Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.


20.5 Ammoxidation of Unconventional Molecules 801
The elements claimed are the same as those reported in References [120, 121] .
The preparation procedure employed is known to lead to the formation of
VOPO
4
, rather than (VO)
2
P
2
O
7
. The presence of Sb, however, may lead to a modi-
fi cation of the structural features. Indeed, the authors claim the presence of
vanadyl pyrophosphate as the major phase present in catalysts, with a minor
amount of vanadium phosphate. The atomic ratio between the components of the
γ - alumina - supported active phase was V/Sb/P 1/1.9/1.18. The reaction conditions
were 425 ° C (at which the best yields were reported), and a feed ratio of reactant/
air/ammonia of 0.6 – 1.0/4.2/1.5. The following results were claimed under these
conditions:
• from cyclohexanol, conversion 78%, selectivity to adiponitrile 75%, selectivity to
hexanenitrile 18%. The by - products were CO, CO
2
and cracking products,
• from cyclohexanone, conversion 60%, selectivity to adiponitrile 48%, selectivity
to hexanenitrile 30%. The by - products were CO, CO
2
and cracking products.
This performance is outstanding, but it has never been successfully reproduced
by other teams active in this research fi eld.
The reaction of cyclohexanol ammoxidation has been investigated by Chen and
Lee, with a V
2
O
5
catalyst [125] . The feed mixture comprised cyclohexanol/oxygen/
ammonia in the ratio 1.2/9/15. A large excess of ammonia was thus used. Cyclo-
hexanone, adiponitrile, adipic acid, benzene and CO
2
formed. The best yield to
adiponitrile was 4%, with a conversion of approximately 52% (as inferred from
the sum of the yields), at W / F = 0.45 g s cm
− 3
and 365 ° C. At a higher W / F value,
the maximum yield to cyclohexanone was reached (19%). All these products under-
went consecutive reaction to benzene and CO
2
. In a pulse reactor, the yield to
adiponitrile was 6.3%, at 96% reactant conversion. Cyclohexanone was also used
in the pulse reactor, but the predominant product was CO
2
. The authors proposed
that the reaction pathway includes the dehydrogenation of cyclohexanol to cyclo-
hexanone, its oxidative cleavage to adipic acid and subsequent transformation to
the dinitrile. The formation of benzene occurs via dehydration to cyclohexene,
followed by dehydrogenation. The ammoxidation of cyclohexene exclusively yielded
benzene.
The ammoxidation of cyclohexanol or cyclohexanone is also reported in one
patent [126] , using a cyclohexanone/air/ammonia/H
2
O ratio of 1/10/2.5/10 (the
presence of added steam is noteworthy), 450 ° C, and a contact time of 1 s. Several
catalysts were used, as reported in Table 20.6 . The products obtained were aniline,
phenol and adiponitrile.
The nature of the products formed is rather unusual. The formation of aniline
implies a reaction between the ketone and ammonia to yield the cyclohexanonei-
mine. The latter then rearranges with aromatization, to yield aniline. Aromatiza-
tion occurs with cyclohexanone, leading to the formation of phenol. Therefore, the
formation of the aromatic ring is quicker than the opening of the aliphatic cycle.
This is the key point of the reaction involving cyclic reactants: the competition
between the parallel reactions of ring opening and aromatization controls the
selectivity.

802 20 Catalytic Ammoxidation of Hydrocarbons on Mixed Oxides
20.5.3
The Ammoxidation of Cyclohexane and n - Hexane
Some papers and patents report on the ammoxidation of cyclohexane in the gas
phase [124, 127 – 129] or in the liquid phase [130] . In Reference [124] , which was
discussed in the previous section, the ammoxidation of cyclohexane is reported to
give 50% adiponitrile selectivity and 35% hexanenitrile selectivity for a conversion
close to 70%, at 425 ° C.
Osipova and coworkers [127] used a catalyst based on Ti/Sb mixed oxide, cal-
cined at 750 ° C. Under these conditions, titania is in the rutile form, and can host
up to 7 mol% Sb
2
O
5
in solid solution. Higher Sb content led to the development
of also an equimolar Ti/Sb compound (TiSbO
4
), while in systems with more than
50 mol% Sb
2
O
5
, the additional formation of α - Sb
2
O
4
was found. A catalyst with the
composition 70% Sb
2
O
5
– 30%TiO
2
gave the best performance.
Catalytic tests were run in a pulse reactor, at 400 ° C, with a cyclohexane/
oxygen/ammonia feed composition in mol% of 3/6/4 (the balance being He).
The main products obtained were adiponitrile ( ADN ) and benzene, with an
overall selectivity of more than 90% (the cyclohexane conversion is not reported).
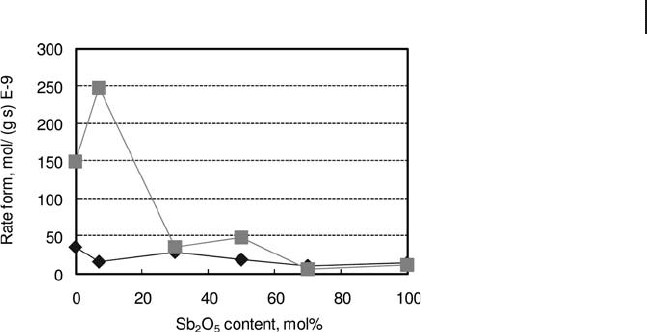
The rates of benzene and ADN formation are plotted in Figure 20.10 as functions
of the Sb
2
O
5
content of catalysts. It is shown that the overall formation of benzene
considerably decreased on increasing the amount of Sb in catalysts. The forma-
tion of ADN decreased, but the decrease was less pronounced than that of
Table 20.6 A summary of catalyst performance reported in [126] for
cyclohexanone ammoxidation.
Catalyst Comment Yield aniline
(%)
Yield phenol
(%)
Yield ADN
(%)
K/Ni/Co/Fe/Bi/P/
Mo/O - silica
Typical catalyst for propene
ammoxidation
3.1 11.6 tr
V/W/Mo/O - silica Typical catalyst for
electrophilic oxidation
10.2 19.2 0.6
P/Mo/O - silica Heteropolycompound 8.9 10.0 –
W/O - silica 5.4 7.8 –
Fe/V/Sb/O - silica
Catalyst for propane and
propene ammoxidation
0.1 18.9 0.3
Sb/Mo/O Catalyst for allylic
(amm)oxidation
– 3.4 –
ADN: adiponitrile
20.5 Ammoxidation of Unconventional Molecules 803
benzene. Moreover, the formation of benzene was much greater than that of
ADN for low Sb contents, while the two rates became comparable for intermedi-
ate and large values of Sb content; therefore, the selectivity to ADN increased on
increasing the Sb content in catalyst.
Osipova and coworkers [127] also found that under non - steady conditions (those
utilized in the pulse reactor), a considerable fraction of ADN remained adsorbed
on the catalyst. A rough estimation for the catalyst with 30% Sb
2
O
5
led to the con-
clusion that the overall amount of ADN produced in the single pulse approximately
corresponded to 1/3 of the reacted cyclohexane. In particular, ADN bound strongly
to the catalyst surface and made heavy (polymeric compounds), at least at 300 ° C
and in the presence of ammonia. Adsorbed ammonia contributed to the polymer-
ization of ADN at the catalyst surface.
Simon and Germain [128] investigated several catalytic systems and found that
cyclohexane conversion is high (70%) even in the absence of catalyst at 460 ° C (feed
cyclohexane/oxygen/ammonia 1/3.6/1.5) yielding mainly cyclohexene, benzene
and CO
x
, with minor amounts of other compounds.
The presence of a catalyst led to the formation of C
4
dinitriles (maleonitrile,
fumaronitrile, succinonitrile), C
5
dinitriles (glutaronitrile) and C
6
dinitriles (muco-
nonitrile, adiponitrile), but the yield of these compounds was very low. In the best
case, with a V/Mo/O catalyst (atomic ratio V/Mo 4/1; phase: V
2
O
5
), the yield to
maleonitrile was 1.9% and 0.8% to fumaronitrile, 17% to benzene, 23% to CO
x
,
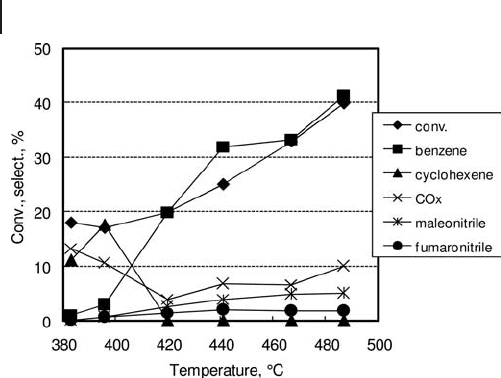
with traces of mucononitrile, at a conversion of 57% at 460 ° C. With the same cata-
lyst, the initial selectivity (extrapolated at zero conversion) to C
4
nitriles was approx
5% (negligible to other nitriles), while the predominant primary products were
benzene and carbon oxides. For temperatures lower than 420 ° C the predominant
product was cyclohexene, while at higher temperatures benzene and CO
x
prevailed
(Figure 20.11 ).
Figure 20.10 Rates of adiponitrile (ADN) ( 䉬 ) and benzene ( 䊏 )
formation as functions of the content of Sb
2
O
5
in catalysts
for cyclohexane ammoxidation. Elaborated from [127a] .
804 20 Catalytic Ammoxidation of Hydrocarbons on Mixed Oxides
The performance was very similar with a Ti/Mo/O catalyst (atomic ratio Ti/Mo
1.14/1; phases TiO
2
and MoO
3
) and with a Bi/Mo/O catalyst (atomic ratio Bi/Mo
0.9/1.) Other catalysts (Sn/Mo/O, Sb/Mo/O, Sn/Sb/Fe/O) were more selective
than V/Mo/O to either benzene or CO
x
.
The mechanism of reaction included the rapid oxy - dehydrogenation of cyclohex-
ane to cyclohexene and benzene. The latter was then transformed to successive
products (see the section on benzene ammoxidation). The opening of the ring may
occur either for cyclohexene or for benzene itself.
Table 20.7 summarizes the performance of catalysts described in a patent [129a]
which reports the formation of saturated dinitriles (C
4
, C
5
and C
6
). All catalysts
described contain Sb oxide as the main component. In References [129b, 129c] ,
the same catalysts are described as those reported in Reference [129a] , but with
the addition of a halogenated compound in the feed stream.
The addition of halogenated compounds increased the conversion of cyclohex-
ane, while the selectivities remained substantially unchanged. This indicates the
presence of a radical - based mechanism for the activation of cyclohexane. In fact,
it is known that the radical chain reaction is enhanced in the presence of halogens,
owing to their tendency to give rise to X • species.
The only example reported in the literature concerning n - hexane ammoxidation
is, once again, Reference [124] . In this paper, for a conversion of approx 12%,
a selectivity to adiponitrile close to 40% and to hexanenitrile of approx 30%
are reported, at 425 ° C and with a molar feed ratio hexane/air/ammonia of
0.6 – 1.0/4.2/1.5. To our knowledge, these interesting results have never been
confi rmed.
Figure 20.11 Effect of temperature on catalytic performance
of V/Mo/O catalyst in cyclohexane ammoxidation [128] .

20.5 Ammoxidation of Unconventional Molecules 805
20.5.4
The Ammoxidation of Benzene
The ammoxidation of benzene is described in some papers and patents [124, 128,
131, 132] . Whilst benzene has been reported to remain unconverted in the pres-
ence of ammonia and oxygen with a V
2
O
5
catalyst [124] , it was effi ciently trans-
formed to nitriles with catalysts based on mixed molybdates [128] .
Figure 20.12 reports the performance of the best catalyst, based on V/Mo/O
(V/Mo atomic ratio 4/1) at 465 ° C, with a feed composition of benzene/oxygen/
ammonia/nitrogen 1/4.4/1.6/17.6 (molar ratios). Among the various systems
investigated, those giving the best performance were based on V/Mo/O and Ti/
Mo/O which gave quite similar performance. Other systems were active but not
selective to nitriles (i.e. Sn/Mo/O), or were neither active nor selective (i.e. Bi/Mo/
O, Sb/Mo/O, Sn/Sb/Fe/O). The C
6
unsaturated dinitriles (mucononitrile) included
the three isomers: cis - cis dicyanobutadiene, cis - trans dicyanobutadiene and trans -
trans dicyanobutadiene.
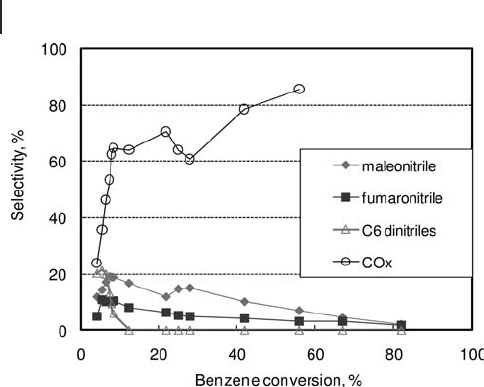
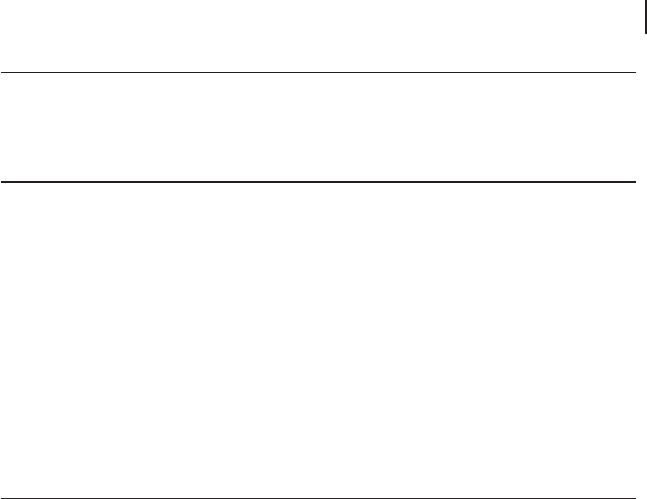
The data apparently indicate that the C
6
dinitrile was a primary product, and the
overall selectivity at low benzene conversion (less than 7%) was around 20%; the
selectivity to mucononitriles (the three isomers) rapidly declined when the benzene
conversion was increased above 7 – 8%, and fi nally became nil. The C
4
unsaturated
dinitriles, maleonitrile and fumaronitrile, reached a maximum selectivity of around
20% and 10% respectively, at conversions lower than 10%. It is not clear whether
they were primary or secondary products, because of the errors made in yield and
selectivity calculation for low benzene conversions (and also errors made in data
extrapolation from fi gures). It is worth noting that the C balance was much lower
than 100%. Therefore, it is likely that some products were not detected.
Table 20.7 A summary of catalytic performance reported in [129a] for
cyclohexane ammoxidation.
Catalyst
T ( ° C), τ (s),
feed
Cyclohexane
conversion (%)
Selectivity
to ADN (%)
Selectivity
to GN (%)
Selectivity
to SN (%)
Sb/Sn/O 2/1 450, 1.95,
a)
19.3 23.7 14.2 3.5
“ 550, 1.95,
a)
27.2 14.1 10.6 2.2
“ 450, 3.9,
a)
21.4 22.9 13.7 3.9
Sb/Sn/O 4/1 445, 2.0,
b)
12.4 18.7 18.3 5.1
Sb/Sn/O 1/2 470, 2.0,
b)
14.3 14.1 15.7 1.3
Sb/Sn/O 3/1 430, 1.5,
c)
19.5 21.7 12.4 5.1
Sb/U/O 2/1 435, 2.0,
b)
21.3 24.3 10.3 4.0
Sb/Ti/O 2/1 440, 2.0,
d)
24.2 19.7 13.5 5.9
a feed composition (mol%) cyclohexane/oxygen/ammonia 5/10/6.6 (balance N
2
).
b feed composition (mol%) cyclohexane/oxygen/ammonia 5/10/10 (balance N
2
).
c feed composition (mol%) cyclohexane/oxygen/ammonia 8.4/10/14 (balance N
2
).
d feed composition (mol%) cyclohexane/oxygen/ammonia 7/14.7/14 (balance N
2
).
GN: glutaronitrile; SN: succinonitrile; ADN: adiponitrile.
806 20 Catalytic Ammoxidation of Hydrocarbons on Mixed Oxides
The C
4
dinitriles were more stable than the C
6
dinitriles. Although their selectiv-
ity decreased with increasing benzene conversion, they were still present at high
benzene conversion. CO
x
formed mainly by decomposition of the nitriles, but a
primary contribution (benzene combustion) cannot be excluded. The authors also
mention a slight increase of the selectivity to C
6
dinitriles on increasing the reac-
tion temperature (giving 5% more when raising from 440 to 500 ° C).
The authors made a hypothesis about the mechanism. They assumed that
benzene undergoes H • abstraction to generate a mono - radical which then reacts
with an NH
2
i
fragment to generate aniline (however, the latter is not found among
the reaction products) and di - radicals. The ortho di - radical is transformed to o -
quinone diimine, the para to p - quinone diimine. The opening of the ring in the
former case generated the C
6
dinitriles, and in the latter case maleonitrile + 2CO
2
.
The meta di - radical is supposed to give CO
x
. In the case of benzene oxidation, the
same authors reported that only p - quinone and maleic anhydride were found as
the products (oxidation of the para di - radical), while o - quinone and muconic acid
(oxidation of the ortho di - radical) were not found.
Unger [131] investigated several catalysts for the ammoxidation of benzene
(Table 20.8 ). The report shows that the performance of catalysts was not much
different from that reported in Reference [128] . However, in the only example
where the conversion was reported, the selectivity to mucononitrile was 17% (for
a yield of 7%), at 40% conversion. In this case, therefore, the compound can be
saved from consecutive degradation. It is likely that one key factor in saving muco-
nonitrile from consecutive degradation is the reactor confi guration and catalytic
bed arrangement. This is also clearly demonstrated in Reference [128] .
Reference [132] also describes catalysts and methods for the ammoxidation of
benzene to mucononitrile. The performance seems to be greatly affected by the
type of reactor used and the reactor conditions.
Figure 20.12 Catalytic performance of V/Mo/O catalyst in benzene ammoxidation [128] .
20.5 Ammoxidation of Unconventional Molecules 807
Catalysts based on Fe/Sb/O, Sn/Sb/O and U/Sb/O were fairly active and selec-
tive to C
4
unsaturated dinitriles, but non - selective to mucononitrile. Catalysts made
of Bi/Mo/P/O, alumina - supported Mo/O and alumina - supported W/O had low
activities.
20.5.5
Ammoxidation of C
2
Hydrocarbons
Although industrial interest in the synthesis of acetonitrile directly from C
2
hydro-
carbons is currently limited, with acetonitrile being mainly produced as a by -
product in acrylonitrile production, there are a number of indications regarding
the future need of direct production of acetonitrile by C
2
hydrocarbon (ethane, in
particular) ammoxidation. In fact, acetonitrile is used as a solvent and also as an
intermediate in the production of many chemicals, ranging from pesticides to
perfumes. Production trends for acetonitrile generally follow those of acrylonitrile,
but the growth rate for acetonitrile use is higher than that of acrylonitrile. The four
Table 20.8 Summary of results reported in [131] for benzene ammoxidation.
Catalyst Benzene
conversion
(%)
T ( ° C), τ (s),
feed comp
Selectivity to
mucononitrile
(%)
Selectivity
to maleic
anhydride
(%)
Selectivity to
maleonitrile
(%)
Bi/Mo/P/O - BPO
4
15 – 20 450, 1.4,
a)
8 0
g)
1 8 0
Co/Mo/O - SiC ng 450, 1.4,
a)
9 5
g)
5 0
Co/Mo/O - Al
2
O
3
ng 450, 1.5,
b)
7 5
g)
2 0 0
V/P/O - Al
2
O
3
ng 450, 1.5,
c)
7 0
g)
(mainly
cis - cis)
30 0
V/P/O - Al
2
O
3
ng 450, – ,
d)
7 8
g)
1 2 1 0
Bi/Mo/P/O - BPO
4
ng 450, – ,
e)
9 9
g)
0 0
V/Mo/O - BPO
4
40 450, – ,
f)
1 7
h)
n g n g
a Feed: benzene/oxygen/ammonia/nitrogen 30/120/70/200.
b Feed: benzene/oxygen/ammonia/nitrogen 30/60/60/250.
c Feed: benzene/oxygen/ammonia/nitrogen 30/70/70/200.
d Feed: benzene/oxygen/ammonia/nitrogen 15/90/110/425.
e Feed: benzene/oxygen/ammonia/nitrogen 17/60/80/450.
f Feed: benzene/oxygen/ammonia/nitrogen 15/50/50/200.
g the selectivity is expressed only in reference to the condensable (high - boiling) products.
h this is a true selectivity.
ng = not given.

808 20 Catalytic Ammoxidation of Hydrocarbons on Mixed Oxides
main producers of acetonitrile in the United States are: Ineos, DuPont, J.T. Baker
Chemical and Sterling Chemicals.
Acetonitrile can be produced by catalytic ammoxidation of ethane and propane
over Nb - Sb mixed oxides supported on alumina, with selectivities to acetonitrile
of about 50 – 55% at alkane conversions of around 30% [133] . In both cases, CO
forms in approximately a 1 : 1 molar ratio with acetonitrile, owing to a parallel
reaction from a common intermediate. When feeding n - butane, the selectivity to
acetonitrile halves. Bondareva and coworkers [134] also studied ethane ammoxida-
tion over similar types of catalyst (V/Mo/Nb/O).
A different type of catalyst is constituted by Co ion - exchanged zeolites and
mesoporous materials (MCM - 49). Co - ZSM - 5 was found to be selective in ethane
ammoxidation [135] . A good correlation between the acidity and the catalytic activ-
ity was observed. The strength of ammonia bonding to the catalyst appears to have
a crucial effect on the activity of Co - ZSM - 5. Li and Armor [136] reported that
dealuminated zeolite was active for the ammoxidation of ethane to acetonitrile.
Pan and coworkers [137] instead studied ion - exchanged Co - Na - MCM - 49 and Co -
H - MCM - 49 materials for the same reaction, reporting that the presence of
ammonia in the feed considerably improved the selectivity and total yield of eth-
ylene and acetonitrile.
It should be mentioned fi nally that acetonitrile could be also prepared by the
catalytic ammoxidation of bioethanol over vanadium - alumino - phosphate ( VAPO )
catalysts [138] , which is an alternative starting from biomass - derived raw
materials.
20.5.6
Conclusions on the Ammoxidation of Unconventional Molecules
Analysis of the literature published in the fi eld of catalytic, gas - phase ammo-
xidation of ‘ unconventional ’ molecules allows the following conclusions to be
drawn:
1. The mechanism of catalytic ammoxidation generally accepted in literature for
activated methyl groups (allylic position in olefi ns, or side position in
alkylaromatics) includes the generation of a
−CH
2
i
radical as the fi rst, rate -
determining step. The data analyzed in the present report confi rm that the
generation of a radical species is also the key step for the ammoxidation of
other molecules, such as butenes, cyclohexane, cyclohexanol and n - hexane. The
fi rst step is less evident in the case of butadiene or benzene (and also in the
case of ethylene ammoxidation to acetonitrile). From benzene, the formation
of the Ph · radical is hypothesized in literature. By analogy, the formation of a
–
C
=
CH · species can be postulated from butadiene. Catalysts able to perform
this type of attack are a function of the type of molecule used, but in general
V oxide is one key component of the catalysts.
2. Once the radical species has been generated, this is followed by π - allyl complex
formation in the case of substrates in which the double bond is in the α - position
with respect to the radical. Insertion of N into the substrate generally occurs
20.5 Ammoxidation of Unconventional Molecules 809
by insertion of the NH
2 −
species from an M
=
NH species (isoelectronic with
the M
=
O species); M ions known to generate this moiety are Mo
6+
and Sb
5+
.
However, it seems that the mechanism may indeed include the insertion of an
NH
2
i
species. The formation of the latter does not require the presence of metal
ions able to generate the M
=
NH species. This may explain why with some
molecules investigated, the performance of catalysts that do not include either
Mo or Sb is at least comparable (and often better) than that of catalysts including
these elements. So, the key - point is the generation of radical species that can
either react to generate the nitrile precursors or react to yield the by - products.
It is worth mentioning that the same hypothesis of a radical mechanism and
reaction with NH
2
i
has also been made for ammoxidation of alkylaromatics
[97c] .
3. An alternative mechanism may include fi rst an O - insertion step, followed
by the transformation of the oxidized compound into the cyano - containing
compound. An example might be the oxidation of butadiene to maleic anhydride
followed by hydrolysis to the acid, the formation of the diamide and the
oxydehydrogenation to the dinitrile.
4. There are several contradictions in literature concerning the nature of the
products obtained. Table 20.9 tries to summarize the literature information,
with an indication of the best catalyst reported and of the best yield to nitriles.
The main discrepancy concerns the nature of nitriles obtained. In general, it
is possible to infer that starting from more reactive C
6
compounds (e.g.
cyclohexane), which may also undergo a relevant number of transformations,
Table 20.9 A summary of literature data on ‘ unconventional ’ ammoxidation reactions.
Reactant Major products Best catalyst Best
yield
(%)
Butadiene maleonitrile + fumaronitrile (crotonitrile) V/W/Cr/P/O - TiO
2
6 7
n - Butane maleonitrile + fumaronitrile (crotonitrile) V/W/Cr/P/O - TiO
2
2 6
Cyclohexanol adiponitrile + hexanenitrile (aniline, phenol) V/Sb/P/O - Al
2
O
3
7 2
a)
Cyclohexanone adiponitrile + hexanenitrile(aniline, phenol) V/Sb/P/O - Al
2
O
3
4 7
a)
Cyclohexane adiponitrile + glutaronitrile + succinonitrile
+ maleonitrile + fumaronitrile (benzene,
cyclohexene)
Ti/Sb/O 10
n - Hexane adiponitrile + hexanenitrile V/Sb/P/O - Al
2
O
3
8
a)
Benzene mucononitrile + maleonitrile + fumaronitrile V/Mo/O 9
a Reference [124] is very controversial.

810 20 Catalytic Ammoxidation of Hydrocarbons on Mixed Oxides
the formation of both saturated and unsaturated nitriles (mono and di) with
the number of C atoms ranging from 4 to 6 is possible. This is a further
indication of the radical nature of the mechanism of the reaction. No author
has reported the formation of C
1
, C
2
or C
3
nitriles (cyanhydric acid, acetonitrile
and acrylonitrile), but we believe that this is highly likely. When the reaction is
instead carried out over benzene, the number of nitriles that can form is
lower.
20.6
Use of Other Oxidants for Ammoxidation Reactions
There is very limited literature data on the possible use of other oxidants in ammo-
xidation reactions, because air (O
2
) is the preferable source from an economic
point of view. However, it may be interesting to cite direct propane ammoxidation
with N
2
O and O
2
over steam - activated Fe - silicalite zeolite [139] . Yields of acryloni-
trile and acetonitrile below 5% were obtained using N
2
O or O
2
as the oxidant. Co -
feeding N
2
O and O
2
boosts the performance of Fe - silicalite compared to the
individual oxidants, leading to acetonitrile yields of 14% and acrylonitrile yields of
11% (propane conversions of 40% and product selectivities of 25 – 30%). The ben-
efi cial effect of O
2
on the propane ammoxidation with N
2
O contrasts with other
N
2
O - mediated selective oxidations over iron - containing zeolites (e.g. hydroxylation
of benzene and oxidative dehydrogenation of propane), where a small amount of
O
2
in the feed dramatically reduces the selectivity to the desired product. It is
shown that the productivity of acrylonitrile, and especially acetonitrile, expressed
as mol(product) h
− 1
kg(cat)
− 1
, is signifi cantly higher over Fe - silicalite than over
active propane ammoxidation catalysts reported in the literature.
20.7
Conclusions
Catalytic vapor - phase ammoxidation on mixed oxides is an important class of
industrial processes. Propene ammoxidation to acrylonitrile is a well established
process for the synthesis of this widely used monomer and intermediate. Over the
40 years since its commercial introduction, the yield to acrylonitrile has nearly
doubled to over 80% with the fourth generation of catalysts. This is due to the
intensive research effort and understanding of the several factors underpinning
catalytic activity. Commercial catalysts contain over 20 elements, the presence of
all of which is necessary to optimize the catalytic behavior.
A new current challenge is the shift from propene to propane as feedstock. Since
the early 1990s it has been necessary to develop new generations of multi - compo-
nent catalysts to improve the performance. However, both technical and economic
conditions (the price differential between propane and propene) now exist for the
commercial introduction of direct propane to acrylonitrile processes. Initial exam-
