixing of the
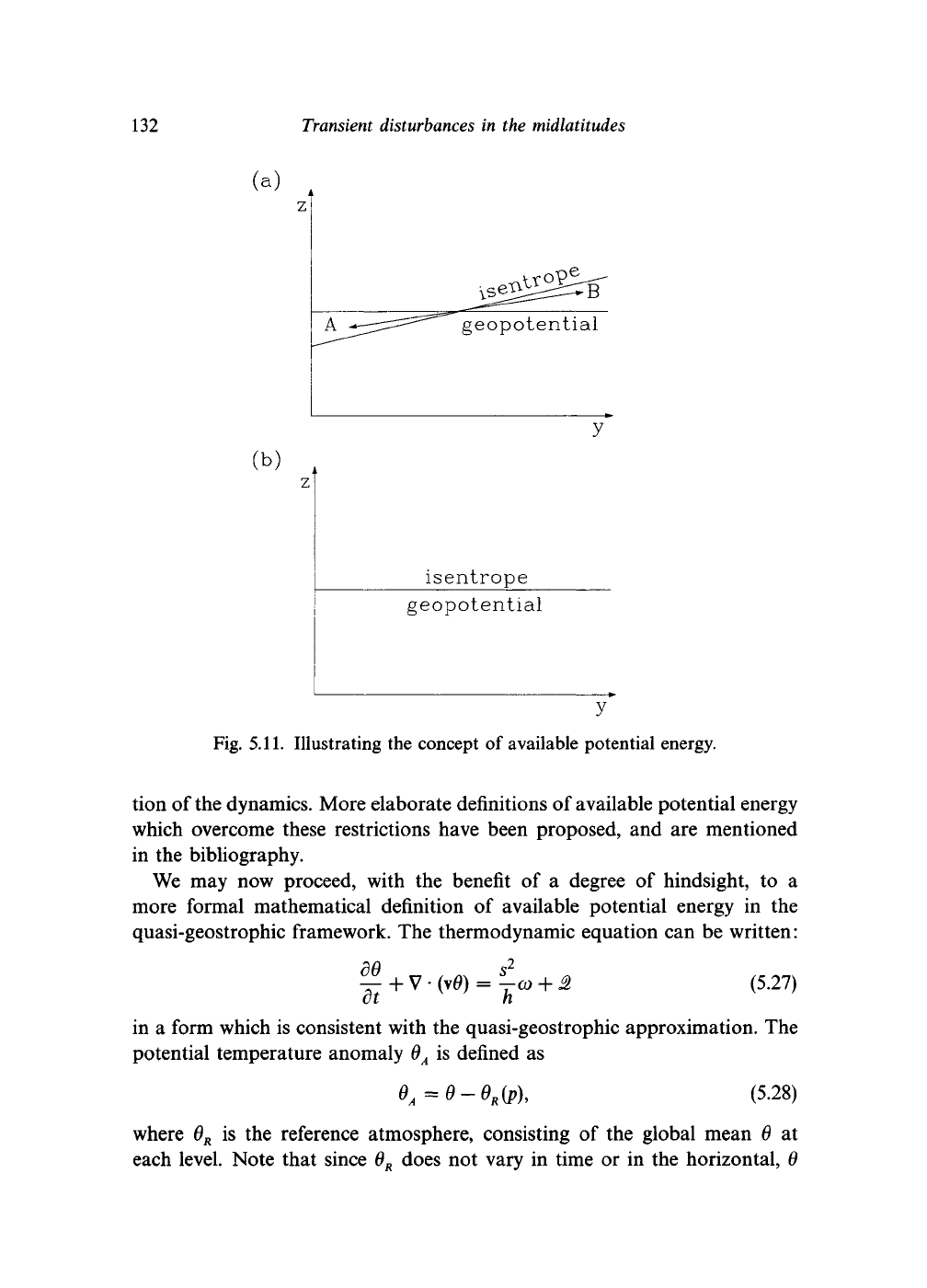
atmosphere. Figure 5.11 illustrates the concept. Whether such mixing is in
fact possible depends upon the dynamic constraints to which the system is
subject. In Fig. 5.11
(a),
the isentropes are not parallel to the geopotentials.
We may imagine adiabatic processes which could exchange the wedge of
potentially warmer air marked A for the wedge of potentially colder air
marked B. The result would be to replace the mass of air B with somewhat
less dense air from A, while the air mass A would be replaced by denser air
from
B.
The mean mass distribution would have been lowered slightly and so
a certain amount of potential energy would have been destroyed. Once the
isentropes are parallel to the geopotentials, no further adiabatic exchanges
of air masses with different potential temperatures are possible. Accordingly,
the potential energy released in going from the state shown in Fig. 5.11 (a)
to the state in Fig. 5.11(b) is indeed the 'available potential energy'.
These arguments are restricted to the usual situation of a statically stable
atmosphere, in which the static stability is not supposed to vary on pressure
surfaces. This is a consistency requirement for a quasi-geostrophic descrip-
132
Transient disturbances
in
the midlatitudes
(a)
A
geopotential
y
isentrope
geopotential
y
Fig. 5.11. Illustrating the concept of available potential energy.
tion of
the
dynamics. More elaborate definitions of available potential energy
which overcome these restrictions have been proposed, and are mentioned
in the bibliography.
We may now proceed, with
the
benefit
of a
degree
of
hindsight,
to a
more formal mathematical definition
of
available potential energy
in the
quasi-geostrophic framework. The thermodynamic equation can be written:
ot
(5.27)
in
a
form which is consistent with the quasi-geostrophic approximation. The
potential temperature anomaly 9
A
is defined as
0
A
= 0-0
R
(p)
9
(5.28)
where 9
R
is
the reference atmosphere, consisting
of
the global mean
9 at
each level. Note that since 9
R
does not vary
in
time
or in
the horizontal,
9
5.3 Atmospheric energetics
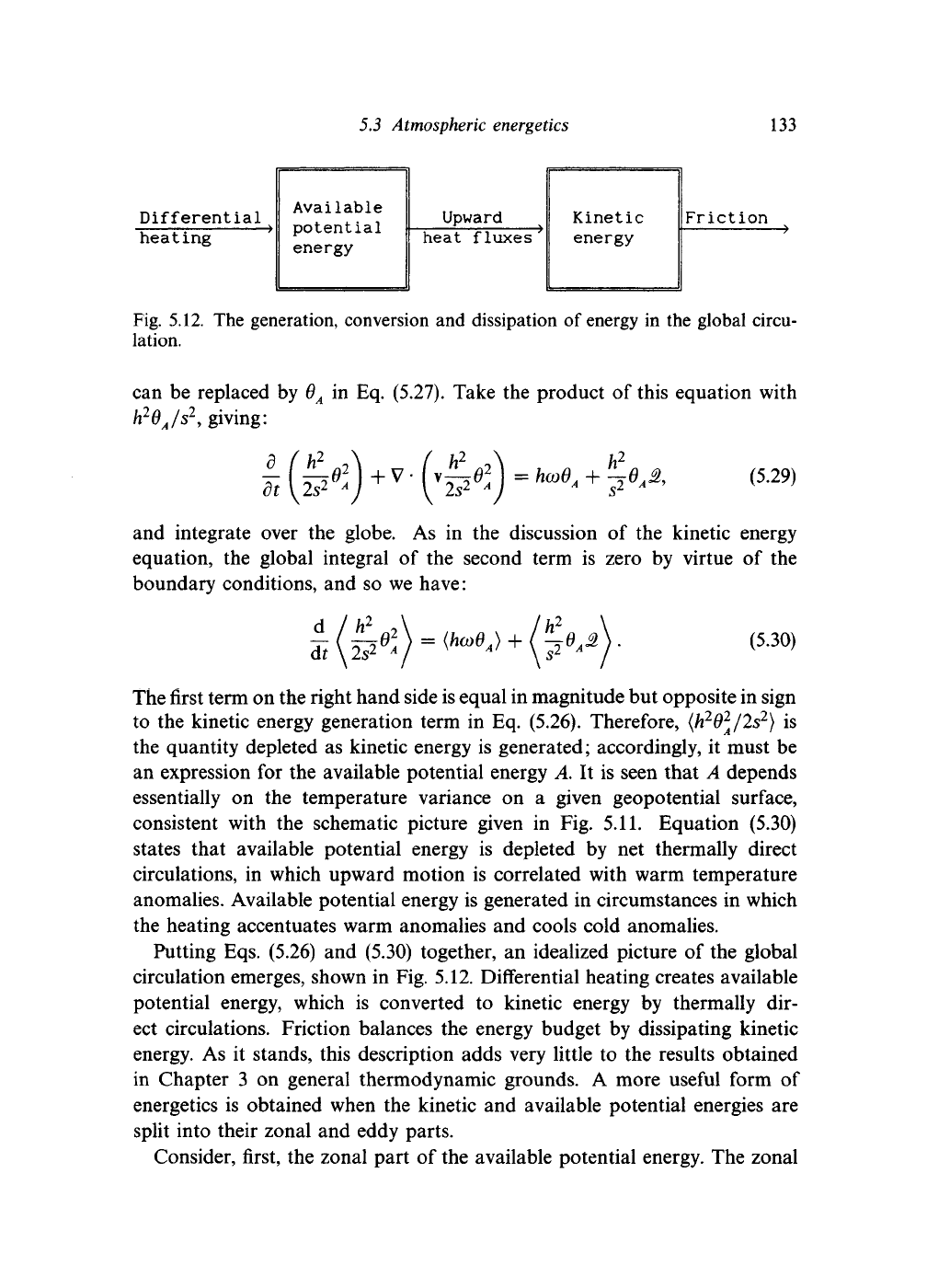
133
Differential
heating
Available
potential
energy
Upward
heat fluxes
Kinetic
energy
Friction
Fig. 5.12. The generation, conversion and dissipation of energy in the global circu-
lation.
^
^,0
Z
+V- v
can be replaced by 6
A
in
Eq. (5.27). Take the product
of
this equation with
h
2
6
A
/s
2
, giving:
(5.29)
and integrate over the globe.
As in
the discussion
of
the kinetic energy
equation, the global integral
of
the second term
is
zero
by
virtue
of
the
boundary conditions, and so we have:
(5.30)
The first term on the right hand side is equal in magnitude but opposite in sign
to the kinetic energy generation term in Eq. (5.26). Therefore, (h
2
9
2
/2s
2
)
is
the quantity depleted as kinetic energy is generated; accordingly,
it
must be
an expression for the available potential energy A.
It
is seen that A depends
essentially
on the
temperature variance
on a
given geopotential surface,
consistent with
the
schematic picture given
in
Fig. 5.11. Equation (5.30)
states that available potential energy
is
depleted
by net
thermally direct
circulations,
in
which upward motion
is
correlated with warm temperature
anomalies. Available potential energy is generated in circumstances in which
the heating accentuates warm anomalies and cools cold anomalies.
Putting Eqs. (5.26) and (5.30) together, an idealized picture of the global
circulation emerges, shown in Fig. 5.12. Differential heating creates available
potential energy, which
is
converted
to
kinetic energy
by
thermally
dir-
ect circulations. Friction balances the energy budget by dissipating kinetic
energy. As
it
stands, this description adds very little to the results obtained
in Chapter
3
on general thermodynamic grounds.
A
more useful form
of
energetics is obtained when the kinetic and available potential energies are
split into their zonal and eddy parts.
Consider, first, the zonal part of the available potential energy. The zonal

134
Transient disturbances
in
the
midlatitudes
mean
of
the thermodynamic equation
is:
[0],
+
\v\[0]
y
=
jtt-
WF]
y
+ [I],
(5.31)
where the subscript notation
is
used
to
denote partial differentiation and
the
eddy terms have been
put on to the
right hand side.
For
brevity,
the
eddy
fluxes have been written
in a
form which includes both
the
transient
and
steady eddy contributions, since,
to a
good approximation:
[
v
*9*]
=
[v*e
] +
[v'O'l
(5.32)
The zonal available potential energy, denoted
AZ, is
obtained
by
multi-
plying
Eq.
(5.31)
by
h
2
[9]/s
2
and
integrating over
the
entire globe.
The
multiplication yields:
The second term
on the
left hand side integrates
to
zero,
by
arguments
similar
to
those used above which make
use of
the boundary conditions
on
[v].
The global average
of
the second term
on the
right
can be
rewritten
in
the more convenient form:
(^^e"]
y
\
=
-(K[e]ybfW]\.
(5.34)
This identity
is
easily established
by
integrating
by
parts with respect
to y
and using the conditions that
v*
= 0 at
the edges
of
the domain (or, equally,
that
the
boundary conditions
on all
variables
are
cyclic
in y).
Finally, from
Eq. (5.33) we can show that:
j
t
=
(h[6][co])
-
/^[0M^]\
+ O^Wffl)
•
(5.35)
With
the
exception
of the
second term,
all the
terms
are
very straight-
forwardly related to the zonal mean version of the various terms in Eq. (5.30).
This second term
is a
new effect;
it
represents
the
conversion
of
zonal into
eddy available potential energy
by the
action
of an
eddy component
of
meridional wind
in the
presence
of a
basic poleward temperature gradient.
The difference between Eqs. (5.35)
and
(5.30) yields
an
expression
for the
rate
of
change
of
the eddy available potential energy, AE, where
(&
[¥
~
2]
)-
(5
-
36)
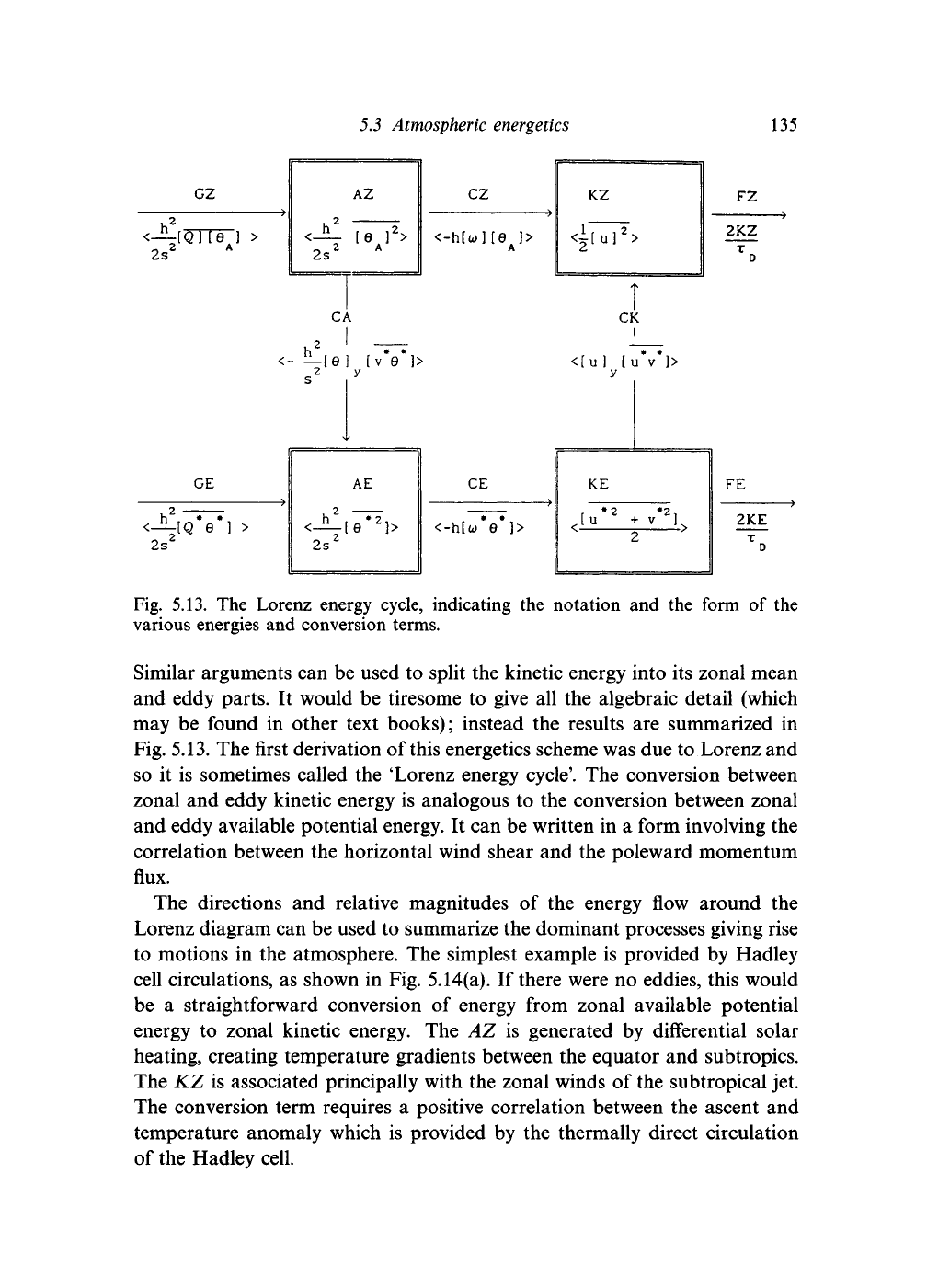
5.3 Atmospheric energetics
135
GZ
,2
<—[Q] [e ] >
2s
2
A
<h
2
2s
2
AZ
[e
A
]
2
>
cz
<-h[u>]
[0
A
]>
KZ
2
U
FZ
2KZ
GE
2s
2
Fig. 5.13. The Lorenz energy cycle, indicating the notation and the form of the
various energies and conversion terms.
Similar arguments can be used to split the kinetic energy into its zonal mean
and eddy parts. It would be tiresome to give all the algebraic detail (which
may be found in other text books); instead the results are summarized in
Fig. 5.13. The first derivation of this energetics scheme was due to Lorenz and
so it is sometimes called the 'Lorenz energy cycle'. The conversion between
zonal and eddy kinetic energy is analogous to the conversion between zonal
and eddy available potential energy. It can be written in a form involving the
correlation between the horizontal wind shear and the poleward momentum
flux.
The directions and relative magnitudes of the energy flow around the
Lorenz diagram can be used to summarize the dominant processes giving rise
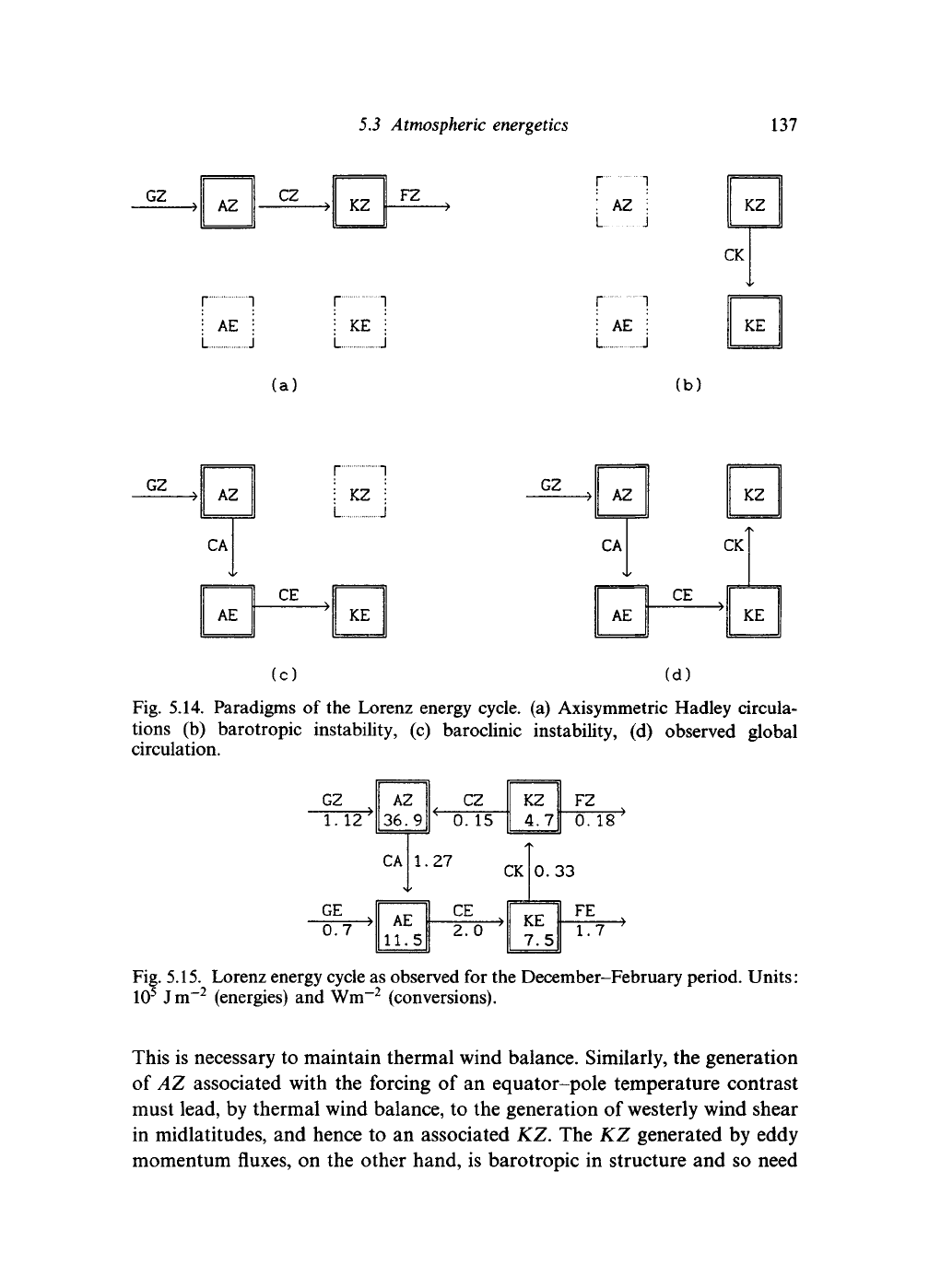
to motions in the atmosphere. The simplest example is provided by Hadley
cell circulations, as shown in Fig. 5.14(a). If there were no eddies, this would
be a straightforward conversion of energy from zonal available potential
energy to zonal kinetic energy. The AZ is generated by differential solar
heating, creating temperature gradients between the equator and subtropics.
The KZ is associated principally with the zonal winds of the subtropical jet.
The conversion term requires a positive correlation between the ascent and
temperature anomaly which is provided by the thermally direct circulation
of the Hadley cell.

136 Transient disturbances in the midlatitudes
During the 1930s and 1940s, there was considerable debate about the
origin of eddy motions in the atmosphere. One possibility was that the
strong shears associated with the poleward edge of the subtropical jet would
prove hydrodynamically unstable. Such 'barotropic instability' would result
in conversion of KZ to KE and would require that the momentum fluxes
would be negatively correlated with the wind shears. In terms of the eddy
structures, we would see eddies tilting against the background wind shear.
Fig. 5.14(b) illustrates the Lorenz energy diagram associated with this view
of the global circulation.
The second possibility, which rapidly became the accepted mechanism
following the work of Charney and Eady in the latter part of the 1940s, was
that 'baroclinic instability' generated the eddies. The energetics of baroclinic
instability are illustrated in Fig. 5.14(c); temperature fluxes correlated with
a poleward temperature gradient convert AZ to AE. Upward heat fluxes
then convert AE to KE. Baroclinic instability theory, which will be outlined
in Section 5.4, indicates the conditions under which such a pattern of
conversions is dynamically consistent.
Figure 5.15 gives observed values of the various energies and conversions,
estimated from observed data. The vertical velocities were inferred from
the horizontal wind and mass fields during initialization prior to carrying
out a numerical forecast integration (see Section 2.2). It will be seen that
the dominant conversions are those associated with baroclinic instability,
although only quite a small fraction of AZ is actually converted into other
forms of energy. The conversion between AZ and KZ is small and its sign
is somewhat uncertain. This arises from the near cancellation between a
negative contribution to
(/*[#]
[co])
from the Hadley cell and the positive con-
tribution from the thermally indirect Ferrel circulations of the midlatitudes.
There is no trace of barotropic instability, the conversion CK having the
wrong sign for this. Rather, the momentum fluxes are associated with the
transient eddies returning kinetic energy to the zonal flow and so helping to
maintain the tropospheric jets in midlatitudes.
An analysis of
the
energetics of
the
global circulation is helpful in revealing
the dominant pattern of energy conversions in the atmosphere. If care is
taken, these may be regarded as the signatures of basic physical processes
occurring, such as baroclinic instability. But the usefulness of the energetics
approach is limited in a number of respects.
First, it must be appreciated that the kinetic energies and available poten-
tial energies cannot vary independently in the midlatitudes. For instance, in
Section 5.2, we found that the poleward thermal advection of a developing
baroclinic wave is inevitably accompanied by an upward temperature flux.
5.3 Atmospheric energetics
137
GZ
AZ
r
i
! AE i
L
j
cz
(a)
KZ
FZ
r
i
I KE i
L
j
r
i
: AZ
i
L
J
r
]
! AE !
L
J
(b)
KZ
CK
KE
GZ
AZ
CA
AE
CE
r
1
KZ
i
L
j
KE
(c)
GZ
AZ
(d)
KZ
CA
AE
CK
CE
KE
Fig. 5.14. Paradigms of the Lorenz energy cycle, (a) Axisymmetric Hadley circula-
tions (b) barotropic instability, (c) baroclinic instability, (d) observed global
circulation.
GZ
1.
12
AZ
36.9
CA
1.
AE
11.5
CZ
(
0.
15
KZ
4.7
27
CK
CE
2.0
>
FZ
0.
18
)
0.33
KE
7.5
FE
1.7
)
Fig. 5.15. Lorenz energy cycle as observed for the December-February period. Units:
10
5
Jm"
2
(energies) and Wm"
2
(conversions).
This is necessary to maintain thermal wind balance. Similarly, the generation
of AZ associated with the forcing of an equator-pole temperature contrast
must lead, by thermal wind balance, to the generation of westerly wind shear
in midlatitudes, and hence to an associated KZ. The KZ generated by eddy
momentum fluxes, on the other hand, is barotropic in structure and so need

138 Transient disturbances in the midlatitudes
not be accompanied by any change of AZ. It is perhaps preferable to reject
the distinction between eddy available potential and kinetic energy, defining
instead a single 'eddy energy':
)
(5.37)
Eddy energy is created by the negative correlation between the temperature
flux and the temperature gradient, and is destroyed if there is a positive cor-
relation between momentum flux and horizontal wind shear. But, of course,
this is only a diagnostic relationship. In order to decide whether baroclin-
ically unstable waves can form on any particular zonal flow, it is necessary
to show that energy releasing disturbances, with the necessary phase tilts
and other structures, can be maintained in a dynamically consistent fashion
in that flow. Our discussion in the next section will address these issues.
Second, the analysis is a truly global diagnostic. It is very difficult to
construct an energetics scheme for a limited area. The boundary fluxes
rapidly become the dominant terms. But the global budget is likely to
include a number of processes with quite different signatures in different
regions. An example was given above, where it was shown that the Hadley
and Ferrel cells make large contributions to the AZ to KZ conversion which
nearly cancel. The net AZ to KZ conversion is therefore a small residual
between large effects of opposite sign arising from the Hadley cell in the
tropics and the Ferrel cell in the midlatitudes. The magnitude and even the
sign of this conversion are very uncertain.
5.4 Theories of baroclinic instability
The energetics discussed in the last chapter revealed that, because of the
horizontal temperature gradients in a stably stratified atmosphere, potential
energy is available in principle for conversion into eddy energy. The eddy
fluxes required for the necessary conversions of energy also define the sort of
structures that must characterize such growing eddies. But these considera-
tions alone do not guarantee that growing eddies can in fact be sustained. The
demands of thermal wind balance restrict the scale and shape of dynamically
possible eddies. In this section, we will outline a more predictive approach
which will define the conditions under which unstable eddies can be expected,
and which will determine their scale and rate of growth.
The philosophy behind these theories is to imagine small perturbations
growing on a zonally uniform basic state. It is found that certain classes
