Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.


of synthesis and activation and the modality of doping. These catalysts are pre-
pared by hydrothermal synthesis, which results in nucleation and growth of M1
and M2 phases with well defi ned crystal morphologies.
Several articles on synthesis and characterization of Mo/V/M/O (M = Te, Sb,
Al) catalysts have been published recently [28] . The effects of metal oxide precursor
sources, synthesis conditions and post - synthesis treatments are subjects of current
studies. Asahi [29] has modifi ed the composition of the Mitsubishi catalyst, by
incorporation of Sb in place of Te in the M1 phase; this catalyst is more stable
than the original one, and hence provides longer catalyst lifetime.
Standard Oil (later BP America, now Ineos) developed V
–
Sb - based catalysts
which show a high selectivity to acrylonitrile especially when using high propane
concentration [30, 31] . Single and dual catalyst compositions were patented, with
the second catalyst having the function of converting the propene intermediate to
acrylonitrile [30] . Claimed catalyst compositions are mixed oxides such as (i)
Cr
a
Mo
b
Te
c
M
d
, where M is Mg, Ti, Sb, Fe, V, W, Cu, La, P, Ce or Nb; (ii) VSb
a
M
b
,
where M is one or more of Sn, Ti, Fe, Mn or Ga; (iii) Bi
a
Fe
b
Mo
c
A
d
B
e
, where A is
one or more alkali or alkaline metals, boron, W, Sn or La and B is one or more of
the elements Cr, Sb, Pb, P, Cu, Ni, Co, Mn or Mg; and (iv) Bi
a
FeMo
12
V
b
D
c
E
d
F
e
G
f
,
where D is one or more of the alkali metals; E is one or more of Mn, Cr, Cu, Zn,
Cd or La; F is one or more of P, As, Sb, F, Te, W, B, Sn, Pb or Se; and G is one
or more of Co, Ni or an alkaline earth metal. The gas feed composition was usually
propane/ammonia/oxygen/water in a 5/1/2/11 molar ratio, with excess alkane and
water used as a diluent.
A third catalytic system, based on vanadium aluminum oxynitrides ( VALON ),
has also been proposed [32] . Maximum acrylonitrile yield was about 30%, but with
an acrylonitrile productivity four times higher than V - Sb - W - Al - O catalysts and one
order of magnitude higher than Mo/V/Nb/Te/O catalysts [33] .
Other companies have studied and developed proprietary formulations, but in
general catalytic systems belong either to the antimonate family (Standard Oil,
Rhodia, BASF, Nitto, Monsanto) [34 – 38] or to the molybdate family (Mitsubishi,
Asahi).
All catalysts claimed are ‘ multi - functional ’ systems. Indeed, the formation of
acrylonitrile from propane occurs mainly via the intermediate formation of
propene, which is then transformed to acrylonitrile via the allylic intermediate. It
follows that the catalyst possesses different kinds of active site: one site that is able
to activate the paraffi n and oxidehydrogenates it to the olefi n, and one site that
(amm)oxidizes the adsorbed olefi nic intermediate. This second step must be very
rapid to limit, as much as possible, the desorption of the olefi n. In order to develop
an effective cooperation between the two sites, it is necessary to have systems in
which they are in close proximity. The multi - functionality is achieved either
through the combination of two different compounds (phase - cooperation), or
through the presence of different elements inside a single crystalline structure. In
antimonate - based systems, the cooperation between the metal antimonate (having
the rutile crystalline structure), responsible for propane oxidative dehydrogenation
to propene and propene activation, and antimony oxide, active in allylic ammoxida-
tion, is made more effi cient through the dispersion of the latter compound over
20.3 Propane Ammoxidation to Acrylonitrile 781
782 20 Catalytic Ammoxidation of Hydrocarbons on Mixed Oxides
the former one; in this case, an optimal ratio between the two compounds exists,
which makes the cooperation more effi cient. In metal molybdates (the Mitsubishi
catalyst), one single crystalline structure contains the elements required for the
activation and oxidative dehydrogenation of the hydrocarbon (vanadium), and
those active for the transformation of the olefi n and the allylic insertion of the
NH
2 −
species (molybdenum and either tellurium or antimony). Niobium has the
role of improving the structural stability of the compound.
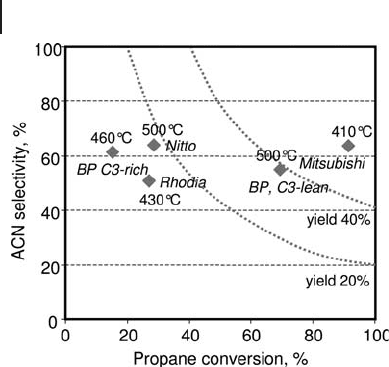
The selectivity to acrylonitrile versus propane conversion is plotted in Figure
20.7 for the best performances reported in patents issued by different companies,
and the temperature of reaction is also reported. In general, best per - pass yields
obtained with propane - rich conditions are between 20 and 25%. The yield to acry-
lonitrile reported is close to 60% at 87 – 89% propane conversion, with a selectivity
of 60 – 64% at 410 ° C, under propane - lean conditions, reported in Mitsubishi
patents with a catalyst made of mixed molybdate.
20.3.1
Mo/V/Te/Sb/(Nb)/O Catalysts
The elements constituting this catalyst are the basis for several crystalline struc-
tures, as illustrated in Figure 20.8 , which summarizes the main mixed oxides that
can be obtained by combination of V, Mo, Nb and Te(Sb). The fi gure shows the
various bi - component systems in a triangular diagram of composition, as well as
the areas which include tri - and multi - component systems, exhibiting superior
performance in ethane oxidation, and in propane oxidation and ammoxidation.
Many of the reported structures are related; for example orthorhombic
Mo
5 − x
(V,Nb)
x
O
14
solid solutions are isostructural with θ - Mo
5
O
14
and with several
bi - component Sb/Mo, Nb/Mo and Te/Mo mixed oxides. The M1 phase is
Figure 20.7 A summary of best performances in propane
ammoxidation claimed by different companies.
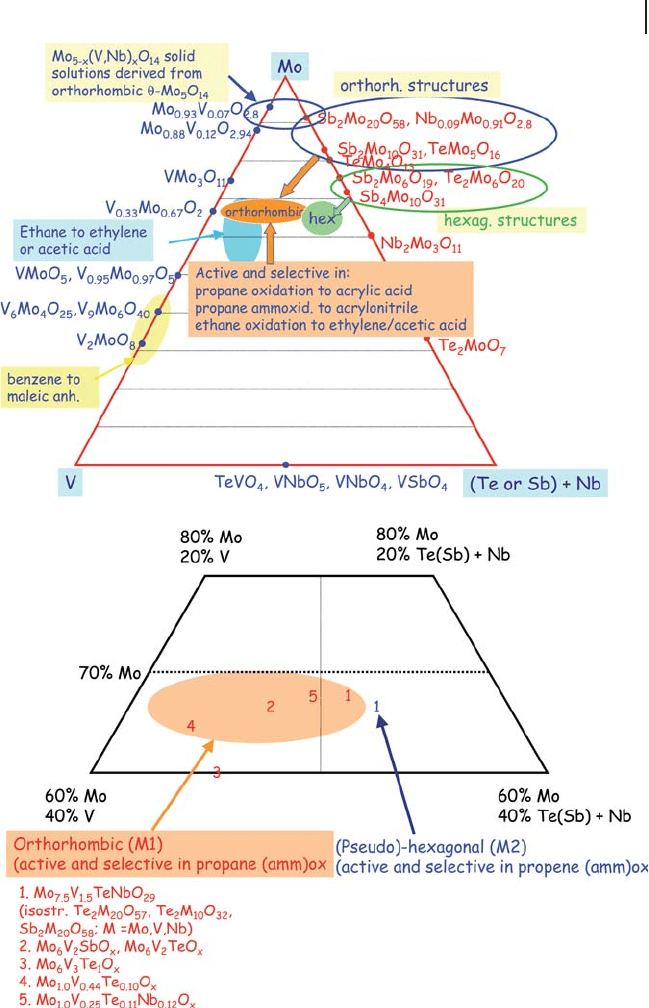
Figure 20.8 Top: Triangular diagram of composition for the
Mo/V/Nb(+Te or Sb)/O system, indicating the stoichiometry
of compounds which form, and the area of existence for
systems active and selective in alkanes oxidation and
ammoxidation. Bottom: detail of the top Figure.
20.3 Propane Ammoxidation to Acrylonitrile 783

784 20 Catalytic Ammoxidation of Hydrocarbons on Mixed Oxides
structurally derived from the latter oxides, and the M2 phase is related to other
Mo - leaner Te/Mo and Sb/Mo mixed oxides.
Two main preparation methods have been used to synthesize Mo/V/Te/(Nb)/O
catalysts active in propane ammoxidation: (a) the dry - up and (b) the hydrothermal
synthesis. The dry - up method involves mixing aqueous slurries of metal oxide
precursors followed by a gradual evaporation of the combined aqueous slurry.
Solvent evaporation leads to nucleation and growth of precursor metal oxide
phases, which require further heat treatment to obtain active catalysts.
For the synthesis of Mo/V/Te/Nb/O catalysts, ammonium paramolybdate,
vanadyl sulfate, ammonium niobium oxalate and tellurium oxide or telluric acid
are mixed as aqueous solutions or slurries and dried at about 150 ° C. These mixed
slurries are then calcined at 500 – 650 ° C in a N
2
stream [39] . In addition to the
dominant M1 and M2 phases, impurity phases, for example MoO
3
, Mo
6
V
9
O
40
and
Mo
3
Nb
2
O
11
, are also observed [28c] . It should be noted that Mo - V - Te - O catalysts
cannot be obtained by the dry - up method.
The preferable method for catalyst preparation is the application of hydrother-
mal conditions [28a, 40] , because they allow the syntheses of single - phase complex
metal oxides, such as binary Mo/V/O, ternary Mo/V/Te/O and quaternary Mo/V/
Te/Nb/O, which have various crystal structures [40g, 40h] and performance [41] .
Mo
1.0
V
0.44
Te
0.1
O
x
has an orthorhombic structure, Mo
1.0
V
0.81
Te
0.64
O
x
a hexagonal
structure and Mo
1.0
V
0.25
O
x
a tetragonal structure. All these basically have the same
layered structure, in which networks of corner - shared MO
6
(M = Mo, V) octahedra
form slabs and the octahedra between the slabs also share corner oxygen forming
linear infi nite chains of octahedra along the c - direction [41] . The structures differ
in the arrangement of octahedra within the slabs. In the orthorhombic structure,
the MO
6
(M = Mo, V) network in the slab is constructed with pentagonal and hex-
agonal rings of octahedra. The pentagonal bipyramidal sites may be occupied by
Mo and V. Te, as the third constituent element, is exclusively located in hexagonal
channels, whereas the heptagonal channels remain empty. The other structures
do not possess the heptagonal rings and showed much worse performance, indi-
cating that catalysts with the particular arrangement of MO
6
(M = Mo, V) octahedra
forming slabs with pentagonal, hexagonal and heptagonal rings in the (001) plane
of an orthorhombic structure is the active and selective phase in propane and
ethane conversion [41] . Mo and V are indispensable elements for the structure
formation. Te, located in the central position of the hexagonal ring, promoted the
conversion of intermediate propene. Introduced Nb occupied the same structural
position as V and the resulting catalyst clearly showed improved selectively.
Mo/V/Te/(Nb)/O catalysts owe their activity and selectivity towards propane
conversion to the essential presence of so - called M1 (orthorhombic) and M2
(pseudo - hexagonal) phases [42] . The M1 phase alone is capable of selective trans-
formation of propane [6b] , and the presence of the M2 phase is claimed to improve
the selectivity under more demanding conditions, such as high conversion [43] .
Signifi cantly improved acrylonitrile yields from propane above those obtained with
pure M1 phase were observed using as catalyst a physical mixture with a composi-
tion of 50 wt% M1/50 wt% M2 and a surface area ratio of about 4/1. The two phases

must be thoroughly ground and brought into intimate contact with each other on
a micro - /nano - scale for synergy to occur.
The orthorhombic and hexagonal structures of the M1 and M2 phases was fi rst
recognized by workers at CNRS and Elf Atochem [44] using electron microscopy.
They were even able to propose schematic structures for these materials, but these
models do not indicate which cation is found at a particular site and thus provide
no knowledge of cation co - ordination or valence state.
The M1 and M2 compounds are not simple phases. They have large unit cells
and do not form single crystals. Using combinatorial synthesis, it was possible to
fi nd ways to prepare the M1 and M2 phases separately [45] and subsequently
perform structural analysis combining neutron and X - ray powder diffraction data.
The result for the M1 phase shows that the material has a layer structure with two
sets of channels, one approximately hexagonal, the other roughly heptagonal. The
channels allow the accommodation of large metal cations. This is where the large
Te
4+
cation sits. The large channel provides access to the cation ’ s lone pair electrons,
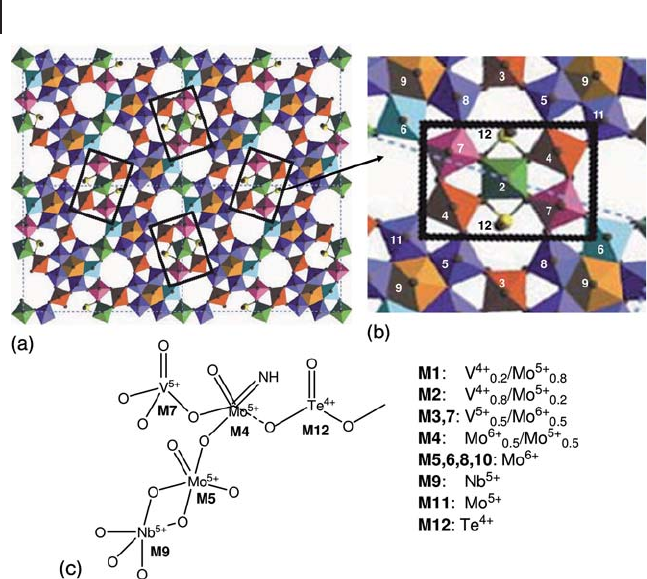
which is likely quite important in the ammoxidation reaction (Figure 20.9 ).
The MoVNbTeO
x
system for propane ammoxidation comprises three crystalline
phases: orthorhombic Mo
7.8
V
1.2
NbTe
0.94
O
28.9
( M1 ) ( Pba 2: a = 21.1337 Å ; b = 26.6440 Å ;
c = 4.01415 Å ; z = 4), pseudo - hexagonal Mo
4.67
V
1.33
Te
1.82
O
19.82
( M2 ) ( Pmm 2:
a = 12.6294 Å ; b = 7.29156 Å ; c = 4.02010 Å ; z = 4) and a trace of monoclinic
TeMo
5
O
16
( P 21/ C : a = 10.0349 Å ; b = 14.430 Å ; c = 8.1599 Å ; β = 90.781 ° ; z = 1)
[45c] . The catalytically active and selective centers reside on the surface of the basal
plane of the M1 phase and are composed of an assembly of fi ve metal oxide
octahedra (
2
032
5
068
6
VMo
..
++
,
1
062
4
038
5
VMo
..
++
,
1
05
6
05
5
Mo Mo
..
++
) and two tellurium – oxygen
sites 2
094
4
Te
.
+
()
, which are stabilized and structurally isolated from each other (site
isolation) by four Nb
5+
centers, each surrounded by fi ve molybdenum – oxygen
octahedra (Figure 20.8 b). The V
5+
surface sites, distinguished through their (V
5+
=
O ↔ V
4+
–
O) resonance structure, are the paraffi n activating sites capable of methy-
lene - H abstraction; the Te
4+
sites (lone pair of electrons) are required for the α - H
abstraction of the chemisorbed propene molecule, once formed; and the adjacent
Mo
6+
sites for NH insertion into the π - allylic surface intermediate. Within this
structure are contained all key catalytic elements needed to transform propane
directly to acrylonitrile, strategically arranged and within bonding distance of each
other, generating the active center of the M1 phase [45c] .
Under mild operating conditions, the M1 phase alone is enough to effectively
convert propane directly to acrylonitrile. Under demanding conditions symbiosis
between the M1 and M2 phases occurs, with the latter serving as a co - catalyst or
mop - up phase to the former, transforming unconverted, desorbed propene to
acrylonitrile. The M2 phase is incapable of propane activation, lacking V
5+
sites,
but is a good propene ammoxidation catalyst. A maximum acrylonitrile yield from
propane of 61.8% (86% conversion, 72% selectivity at 420 ° C) was achieved with a
nominal catalyst composition of Mo
0.6
V
0.187
Te
0.14
Nb
0.085
O
x
, identifi ed by a combina-
torial methodology, which is composed of 60% M1, 40% M2 and a trace of
TeMo
5
O
16
[45c] . The Te environment is consistent with that observed by Millet and
coworkers using EXAFS in mixed - phase samples [46] .
20.3 Propane Ammoxidation to Acrylonitrile 785
786 20 Catalytic Ammoxidation of Hydrocarbons on Mixed Oxides
20.3.2
Rutile - Type Antimonate Catalysts
Several papers have been published dealing with the study of metal antimonates
having the rutile structure. In particular, V/Sb/O and Fe/Sb/O systems have been
the subject of many investigations, aimed at understanding the nature of these
mixed oxides, and at the identifi cation of the active species.
Indeed, the preparation of a truly stoichiometric metal antimonate, MSbO
4
(M = metal), is a diffi cult task. The method of preparation employed affects the
nature of the catalysts prepared, but in general non - stoichiometry is a particular
feature of these systems [47 – 50] . The most striking case is the V/Sb/O system, for
which the composition V
0.92
Sb
0.92
O
4
( quasi - VSbO
4
) has been reported for the cata-
lyst with the V/Sb atomic ratio equal to 1/1 [49] . This cation - defi cient structure,
which has 0.04 cationic positions unoccupied per O
2 −
anion, contains Sb
5+
, while
Figure 20.9 (a) Unit cell of the M1(orthorhombic) phase
along the c axis with the regions considered to be the active
centers in propane ammoxidation shown in rectangular
boxes. (b) Enlarged view of the proposed active center in M1
phase (Mo
7.5
V
1.5
NbTeO
39
) in [001] projection with indication
(numbers) of the different atoms summarized below in the
scheme of the active site. (c) Elaborated from [6a, 45] .

vanadium is present both as V
4+
and as V
3+
. Electroneutrality is preserved for the
composition VVSbO
028
3
064
4
092
5
4.. .
++ +
[51] .
However, the ratio between V
3+
and V
4+
can vary depending on the method of
preparation; of particular importance are the atmosphere and temperature of
thermal treatment. The preparation procedure will affect the number of cationic
vacancies (for V
tot
/Sb ratio equal to 1.0) within the V
1 − y
Sb
1 − y
O
4
series, which is
theoretically comprised between the two limiting compounds VVSbO
1
3
0
4
1
5
4
++ +
(the
stoichiometric compound, with no cationic vacancies and only V
3+
) and
VV Sb O
0
3
089
4
089 4
++
..
(with only V
4+
, and the maximum of 0.22 vacant cation positions),
with y varying between 0 and 0.11 [47, 48, 52] . This series of catalysts is preferen-
tially formed by treatment in air. Therefore, the presence of cation vacancies is a
consequence of V having an oxidation state higher than 3+ which explains why
V/Sb/O systems give such large deviations from stoichiometry, at least under
conditions that are not the most stable from the thermodynamic point of view.
Another possibility is to have a ratio between Sb
5+
and V different from 1.0.
The V
0.9+ y
Sb
0.9
O
4
non - stoichiometric series has been described, with y theoretically
ranging from 0 to 0.2, and correspondingly with a degree of vacant cation posi-
tions ranging from 0.2 (in V
0.9
Sb
0.9
O
4
, with VV
01
3
08
4
..
++
, where the ratio V
tot
/Sb is 1.0)
to 0 (V
1.1
Sb
0.9
O
4
, with full occupancy of the cation sites, and with vanadium as
VV
09
3
02
4
..
++
) [53] . In practice, vacant positions in V
0.9
Sb
0.9
O
4
(0.2 per unit formula) are
progressively occupied by V ions, with a corresponding increase of the V/Sb ratio
and of the V
3+
/V
4+
ratio. The latter is mainly affected by the atmosphere of thermal
treatment, and therefore this parameter fi nally affects the extent of cation site
occupancy in the rutile structure.
Berry and coworkers [47] described a compound of composition VSb
1 − y
O
4 − 2 y
,
where y ≈ 0.1, which does not formally contain cation vacancies, and which is
obtained when the thermal treatment of the catalyst is carried out with oxygen -
impure nitrogen. In practice, it is assumed that the ratio between V
3+
/Sb
5+
is equal
to 1.0, and the solid solution is enriched with V
4+
ions (and obviously with addi-
tional O
2 −
ions); which represents a solid solution between VSbO
4
and VO
2
(both
rutile compounds). Only in the presence of oxygen - free nitrogen is it possible to
obtain monophasic compounds belonging to the series VSb
1 − y
O
4 − 1.5 y
. For y = 0.1
the compound V
1.039
Sb
0.935
O
4
is formed, with a small degree of cation site unoccu-
pancy (0.026 vacancies per unit formula), a V/Sb ratio higher than 1.0 and again
the presence of V
4+
.
In most cases described in the literature, the rutile V/Sb/O system has been
reported to possess either equimolar amounts of V and Sb, or an excess of V.
Moreover, Sb is also present exclusively as Sb
5+
. Excess Sb, if preparations are
carried out with an Sb/V atomic ratio higher than 2 and calcination is performed
in air, forms either α - Sb
2
O
4
or β - Sb
2
O
4
(the latter at calcination temperatures
higher than 800 ° C). Incorporation of small amounts of V in these Sb oxides is
also likely, lowering the temperature of the α β transformation. Antimony oxide
is also present in the form of amorphous oxide dispersed over the rutile, as sug-
gested by IR spectroscopy, for Sb/V ratios between 1 and 2 [54] .
20.3 Propane Ammoxidation to Acrylonitrile 787

788 20 Catalytic Ammoxidation of Hydrocarbons on Mixed Oxides
The case of FeSbO
4
is different. It is known that the structure can host an excess
of Sb. Berry and coworkers have demonstrated that on increasing the Sb/Fe ratio,
an increase in cell volume of the tetragonal structure occurs [55] . Moreover, instead
of a random distribution of iron and antimony in the cation sites, cation ordering
has been found, with development of a tri - rutile - like structure. The change from
the mono - to the tri - rutile - like ordering, from the Fe
3+
SbO
4
stoichiometric com-
pound to the Fe
2+
Sb
2
O
6
(with an Sb/Fe atomic ratio equal to 2) limiting compound
occurs with the reduction of Fe
3+
to Fe
2+
. Therefore, it is possible that the forma-
tion of Sb - enriched solid solutions preferentially occurs with metal ions which can
exist in stable 3+/2+ oxidation states. Indeed, the formation of very small amounts
of crystalline (VO)
2+
Sb
2
O
6
has sometimes been observed [54a] . Fe/Sb/O systems
also have been studied as catalysts for propane and propene ammoxidation [34b,
36, 56, 57] .
The degree of structural defects can be checked by means of IR spectroscopy.
Specifi cally, IR bands at 880 and 1015 cm
− 1
are attributed to the Sb
–
O
–
Sb and
Sb
–
O
–
V
4+
stretching vibrations involving co - ordinatively unsaturated O
2 −
ions
adjacent to cationic vacancies [58] . Cationic vacancies play an important role in the
catalytic performance of rutile - type mixed oxides [59 – 61] . For instance, it was
found that an increased concentration of cationic vacancies and isolated V
4+
species
in V/Sb/(Fe)/O systems, due to the introduction of increasing amounts of Fe in
the lattice, led to a proportionally higher activity [60c] . The formation of V
4+
in
Cr/V/Sb mixed oxides had the similar effect of increasing propane conversion
considerably [62] .
The IR band at around 820 – 840 cm
− 1
, also observed in all antimonates, is not
typical of M
–
O stretching vibrations in rutile single oxides, and is also not observed
in any antimony oxide [63] . This band has been attributed to the stretching vibra-
tion of Sb
5+
–
O
–
Sb
3+
[50, 64] , present on the surface of the rutile, and possibly
constituting an active site for the reaction. Indeed, this vibration could be due to
the presence of SbO
x
- rich domains in the outer zones of non - stoichiometric rutile
crystallites. Non - stoichiometric FeSbO
4
prepared by thermal treatment at 800 ° C
with excess antimony in the structure, is characterized by a tetragonal cell larger
than that of stoichiometric FeSbO
4
and by the presence of the IR band at 820 cm
− 1
.
Treatment of this compound at 1000 ° C leads to (i) a decrease in the cell volume,
which becomes similar to that of stoichiometric rutile and (ii) a strong decrease
in the intensity of the mentioned IR absorption band [65] . Therefore, it is possible
that the development to a well - crystallized, ordered rutile structure occurs as a
consequence of a cation redistribution in the lattice at high temperature, with the
disappearance of features related to Sb - enrichment.
The best performances among the variety of compositions which have been
claimed for V/Sb/O - based systems are summarized in Table 20.2 [66] . It can be
seen that Standard Oil (then BP and now Ineos) has claimed the same catalyst
type for both propane - lean conditions, with high conversion of the paraffi n, and
propane - rich conditions, with low propane conversion.
In this system, the main catalyst component is the quasi - VSbO
4
rutile, which
activates the paraffi n and transforms it into the olefi n - like adsorbed intermediate.
The intermediate may either desorb to yield propene, or be transformed to acry-
lonitrile over the SbO
x
‘ overlayers ’ , the amount of which is a function of the excess
Sb with respect to the rutile formula [22b] .
In systems developed by Rhodia [34, 37] the main component is SnO
2
(cassiter-
ite), which is inactive in propane ammoxidation, while it acts as the carrier for the
V/Sb/O and SbO
x
active components. Tin oxide is also able to disperse these
components, through the dissolution of V and Sb ions, thus yielding a multi - func-
tional catalyst where the two active compounds can effectively co - operate in the
reaction.
Other antimonates have been studied as catalysts for this reaction [23, 67 – 70] .
In the case of the Ga/Sb/O system [23, 67] , a decrease in the Ga/Sb ratio (from
Ga
1
Sb
1
O
4
to Ga
1
Sb
49
O
124
) leads to a progressive decrease in activity and increase
in selectivity to acrylonitrile. The best yield has been obtained at 550 ° C with a cata-
lyst having composition of Ga
1
Sb
49
O
124
, with 28.3% propane conversion and 35.3%
selectivity to acrylonitrile. The performance was improved by adding Ni, P and W
as dopants. Recently, V
5+
- doped Al
0.5
Ga
0.5
PO
4
was found to be active in propane
ammoxidation, although its performance was not outstanding [71] .
Rutile - type Cr antimonate, CrSbO
4
, is fairly active and selective in propane
ammoxidation [72] and adding V considerably improves the catalytic activity. The
(Cr + V) / Sb atomic ratio is the main compositional parameter affecting the nature
of these crystalline compounds [59a, 62, 73] . When almost equimolar ratios were
used (e.g. in Cr/V/Sb 1/1/1), the corresponding XRD pattern showed the presence
of a single, well - crystallized, rutile - type three - component CrVSbO
6
compound,
which in practice corresponds to an equimolar solid solution between CrSbO
4
and
VO
2
(both characterized by the rutile - type structure). In Cr/V/Sb/O samples with
atomic ratios of Cr/V/Sb 1/ x /1, the general formula Cr
1
V
x
Sb
1
O
4+2 x
was extrapo-
lated, with compositions ranging from CrSbO
4
to CrVSbO
6
. In these catalysts, V
was present mainly as V
4+
, and samples were extremely active in propane ammo-
xidation but poorly selective to acrylonitrile, leading to the predominant formation
of propene and carbon oxides. When samples were instead prepared with an
atomic ratio (Cr + V) / Sb < 1, V was present mainly in the rutile mixed oxide as a
V
3+
species. The main peculiarity of these Cr - based antimonates is their ability to
Table 20.2 Performance of some V / S b / O - based catalysts described in the literature.
Catalyst formula T ( ° C) C
3
/NH
3
/O
2
/H
2
O/inert
(mol%)
C
3
H
8
conversion
(%)
Selectivity
to AN (%)
Ref.
VSb
5
W
0.5
Te
0.5
Sn
0.5
O
x
–
SiO
2
500 6.5/13/12.9/19.4/48.4 68.8 56.7 [31a]
VSb
1.4
Sn
0.2
Ti
0.2
O
x
460 51/10.2/28.6/10.2/0 14.5 61.9 [29b]
VSb
1.4
Sn
0.2
Ti
0.1
O
x
480 6.4/7.7/18.6/0/67.3 40.3 47.5 [31h]
VSb
5
Bi
0.5
Fe
5
O
x
–
Al
2
O
3
440 7.5/15/15/20/42.5 39 77 [34d]
VSb
5
Sn
5
O
x
450 8/8/20/0/64 30 49 [37b]
20.3 Propane Ammoxidation to Acrylonitrile
789

790 20 Catalytic Ammoxidation of Hydrocarbons on Mixed Oxides
host excess Sb in the structure, thereby developing non - stoichiometric compounds,
especially in samples with lower V content. The formation of cationic vacancies
was evidenced by an intense Raman band at 880 – 920 cm
− 1
, especially in samples
having very low (Cr + V) / Sb ratio (i.e. a large excess of Sb) and higher relative
amounts of V (i.e. a Cr/V ratio close to 1).
Also with these systems, an excess of Sb with respect to the stoichiometric
requirement for the rutile formation is necessary, in order to reach good selectivity
to acrylonitrile and low selectivity to carbon oxides.
An important dopant for rutile - based mixed oxides is Nb oxide [59a] . The
Nb/Sb/O system is active in the ammoxidation of ethane to acetonitrile [74] , and
the combination of Bi/Mo/O and alumina - supported Nb
2
O
5
gives good perfor-
mance in the ammoxidation of isobutane to methacrylonitrile [75] . Nb is one
component of the V/Nb/Sb/O catalyst for propane ammoxidation developed by
Nitto Chemical Industries [35] . When used as the support for V/Sb mixed oxide,
Nb
2
O
5
formed new phases by reaction with V and Sb under catalytic reaction
conditions [76] . These phases, of unclear nature, affected catalytic performance
in propane ammoxidation. When Nb was instead added as a promoter for the
alumina - supported V/Sb/O system [77] , the interaction between the active com-
ponents V, Sb and Nb led to an improvement of catalytic performance with
respect to undoped V/Sb/O. However, the Nb
–
Sb interaction may also lead to
the development of SbNbO
4
, which is not active for propane ammoxidation, and,
in addition, removes Sb and Nb sites that would have been co - ordinated with V
to form effi cient V – Sb or V – Nb species. Nb also develops mixed (Cr)V/Nb/Sb
oxides with the rutile structure, and hence modifi es the properties of Sb cations
in allylic ammoxidation [59a] .
The incorporation of other elements, either trivalent or tetravalent, in the rutile
lattice may substantially improve the performance of V antimonate [21] . This is
the case for Al
3+
, Ti
4+
and W
4+
. In the Al - Sb - V
–
O system, the active phase was
identifi ed as having the composition Al
1 − x
SbV
x
O
4
with 0 < x < 0.5 [78] . (Al,V)SbO
4
can be described as a solid solution between rutile - type AlSbO
4
and quasi - VSbO
4
.
A yield to acrylonitrile of almost 40% has been reported for an Al - Sb - V - W oxide
catalyst, using feed ratios of propane, oxygen and ammonia that are stoichiometric
with respect to the formation of acrylonitrile [26b] . A comprehensive study of the
Al - Sb - V - W - O system has shown that the active phase in this system is Sb
0.9
V
0.9 − x
W
x
O
4
,
which is a solid solution between V
0.92
Sb
0.92
O
4
and rutile WO
2
. Titanium substitu-
tion in rutile V/Sb/O produced an improved selectivity to acrylonitrile [79] ; Ti
4+
replaced for both V
4+
and V
3+
/Sb
5+
pairs, forming solid solutions of continuous
composition.
It was demonstrated that the better catalytic properties of (Al,V)SbO
4
and of
quasi - Sb(V,W)O
4
compared with the pure rutile V/Sb/O phase could be rational-
ized in terms of the ‘ site - isolation ’ theory. According to this theory, which was
originally formulated by Callahan and Grasselli [6c, 80] , a catalyst that is selective
for partial oxidation can be developed by creating structural isolation of the active
site. Thus, the improvement of the selectivity to acrylonitrile by substitution of
some of the V atoms with Al or W can be explained by the fact that substitution
