Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.


creates isolation of the remaining vanadium atoms to a suitable degree, preventing
combustion of the reactants and of the desired product.
Recently, an operando Raman technique was used to study the relationship
between surface changes during reaction and catalytic performance using VSbO
4
-
based catalysts [81] . It was observed that the active rutile VSbO
4
phase forms
during catalytic operation by reaction - induced interaction between surface vana-
dium and antimony oxide species.
20.4
Alkylaromatic Ammoxidation
Various aromatic nitriles such as benzonitrile, phthalonitrile, isophthalonitrile,
terephthalonitrile and nicotinonitrile have applications as chemical intermediates.
Nicotinonitrile can be hydrolyzed to nicotinoamide or nicotinic acid, which are
used in the synthesis of vitamin B. Isophthalonitrile is used in the production of
herbicides/fungicides. Phthalonitrile is an intermediate for phthalocyanine pig-
ments. In addition, substituted aromatic nitriles have applications in the synthesis
of several intermediates for applications as fi ne chemicals and in the life
sciences.
20.4.1
Alkylbenzenes and Substituted Alkylbenzenes
Various catalysts have been reported to be active in this class of reaction (Scheme
20.1 ): (i) vanadium supported on TiO
2
(preferably in the anatase form) or ZrO
2
and modifi ed by various dopants such as Cs, P, W; (ii) multi - component molyb-
date; (iii) doped vanadium antimonate; (iv) bulk V - P - O; (v) metal - containing (in
particular Cu and Mo) zeolites; (vi) supported heteropolyacids (PV
3
Mo
12
O
x
on
silica) and (vii) metal phosphates [82 – 91] . Other proposed catalysts are AgAlBO
x
and perovskite materials such as YBaCu
3
H
6
and metal fl uorides such as CeF
3
, AlF
3
and MgF
2
[92] . However, the best catalysts belong to groups (i), (iii) and (iv), even
though it should be noted that, depending on the specifi c substituents on the
aromatic, a different ranking of these catalysts can be observed.
Various reviews have discussed in detail the characteristics and performance
of these catalysts and the reaction mechanism in alkylaromatic ammoxidation
[93 – 95] . Over V
2
O
5
/Al
2
O
3
catalysts, the reaction mechanism proceeds via the
20.4 Alkylaromatic Ammoxidation 791
Scheme 20.1 Synthesis of aromatic nitriles by catalytic ammoxidation.

792 20 Catalytic Ammoxidation of Hydrocarbons on Mixed Oxides
reaction of surface ammonium ions with benzoate ions, which are detected as
surface species by IR spectroscopy [96] . Adsorbed benzylamine and benzaldehyde
species were identifi ed as reaction intermediates [97] , while a kinetic study by
Otamiri and Andersson [98] suggests that vanadium imido (V
=
NH) or hydroxyl-
amino (V
–
NHOH) species may play a role as nitrogen insertion sites on the cata-
lyst surface. On vanadium phosphate catalysts, in particular (VO)
2
P
2
O
7
, the
preferred role of a benzaldehyde - like species was also pointed out, whereas the
reaction pathway via benzamide or benzylamine intermediates was suggested to
be unlikely [99] .
Adsorbed ammonia can exist in three different kinds of nitrogen - containing
surface species such as a protonated cation NH
4
+
()
, and co - ordinately adsorbed
NH
3
and
–
NH
2
groups. In addition, vanadium imido or hydroxylamino could be
also present, especially upon interaction of ammonia with the catalyst at high
temperature. All these species may potentially act as N - insertion species, and could
be present and react on the catalyst surface during catalytic reaction. Therefore,
unique mechanistic conclusions are diffi cult to obtain because multiple reaction
pathways are clearly present, the relative reaction rates of which depend on the
reaction conditions. However, in general terms it may be proposed that owing to
the stronger chemisorption of the aromatic ring, the mechanism of reaction in
alkylaromatic ammoxidation is different from that observed in propene and
propane ammoxidation.
Besides selectivity to aromatic nitriles, the minimization of the direct ammonia
oxidation to N
2
side - reaction is a critical factor, because otherwise runaway condi-
tions may be possible. There is always competition between NH
3
oxidation and
ammoxidation on the catalyst surface. Furthermore, contact between ammonia
and the catalyst surface, particularly at high temperatures, causes a partial reduc-
tion of the oxide surface because of NH
3
oxidation to N
2
. Therefore, control of the
rate of unselective oxidation of ammonia to N
2
is an important factor in determin-
ing the selectivity of the nitrile product, because this side reaction limits the avail-
ability of the surface ammonia species that are necessary for nitrile synthesis.
Typical performance is a selectivity higher than 90% at a conversion in the 50 –
90% range, mainly depending on the type of substrate. Substituted pyridines yield
better selectivities at high conversion than the equivalent alkylaromatics. The
nature and position of the substituents in substituted alkylaromatics also play an
important role in determining selectivity and activity. The commercial application
of this technology is mainly hindered by the relatively small plant necessary for
these products as compared to full - scale processes. The further implementation
of the process of alkylaromatic catalytic ammoxidation would thus require the
development of multi - purpose small - size continuous plants using small fl uidized
bed - reactors (to better control temperature and allow easier substitution of the
catalyst).
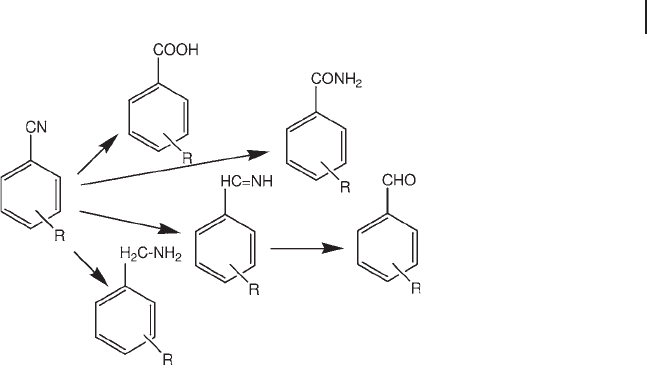
Aromatic nitriles are relatively stable under oxidation conditions even at higher
temperatures compared to the equivalent oxy products. Therefore these com-
pounds are often used as intermediates for subsequent hydrogenation or reduction
steps (Scheme 20.2 ).
The reactivity of an alkylbenzene in the ammoxidation reaction increases with
increasing size of the alkyl groups in the side chain. The rate of ammoxidation of
alkylbenzenes mainly depends on the chemical nature, size and structure of sub-
stituent [92a] . The ammoxidation of substituted toluenes such as 1,2 - ,1,3 - or 1,4 -
methyl - substituted toluenes can result in mono - or dinitriles [100] . There are a few
reports on shape - selective ammoxidation, one such example being the ammoxida-
tion of m - and p - xylenes over Cu - containing ZSM - 5 zeolites to their corresponding
mono - and dinitriles [87, 88] . Besides dialkyl - substitutions, higher methylated
benzenes (e.g. mesitylene) can also be ammoxidized. However, with increase in
the number of methyl groups the number of products will also increase. Further-
more, ammoxidations of substituted methylaromatics such as α - methylstyrene to
atroponitrile [101a] , and β - methylstyrene or allylbenzene to cinnamonitrile [101b]
have also been reported in the literature. In these cases also, processes have never
reached the commercial stage.
A commercially interesting area of application of alkylaromatic conversion is
that of ammoxidation of halogen - substituted toluenes. The activity and the nitrile
selectivity of the ammoxidations of substituted toluenes depend strongly on the
position of the substituent, because it infl uences the accessibility of the methyl
group and especially the strength of the chemisorption and the stabilization of
reaction intermediates. Electron - withdrawing substituents enhance the nitrile
yield, whereas electron - donating substituents cause an increased total oxidation.
Therefore, halogen - substituted toluenes are easily converted into nitriles [102] . The
conversion rate of p - halotoluenes was found to be nearly independent of the nature
of the halogen substituent, but the selectivity decreases in the sequence p - Cl > p -
Br
>>
p - I over V - P - O catalysts. The ammoxidation of the isomeric chlorotoluenes
shows a dependence of the position of substitution in conversion ( p
>>
o > m ) and
selectivity ( p > o > m .)
Scheme 20.2 Substituted aromatic nitriles as intermediates
for aromatic acids, amides, imines, aldehydes and amines.
Elaborated from [82a] .
20.4 Alkylaromatic Ammoxidation 793

794 20 Catalytic Ammoxidation of Hydrocarbons on Mixed Oxides
The reaction of dichloro - substituted toluenes is much more infl uenced by the
geometric position of the substituents. Closer proximity of the substituents to the
methyl group results in lower conversions and nitrile selectivities (2,6 - di - Cl < 2,5 -
di - Cl < 2,3 - di - Cl < 2,4 - di - Cl < 3,4 - di - Cl) [102b, 103] . 2,6 - Dichlorobenzonitrile is of
particular industrial importance for the production of herbicides and pesticides,
and for the preparation of special kinds of engineering plastics with high thermal
resistance. Halogen - substituted xylenes can also be ammoxidized to their corre-
sponding nitriles, for example 3,4,5,6 - tetrachlorophthalodinitrile is formed from
the corresponding chloro - substituted xylene in 45% yield [104] . Recently, especially
in China, there has been renewed interest in these reactions.
The ammoxidation of toluenes substituted with electron - donating groups, for
example hydroxy - and alkoxy - substituted toluenes is rather less selective. However,
under carefully chosen conditions (choice of the catalyst, feed composition, reac-
tion conditions) adequate yields of nitriles can be achieved. Stability of the catalyst
performances is typically an issue.
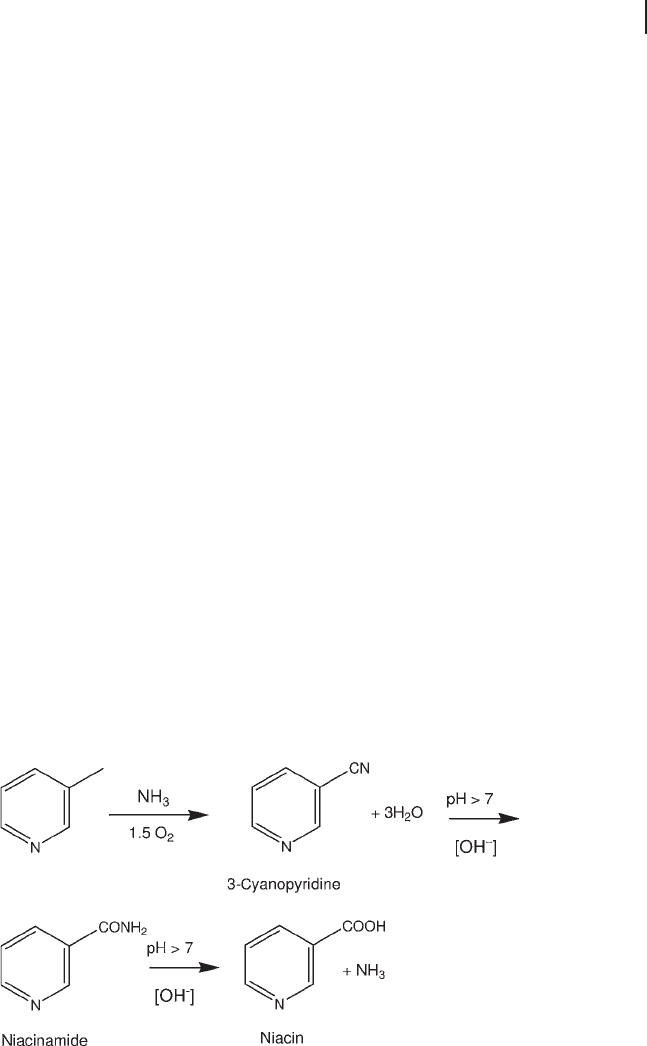
Aromatic imides are another type of product which can be synthesized by cata-
lytic ammoxidation. o - Xylene is converted over vanadium - titanium oxide catalysts
to tolunitrile and then, depending on catalyst composition and reaction conditions,
phthalimide or phthalonitrile can be selectively synthesized (Scheme 20.3 ) [94] .
The product distribution between nitrile and imide depends upon the reaction
conditions and the nature of the catalyst used [105] . The infl uence of various reac-
tion parameters such as (i) reaction temperature; (ii) water vapor addition in the
feed gas; (iii) NH
3
/ o - xylene mole ratio and (iv) space velocity, were studied [105] .
The removal of water vapor from the feed gas has a highly pronounced promo-
tional effect on the selectivity of phthalonitrile. The nitrile selectivity increased
from 2.1 to 34% at the expense of phthalimide (which decreased from 53 to 9%)
with the complete removal of water vapor in the reactant feed mixture. This obser-
vation gives an indication that phthalonitrile being formed in the reaction is
further hydrolyzed to phthalimide via the amide intermediate in presence of water
Scheme 20.3 Reaction network in o - xylene conversion to
phthalimide and phthalonitrile. Adapted from [84] .
vapor. Another interesting aspect is that titania - supported catalysts always gave
signifi cantly higher selectivities of phthalimide compared to phthalonitrile.
Phthalimide yields of up to 80% were reached on a VPO/TiO
2
catalyst [105] .
20.4.2
Alkylaromatics Containing Hetero - Groups
Another industrially relevant area of application of catalytic ammoxidation is the
synthesis of cyano - pyridine. A relevant example is the new green process for pro-
ducing nicotinic acid (niacin) developed by Lonza Ltd. Co - enzyme I ( nicotinamide -
adenine dinucleotide NAD ) and co - enzyme II ( nicotinamide - adenine dinucleotide
phosphate NADP ) are required by all living cells. They enable both the conversion
of carbohydrates into energy and the metabolism of proteins and fats. Both nico-
tinamide and nicotinic acid are building blocks for these co - enzymes. Since the
human body produces neither nicotinic acid nor the amide, it is dependent on
intake via foodstuffs. Old methods to produce nicotinic acid cause large production
of waste and use toxic reactants. For example, the classic method of preparing
nicotinic acid was by oxidizing nicotine with potassium dichromate, which pro-
duces over 9 tons of waste per ton of nicotinic acid, and Cr
VI
is carcinogenic and
environmentally threatening.
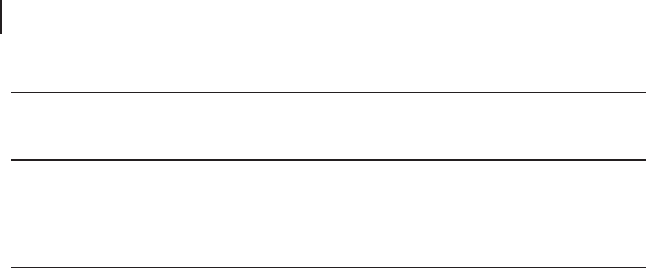
A new route to prepare nicotinic acid starts from 2 - methylglutaronitrile, a major
side - product in the adiponitrile process and, as such, a readily available starting -
material. It is easily hydrogenated to 2 - methylpentanediamine, which is then
condensed to methyl piperidine and dehydrogenated to 3 - picoline. The gas - phase
ammoxidation of the latter to cyanopyridine is followed by hydrolysis to either
nicotinamide or nicotinic acid (Scheme 20.4 ). The cyanopyridine route for the
production of nicotinic acid has the advantage of a signifi cantly better selectivity
with respect to the direct oxidation route from 3 - picoline owing to the easy decar-
Scheme 20.4 Ammoxidation of 3 - picoline and hydrolysis of
cyanopyridine to niacinamide (nicotinamide) and niacin
(nicotinic acid). Adapted from [106] .
20.4 Alkylaromatic Ammoxidation 795

796 20 Catalytic Ammoxidation of Hydrocarbons on Mixed Oxides
borboxylation and further oxidation of nicotinic acid during gas - phase oxidation.
On the other hand, the cyanopyridine route involves more reaction steps and
consumption of ammonia, even if it can be in part recycled.
In addition to ammoxidation of β - picoline (3 - picoline), the conversion of γ - pico-
line (4 - picoline) and α - picoline is also industrially relevant for the manufacture of
various interesting intermediates starting from the corresponding cyanopyridines.
Koei Chemical Company, for example, offers a range of cyanopyridine and cyano-
pyrazines produced by vapor - phase catalytic ammoxidation. The reaction and
related catalysts have been known for a long time. In general, V antimonate - based
catalysts active in propane ammoxidation also show quite good performance in
methylpyridine and m - xylene ammoxidation to cyanopyridine and isophthaloni-
trile, respectively [107] . Vanadium on a variety of supports, such as TiO
2
[108] , ceria
[109] and CeF
3
[110] is active in the reaction, with yields up to about 80%. More
recent proposed catalysts include (i) a vanadium - incorporated ammonium salt of
12 - molybdophosphoric acid supported on titania or zirconia [111] ; (ii) V
2
O
5
sup-
ported on zirconium phosphate [112] ; and (iii) metal phosphates ( α - and β - VOPO
4
)
[113] . Despite the large research effort, no signifi cant improvement in catalytic
performance with respect to earlier investigated catalysts has been achieved.
The reaction mechanism on a vanadia - titania catalyst has been re - investigated
using FTIR spectroscopy [114] , although the conclusions were not markedly dif-
ferent from earlier proposals. The interaction of methylpyrazine with the catalyst
surface involves a consecutive transformation of co - ordinatively bound methylpyr-
azine into oxygenated surface compounds, namely an aldehyde - like complex and
an asymmetric carboxylate. The main reaction product, amidopyrazine, is formed
through the interaction of the surface oxy - intermediates with adsorbed ammonia
species.
In conclusion, research and industrial interest in the ammoxidation of methyl-
pyridine and methylpyrazine is still active. This process could offer a greener
alternative to current processes in the production of intermediates for a variety of
specialty chemicals, especially in rapidly developing locations such as China where
a growing interest in the reaction has been noted [115, 116] .
20.4.3
Ammonolysis vs Ammoxidation
The ammoxidation of methylaromatic compounds has been shown to be a conve-
nient route to produce many nitriles required for further syntheses of side - chain
functionalized products. This method is versatile and can be carried out very easily
because of the stability, and undamaged desorption, of the nitriles formed under
severe gas - phase conditions. A series of interesting new patents on catalysts for
the ammoxidation of alkylaromatics have been issued, indicating that there is still
commercial interest in this reaction [117] .
Another approach developed by Lummus Co is used commercially for isophtha-
lonitrile production from m - xylene [118] . Oxidation is carried out in the absence
of gaseous oxygen (ammonolysis) using lattice oxygen from the catalyst, which is
regenerated in a separate vessel. The approach is analogous to the Dupont process

20.5 Ammoxidation of Unconventional Molecules 797
[119] for maleic anhydride production from n - butane. A fl uidized catalyst is circu-
lated continuously from a reaction zone to a regeneration zone where it is brought
into contact with the aromatic + ammonia feed and air, respectively.
The reactor effl uent contains the nitrile product, intermediates, unconverted
feedstock for recycling, ammonia, water vapor, nitrogen, carbon oxides and traces
of HCN. Separation is carried out by condensation to recover most of the organic
components, which are separated (depending on the specifi c substrate converted)
by distillation, extraction or crystallization. Off - gases are scrubbed with basic and
then acidic solutions to eliminate CO
2
and NH
3
, respectively, and then recycled.
The advantages of ammonolysis over ammoxidation are safer operation and
higher selectivities, but fi xed capital and running costs are higher and, further-
more, the process may be adapted only with diffi culty to multi - purpose plants that
require easy catalyst loading/unloading operations.
20.5
Ammoxidation of Unconventional Molecules
The literature on ammoxidation is very wide. The majority of papers and patents
published in this fi eld deal with propene and propane ammoxidation to acryloni-
trile, of isobutane and isobutene ammoxidation to methacrylonitrile and methyl-
aromatics and methylpyridines (picoline) ammoxidation to the corresponding
cyano - containing compounds, as discussed in the previous sections. A small
amount of literature deals with the ammoxidation of the following molecules:
• linear C
4
aliphatic hydrocarbons ( n - butane, butenes, butadiene) to maleonitrile
and fumaronitrile;
• cyclohexanol and cyclohexanone to adiponitrile;
• n - hexane and cyclohexane to adiponitrile;
• benzene to C
4
and C
6
unsaturated dinitriles.
• ethane ammoxidation to acetonitrile
Since these results are far less well known than those reported for C
3
and alkyl-
aromatic ammoxidation, they are discussed in a more detail below.
20.5.1
The Ammoxidation of C
4
Hydrocarbons
Furuoya and coworkers [120] investigated the ammoxidation of butenes, n - butane
and butadiene using different types of catalyst. The best results were obtained with
a TiO
2
- supported mixed active phase catalyst containing V, W, Cr and P, and using
butadiene as the substrate. The approximate composition of the catalyst giving the
best performance is (atomic ratios): V
1
W
1.1
Cr
1
P
22
Si
0.1
O
x − 87
TiO
2
. There is an equi-
molar amount of V, W and Cr, with a large excess of P. The formation of metal
phosphates during the calcination treatment is highly likely.
The highest yield reported to a mixture of fumaronitrile ( trans - 1,4 - dicyano - 2 -
butene) and maleonitrile ( cis - 1,4 - dicyano - 2 - butene) is 66.8% at 560 ° C (conversion
798 20 Catalytic Ammoxidation of Hydrocarbons on Mixed Oxides
is not reported, but extrapolation from the other results suggests it is likely total),
with a contact time of 1.2 s, a dilute butadiene stream (0.5 mol%) and a butadiene/
NH
3
/O
2
feed ratio of 1/5/20. By - products were acetic acid, HCN, CO, CO
2
and
traces of crotononitrile (these compounds were not detailed). The effects of impor-
tant reaction parameters are summarized in Table 20.3 .
The catalytic system appears to give interesting results. It is worth noting that
in order to obtain a good performance, it is necessary to combine the properties
of several elements. In fact, some of the corresponding bi - component systems
(V/P, W/P, W/Cr) although active in butadiene conversion, were less selective to
nitriles with the best yield being lower than 20%. However, from the data reported,
it is very diffi cult to identify the role of each component in the reaction. In general,
one might believe that V, W and Cr should play the same role of activation of the
hydrocarbon, while P should play the role of an enhancer of surface acidity. The
latter property is indeed very important, owing to the basic characteristics of both
the reactant and the products. However, from the data reported it appears that the
important step is not the activation of butadiene, but rather its transformation to
the nitriles. This means that the adsorption properties of the catalyst and the inser-
tion of N into the molecule are the key steps for the performance.
The considerations reported above suggest that the mechanism of reaction
might not be the same as the well known allylic insertion of a nucleophilic NH
2 −
species onto the activated hydrocarbon, to generate the precursor of the cyano
group. Indeed, none of the elements included in catalyst formulation is able to
produce the M
=
NH species (which is generated by Mo and Sb in propene ammo-
xidation catalysts).
In view of this, the mechanism might consist of the following steps:
• Co - ordination of butadiene to the metal (V or W), with electron transfer from the
diolefi n to the metal ion, and intramolecular rearrangement of the residual
double bond.
• Sequential attack of ammonia from the gas phase onto the activated hydrocarbon
at the C1 and C4 atoms, with formation of amino groups and then nitriles
(possibly via radical reactions).
• Desorption of the compound and re - oxidation of the reduced metal ion by O
2
.
Table 20.3 Summary of results for butadiene ammoxidation
with the catalyst V / W / C r / P / O - T i O
2
[120]
Feed composition
C
4
/NH
3
/air
T ( ° C)
τ (s)
Butadiene conversion
(%)
Yield to MN + FN
(%)
1/5/94 552 1.8 96.8 49.3
1/5/94 517 3.6 nr 49.1
0.5/2.5/97 560 1.2 nr (100%) 66.8
0.5/2.8/96.7 498 1.2 99.7 60.7
FN: fumaronitrile; MN: maleonitrile. The two compounds are reported
to form with a ratio close to 1. τ = contact time, nr = not reproted.

20.5 Ammoxidation of Unconventional Molecules 799
Therefore, the co - ordinating properties (intrinsic Lewis acidity) and the redox
properties (electron transfer) of the metal ion are important for the fi rst step, and
for the transformation of the adsorbed hydrocarbon into the nitrile rather than to
carbon oxides and HCN. It is also worth noting that when a conventional alkene
ammoxidation catalyst is used, such as Bi/Mo/O, the performance is much worse
(yield 4.5% at 450 ° C).
An alternative mechanism might include the following steps: (i) transformation
of butadiene to maleic anhydride (catalyzed by the V/P/O elements included in
the catalyst); (ii) hydrolysis of the anhydride to the diacid (owing to the presence
of water in the reaction environment, and catalyzed by the presence of P);
(iii) transformation of the diacid to the diamide by reaction with ammonia and
(iv) (oxy)dehydrogenation of the diamide to the dinitrile.
The best performance is obtained with very dilute hydrocarbon streams. This
might be due to the fact that an excessive concentration of butadiene might lead
to a high catalyst surface coverage and over - reduction, with a consequent modifi ca-
tion of the adsorption – activation properties. The high selectivity achieved at high
temperature indicates that the two cyan groups are very stable.
The catalyst based on V/W/Cr/P/O - TiO
2
also gives good performance with
n - butene and n - butane reactants (Table 20.4 ).
The catalyst showed comparable selectivities from the various C
4
hydrocarbons.
In the case of n - butane the yield was lower owing to the lower hydrocarbon conver-
sion, but the selectivity remained close to 50% (46%). In this case, vanadium
played the additional role of oxydehydrogenation of butane to butenes. The reactiv-
ity of butene was lower than that of butadiene (both were higher than that of
n - butane) which indicates that the mechanism requires the oxy - dehydrogenation
of butene to yield butadiene, which is the reactive intermediate that undergoes
ammoxidation.
A recent patent by DSM [121] claims catalysts very similar to those reported by
Furuoya [120] . Indeed, the catalyst preparation is the same as the one described
by Furuoya [120b] . The only difference concerns the addition of silica in relevant
amounts, in the form of silica sol. This is claimed to give better reproducibility in
performance for catalysts prepared in the powder form (by spray - drying). The best
performance reported is 95% conversion and 58% yield to nitriles (fumaronitrile +
maleonitrile + succinonitrile, the saturated dinitrile). The authors report the use
of a low O
2
concentration, with a feed composition of 0.50% butadiene, 2.50%
ammonia and 4.4% oxygen (the balance being nitrogen).
Table 20.4 Ammoxidation of C
4
hydrocarbons with the V / W / C r / P / O - T i O
2
system [120] .
Feed composition
C
4
/NH
3
/air
T ( ° C)
τ (s)
C
4
conversion
(%)
Yield to MN + FN
(%)
n - butene: 0.7/2.6/96.7 531 1.2 96.5 56.8
n - butane: 0.6/3.7/95.7 598 1.2 57.0 26.2
800 20 Catalytic Ammoxidation of Hydrocarbons on Mixed Oxides
Colleuille and coworkers [122] investigated catalysts for butadiene ammoxida-
tion which are similar to those also studied in the ammoxidation of benzene (see
below). Table 20.5 summarizes the results reported. The main products were
fumaronitrile and maleonitrile, crotonitrile (the unsaturated mononitrile, 1 - cyano-
propene, with the two trans and cis isomers) and CO
x
, with traces of acrylonitrile
and furan. The residence time used was very low; in this case the best performance
was obtained with a typical propene ammoxidation catalyst, made of Bi/Mo/P/O
under conditions of low butadiene conversion.
Very interesting new types of catalyst, based on Re - Sb - O compounds (SbRe
2
O
6
,
SbOReO
4
· 2H
2
O and Sb
4
Re
2
O
13
), were shown to be selective in the ammoxidation
of isobutylene to methacrylonitrile [123] . The Re - based catalysts were active for
methacrylonitrile synthesis with selectivities of 47.9 – 83.6% at 400 ° C, depending
on composition. Bulk Sb oxides (Sb
2
O
3
and Sb
2
O
4
) showed no activity. Among
these catalysts, SbRe
2
O
6
was most active and selective (83.6%) for methacrylonitrile
formation at 400 ° C. No structural change in the bulk and surface of SbRe
2
O
6
after
i - C
4
H
8
ammoxidation was evidenced by X - ray diffraction, X - ray photoelectron
spectroscopy, scanning electron microscopy or confocal laser Raman microspec-
troscopy. The good performance of SbRe
2
O
6
may be ascribed to its specifi c crystal
structure composed of alternate octahedral (Re
2
O
6
)
3 −
and (SbO)
+
layers. Pulse reac-
tion studies suggested that adsorbed NH
3
species on the SbRe
2
O
6
catalyst facili-
tated the adsorption and subsequent activation of isobutylene.
The same selective behavior in isobutane conversion to methacrylonitrile was
reported, although at very low conversion (selectivity to methacrylonitrile at an
isobutane conversion of 3 to 4% was about 45 to 50%), [123b] .
20.5.2
The Ammoxidation of Cyclohexanol and Cyclohexanone
In one of the most controversial papers published in this fi eld [124] , a catalyst
made of an alumina - supported V/P/Sb/O is reported to give very good perfor-
mance in the ammoxidation of cyclohexanol and cyclohexanone to adiponitrile.
Table 20.5 Summary of performance of catalysts reported in [122] for
butadiene ammoxidation.
Catalyst
T ( ° C), τ (s)
Butadiene
conversion
(%)
Selectivity to
FN + MN (%)
Selectivity to
crotonitrile (%)
Selectivity
to CO
x
(%)
V/Mo/O 450, 0.04 20 18 13 18
Sb/Fe/O 450, 0.04 16 9 11 ng
Bi/Mo/P/O 450, 0.04 9 51 11 18
Feed: butadiene/oxygen/ammonia/nitrogen/steam (molar ratios): 2/19/4/74/1.
FN: fumaronitrile; MN: maleonitrile.
