Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.


Catalytic Ammoxidation of Hydrocarbons on Mixed Oxides
Fabrizio Cavani , Gabriele Centi , and Philippe Marion
771
Metal Oxide Catalysis. Edited by S. David Jackson and Justin S. J. Hargreaves
Copyright © 2009 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
ISBN: 978-3-527-31815-5
20
20.1
Introduction
Ammoxidation, sometimes also termed oxidative ammonolysis, describes the
process of catalytic oxidation of hydrocarbons (particularly alkenes, alkanes, alkyl -
aromatics and alkyl - pyridines) to organic nitriles in the presence of ammonia,
typically using mixed oxide catalysts:
RCH O NH RCN HO−−++→ +
323 2
15 3.
(20.1)
The most important example of this process is the synthesis of acrylonitrile from
propene. Acrylonitrile is a large - volume commodity chemical (within the top
twenty). The world ’ s production capacity for acrylonitrile was 6.14 million tons per
annum in 2005 and the output reached 5.24 million tons, an increase of 0.5% over
2004. The operating rate of production units was more than 85%, lower than the
operating rate of 90% in 2004. The drop in utilization of capacity has been higher
than the growth rate of the new capacity in recent years, and is essentially related
to the market situation and relatively old plant technology. There is a supply defi cit
of acrylonitrile in the world today, but profi tability in the acrylonitrile sector is still
low. The mean spread, that is the difference between the acrylonitrile price and the
raw material cost, between 2002 and 2006 was about $ 150/metric ton. Figure 20.1
reports the world acrylonitrile demand (by use) over the 1995 – 2006 period and
projection up to 2010 [1, 2] . Figure 20.1 also shows that acrylonitrile is a chemical
intermediate used mainly in acrylic fi bres, ABS ( acrylonitrile – butadiene – styrene ),
SAN ( styrene – acrylonitrile ) and NBR ( nitrile – butadiene – rubber ).
There is a mean annual increase in world demand of about 3%, driven mainly
by ABS/SAN resin and other applications. Acrylonitrile is also used to produce
adiponitrile (for manufacture of hexamethylenediamine used in Nylon - 6,6 fi bers
and resins) and acrylamide for water - treatment polymers. Approximately 52% of
the total EU production of acrylonitrile is used in production of fi bres, 15% in
production of ABS and SAN resins, 15% in the production of acrylamide and
adiponitrile, and 18% for other uses [2] .
772 20 Catalytic Ammoxidation of Hydrocarbons on Mixed Oxides
The global market for acrylonitrile is currently quite balanced. After a period of
oversupply in 2001, brought about by the start - up of two large production units,
many closures in Western countries offset capacity growth and brought the market
into better balance. In general, a shift of capacity from Western to Asian countries
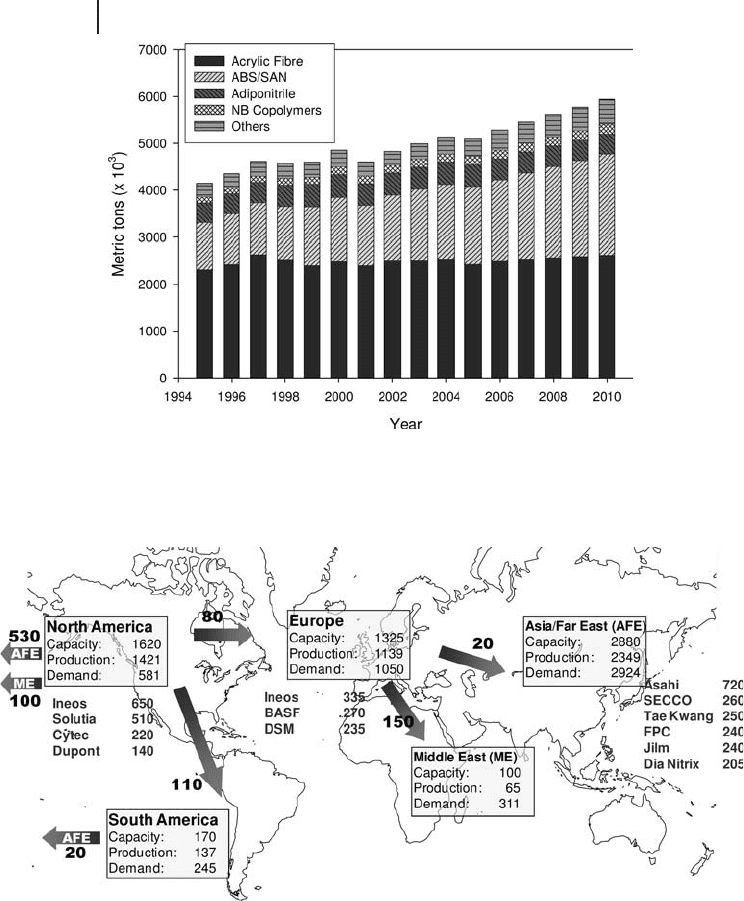
has occurred between 2004 and 2008. Figure 20.2 shows the world acrylonitrile
Figure 20.1 World acrylonitrile demand by derivative
(data estimated from 2006 to year 2010). Data from PCI
Acrylonitrile Ltd [1] .
Figure 20.2 World acrylonitrile trade fl ow by year 2005 and major
manufacturers ’ capacity. Data from PCI Acrylonitrile Ltd [1] .

trade fl ow in 2005 [1] with an indication also of the major manufacturers ’ capacity.
North America is still the largest acrylonitrile exporter and Asia is the major import
area. However, recent and future expansion of production in Asia will result in a
shrinking export market for North America. Costs of acrylonitrile production are
highly dependent upon propene and ammonia prices. Since 2006, prices for acry-
lonitrile have increased substantially (by more than one - third), mainly because of
rising feedstock costs. For this reason, and also because of weak demand, acrylo-
nitrile margins remain poor.
The cost of propene is mainly driven from competing areas of application (Table
20.1 ). Acrylonitrile accounts for only about 9% of propene demand and, because
of the expansion of the market for other applications, a further increase of propene
cost is expected. Global propene demand grew from 16.4 million tons in 1980 to
around 30 million tons in 1990 and about 52 million tons in 2000, with thus an
average annual growth of about 6% per year, which is much higher than the
average annual growth of demand for acrylonitrile. Expected demand for propene
by 2010 will be 81 million tons with demand growing faster than supply. Propene
supply/demand conditions and pricing are strongly dependent on refi nery produc-
tion and the supply/demand balance, operating rates and feedstock in the ethane
industry. Globally, more than 25% of the new crackers currently planned are based
on ethane, and therefore will produce little propene. Propene production by dehy-
drogenation of propane, although increasing, is still an expensive route. Therefore,
higher prices for propene are forecast in the next decade.
Owing to the increasing cost of propene, interest in using alternative cheaper
raw materials, particularly propane, is expanding rapidly. Asahi Kasei Chemicals
Corporation ( AKC ) has begun commercial operation of the world ’ s fi rst propane
process for acrylonitrile production, with the start - up and validation of a propane -
process line at the Ulsan Plant of its Korean subsidiary Tongsuh Petrochemical
Corporation. An existing 70 000 ton/year acrylonitrile line was modifi ed to use the
propane process. Propene, however, will still remain the main raw material to
manufacture acrylonitrile for several years. The most used process is Innovene ’ s
(Ineos) acrylonitrile technology, known in the industry as the SOHIO acrylonitrile
Table 20.1 Propene demand by application (year 2005).
Application Use (thousand metric tons) %
Polypropene 39 289 60
Acrylonitrile 5 684 9
Propene oxide 4 780 7
Cumene 3 629 6
2 - Ethylhexanol 2 424 4
Butanols 2 261 4
Isopropanol 1 350 2
Oligomers 1 327 2
Others 3 566 6
20.1 Introduction 773

774 20 Catalytic Ammoxidation of Hydrocarbons on Mixed Oxides
process, which is used in the manufacture of over 90% of the world ’ s acrylonitrile.
In 1996, this process, which involves the oxidation of propene and ammonia to
acrylonitrile in a fl uid - bed reactor, was designated the eleventh National Historic
Chemical Landmark by the American Chemical Society.
The SOHIO process involves the catalytic oxidative reaction of propene with
ammonia in the vapor phase [3] . Approximately stoichiometric amounts of propene
and ammonia, combined with air, are passed through the reactor in a single - pass
operation with a residence time of just a few seconds.
H C CHCH O NH H C CHCN H O
kJmol
23232 2
1
32 3
515
==++→ +
°=−
−
∆H
(20.2)
The reaction is highly exothermic and the heat of reaction is generally used to
make high - pressure steam, utilized downstream in separation and purifi cation
operations. The main useful by - products from the process are HCN (about 0.1 kg
per kg of acrylonitrile), which is used primarily in the manufacture of methyl
methacrylate, and acetonitrile (about 0.03 kg per kg of acrylonitrile), a common
industrial solvent. Smaller quantities of carbon oxides and nitrogen (from ammonia
combustion) are also obtained. Unreacted ammonia in the reactor effl uent is
neutralized with sulfuric acid. The resulting ammonium sulfate can be recovered
for use as a fertilizer.
Since the reactions generating by - products (carbon oxide formation and
ammonia combustion) are themselves highly exothermic, the total exothermicity
of the reaction is around 530 – 660 kJ mol
− 1
, making control of the reaction tempera-
ture critical.
Prior to 1960, acrylonitrile was produced commercially by processes based on
either ethylene oxide and hydrogen cyanide or acetylene and hydrogen cyanide. In
the late 1950s, Standard Oil (later Sohio and then BP) [4] and Distillers [5] devel-
oped a heterogeneous vapor - phase catalytic process for acrylonitrile by selective
oxidation of propene and ammonia using a catalyst based on bismuth, tin and
antimony salts of molybdic and phosphomolybdic acids and bismuth phospho-
tungstate. The Bi
9
PMo
12
O
52
/SiO
2
system was fi rst reported in 1955 for propene
oxidation. The system works either in a cyclic oxidant process mode, or as a
genuine redox catalyst. The fi rst attempt to use it in a cyclic oxidant mode was
abandoned after demonstration pilot operations, because about 200 kg of the solid
catalyst had to be circulated to produce 1 kg of acrolein [6] . However, the same
system operates successfully as a redox catalyst and was fi rst commercialized for
the production of acrolein from propene in 1957 (Degussa licensee), and subse-
quently commercialized internally in 1959 for the production of acrylonitrile from
propene (licensed worldwide).
Around the same time, but slightly later, Edison (later Montedison) was also
developing a similar process, but based on different catalysts (tellurium -
cerium oxides) [7] . Although at that time the performance of these catalysts were
equal or slightly superior to bismuth molybdenum oxides, the process was never
commercialized.

As a consequence of the introduction of the new catalytic ammoxidation process,
a steep rise in the production of acrylonitrile occurred starting from the year 1960
[6] . Further step changes were observed as a consequence of the introduction of
each new generation of more effective catalysts (the acrylonitrile yield has been
increased over the past 40 years from 50 to over 80%). The substitution of the inef-
fi cient and expensive process of IG Farben (HCN + acetylene) to produce acryloni-
trile by the highly effi cient and environmentally friendly SOHIO processs
(propene + ammonia + air) can be considered as one of the fi rst examples of ‘ green ’
sustainable chemistry. At the time of writing (2008), over 90% of the worldwide
production of acrylonitrile is made using the Sohio ammoxidation process [8] .
20.2
Propene Ammoxidation to Acrylonitrile
Commercial catalysts are made of multicomponent Bi - Fe - Ni - Co molybdates,
also containing several additives: Cr, Mg, Rb, K, Cs, P, B, Ce, Sb and Mn are
those more frequently mentioned in the several patents issued. The mixed
oxide is dispersed in silica (50 wt%) for fl uid - bed reactor applications. Owing
to the high exothermicity of the reaction and by - product formation, most of
the plants use a fl uidized - bed technology. A typical empirical composition is
(K,Cs)
0.1
(Ni,Mg,Mn)
7.5
(Fe,Cr)
2.3
Bi
0.5
Mo
12
O
x
/SiO
2
[9, 10] . The role of the various ele-
ments in the multi - component catalyst, in relation to the multi - step reaction
mechanism, are discussed in detail by Grasselli [10] . Production improvements
since 1980 have stemmed largely from the development of several generations of
increasingly more effi cient catalysts. In addition to mixed metal oxide catalysts
based on multimetal molybdates ( MMM ), other types of commercially used cata-
lysts were based on iron antimony oxide, uranium antimony oxide (in the past),
and tellurium molybdenum oxide. MMM - based catalysts are the most commonly
used nowadays.
Even though the literature on this topic has been mainly focussed on the struc-
tural and chemical - physical properties of Bi molybdates, and on the reactivity of
its various polymorphs (the α , β and γ structures), the industrial catalyst consists
of several divalent and trivalent metal molybdates. Indeed, Bi is present in minor
amounts in catalyst formulations. The two classes of molybdate contribute differ-
ently to catalytic performance: (1) trivalent Bi/Fe/Cr molybdates, having the Schee-
lite - type structure, contain the catalytically active elements while (2) divalent
Ni/Co/Fe/Mg molybdates, having the Wolframite - type structure, mainly enhance
the catalyst re - oxidation rate.
The catalyst also contains excess Mo with respect to the stoichiometric require-
ment for the formation of the molybdates. The excess Mo is considered important
for catalyst performance because:
• it provides a molecular bridge between the co - operating molybdates if their
crystalline match is not perfect;
20.2 Propene Ammoxidation to Acrylonitrile 775

776 20 Catalytic Ammoxidation of Hydrocarbons on Mixed Oxides
• it provides Mo to those phases partly depleted of it because of the redox
cycle, especially under more reducing conditions (Mo is lost in the form of
volatile MoO(OH)
2
). For instance, owing to the reduction of Fe
3+
to Fe
2+
, part of
the Mo is excluded from the molybdate, and is fi nally lost in volatile form.
Excess MoO
3
, when present in the catalyst composition from the beginning,
or when added during reaction, migrates along hydrated silica surfaces towards
Mo - lean catalytically active phases. Often, Mo - enriched make - up MMM catalyst
is preferentially added in place of MoO
3
during reactor operation. Fundamental
understanding of these complex catalysts and the surface - reaction mechanism
of propene ammoxidation has contributed substantially to the development of
new catalyst generations (currently at the fourth generation). Detailed mecha-
nisms for selective ammoxidation of propene over bismuth molybdate and anti-
monate catalysts have been proposed [11] . The rate - determining step is abstraction
of an α - hydrogen of propene by an oxygen in the catalyst to form a π - allyl
complex on the surface [11, 12] . Lattice oxygens from the catalyst participate in
further hydrogen abstraction, followed by oxygen insertion to produce acrolein
in the absence of ammonia, or nitrogen insertion to form acrylonitrile when
ammonia is present [13] . The oxygen removed from the catalyst in these steps
is replenished by gas - phase oxygen, which is incorporated into the catalyst struc-
ture at a surface site separate from the site of propene reaction. In the ammoxi-
dation reaction, ammonia is activated by an exchange with oxygen ions to form
isoelectronic NH
2 −
moieties, which are inserted into the allyl intermediate to
produce acrylonitrile.
The active site on the surface of a selective propene ammoxidation catalyst con-
tains three critical functionalities associated with the specifi c metal components
of the catalyst [14] : an α - H abstraction component such as Bi
3+
, Sb
3+
or Te
4+
; an
olefi n chemisorption and oxygen or nitrogen insertion component such as Mo
6+
or Sb
5+
; and a redox couple, such as Fe
2+
/Fe
3+
or Ce
3+
/Ce
4+
, to enhance transfer of
lattice oxygen between the bulk and surface of the catalyst. Moreover, in general,
it may be considered that the large improvement in the selectivity of these catalysts
derives from the application of seven principles ( ‘ seven pillars ’ ) [6] : lattice oxygen,
metal – oxygen bond strength, host structure, redox activity, multi - functionality of
active sites, site isolation and phase co - operation.
The process schematic of propene ammoxidation is shown in Figure 20.3 [15,
16] . A single - pass confi guration is possible, because over 95 wt% conversion can
occur with selectivities to acrylonitrile which nowadays are well above 80%. Air,
ammonia and propene are sent to a fl uidized - bed reactor, which may contain up
to 70 – 80 tons of catalyst in the form of fi ne spherical particles ( < 40 µ m in diameter)
highly resistant to mechanical attrition. The purity of reactants is very high ( > 90%
for propene and > 99.5% for ammonia). The ammonia to propene molar ratio is
in the range 1.05 to 1.2 and the O
2
/propene ratio in the range 1.9 – 2.1; typically,
oxygen - enriched air is used in industrial operation. Reaction temperature is in the
420 – 450 ° C interval, residence time is between 3 and 8 s, with a linear gas velocity
from 0.2 and 0.5 m s
− 1
and pressure between 1.5 and 3 atm. Since the rate of acry-
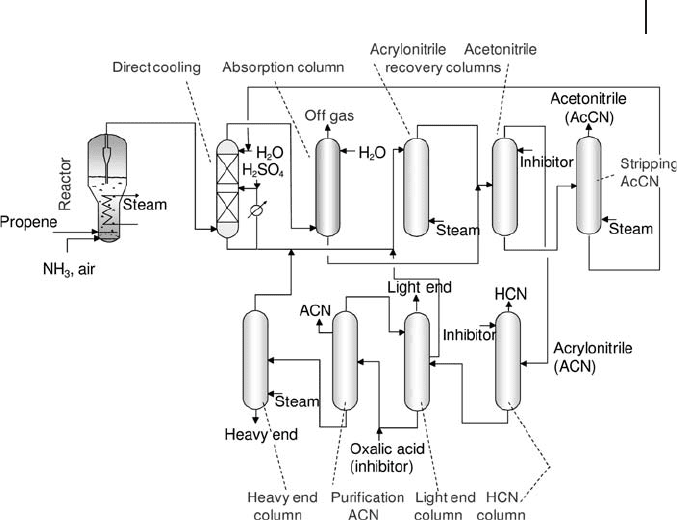
lonitrile synthesis is fi rst order with respect to propene, with higher orders being
shown towards the by - products, an increase in pressure has a negative effect on
selectivity. However, positive pressure is necessary to maintain the correct fl uidiza-
tion characteristics in the reactor.
The feed enters the reactor through separate inlets, in order to minimize homo-
geneous reactions and prevent local fl ammable compositions developing, although
owing to the effect of the solid in inhibiting radical propagation within the
fl uidized - bed reactor, it is possible to work inside the fl ammability region in
the catalytic bed. The fl uidized - bed reactor contains many steam coils to keep the
reaction temperature constant, avoid bubble coalescence and reduce gas back -
mixing. By proper design of these coils a virtually plug - fl ow regime may be possi-
ble. An expansion area to homogenize the gas velocity profi les, and thus minimize
the entrapment of solid particles, and cyclones, to recover the fi nest particles, are
integrated into the fl uidized - bed process.
The hot reactor effl uent is sent to a water absorber where it is quenched counter -
currently, while unreacted ammonia is neutralized with sulfuric acid. The result-
ing ammonium sulfate can be recovered and used as a fertilizer. The off - gases
containing N
2
, carbon oxides and unreacted hydrocarbon are sent to incineration.
The solution of acetonitrile/acrylonitrile is a heteroazeotrope. After settling, an
aqueous and an organic phase are obtained. The fi rst is refl uxed, while the latter,
rich in acrylonitrile and HCN, is sent to the purifi cation step. The aqueous aceto-
Figure 20.3 Schematic fl ow sheet of the SOHIO process of
propene ammoxidation to acrylonitrile. Adapted from [11] .
20.2 Propene Ammoxidation to Acrylonitrile 777

778 20 Catalytic Ammoxidation of Hydrocarbons on Mixed Oxides
nitrile recovered at the bottom is further concentrated by azeotropic distillation.
The crude acrylonitrile is fi rst purifi ed in two in - series columns to separate HCN
and impurities (acetone, acetaldehyde, propionaldehyde, acrolein) and fi nally
further purifi ed under vacuum. Final polymer grade must have a purity higher
than 99.4%. Disposal of the process impurities includes deep - well disposal (not
sustainable), wet air oxidation, ammonium sulfate separation, biological treatment
and incineration [17] .
20.3
Propane Ammoxidation to Acrylonitrile
In the current process technology for the manufacture of acrylonitrile, the propene
feedstock cost represents about 67% of the full (or fi xed) cost of production [18] .
The price differential between propene and propane depends on many factors, but
can be estimated to be on average $ 360 per ton during 2007 [19] , compared with
around $ 320 per ton in 2004 [18] . This price differential makes propane ammoxida-
tion competitive using the currently available catalysts. In fact some plants have
already started to be revamped. For example, at Asahi Kasei Corporation an exist-
ing 70 000 ton/year acrylonitrile line was modifi ed to use the propane process.
Production began in January 2007 [20a] ).
The reaction conditions claimed by the various companies are substantially dif-
ferent. As shown in Figure 20.4 , sometimes propane - rich conditions have been
claimed, as in earlier patents from Standard Oil, while in other cases propane - lean
conditions are preferred. In the former case, the conversion of propane is low, and
therefore recycling of the unconverted paraffi n becomes necessary. Mitsubishi was
the fi rst to claim the use of hydrocarbon - lean conditions, that is conditions in
which very high propane conversions can be reached. In more recent patents, BP
has claimed the use of analogous conditions, using oxygen - rich feed, and propane
as the limiting reactant. However, the lower activity of antimonates makes it neces-
sary to use temperatures which are approximately 50 ° C higher than those employed
with the Mitsubishi catalyst.
Figure 20.4 Feed composition in
propane ammoxidation claimed by
different companies.
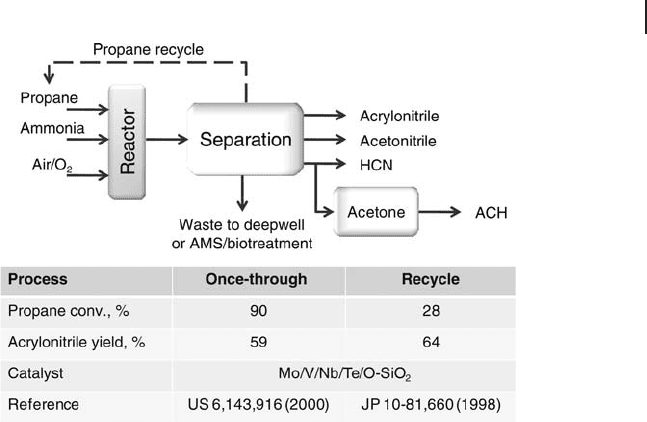
A once - through process is the preferred option [18a] (Figure 20.5 ). The catalyst
and process conditions are tailored to achieve as near total feedstock conversion
as possible. Products are separated and fresh feedstock is introduced into the
reactor on a continuous basis. In addition to providing the lowest capital cost for
a new - build plant, this also provides the best possibility for retrofi tting existing
plants by simply replacing catalyst and feedstock. This option is preferable to the
combination of an alkane dehydrogenation unit with an existing propene - to -
acrylonitrile process, owing to the signifi cant capital cost involved. The signifi cant
improvement in selectivity at high conversion using V/Mo/Nb/Te oxide catalysts,
compared to the earlier generation of catalysts based on V - Sb oxide, also makes
the once - through option preferable to the recycle option (Figure 20.5 ) [18a] .
Although selectivity is higher when operating at lower propane conversion, addi-
tional equipment is required for recovery and recycling of the unreacted feedstock.
Nevertheless, if selectivities to acrylonitrile could exceed 80% at a propane conver-
sion lower than about 30% using new generation catalysts, the recycle process
could become an interesting option. The recycling of unconverted propane is also
an option when high alkane conversion is reached, because this allows not only
the complete recovery of propane but also propene recycling (one side - product of
the reaction, which is a precursor of acrylonitrile), along with carbon dioxide,
which may act as a ballast for the reaction. The Mitsubishi process makes use of
the BOC - PSA technology for the removal of N
2
(both present in the feed and gen-
erated in the reactor by ammonia combustion), while the purge stream is inciner-
ated [20b] . One Mitsubishi patent claims the staged feeding of ammonia along the
catalytic bed [20c] ; this is a necessary option if the catalyst is very active in ammonia
Figure 20.5 Once - through and recycle processes for propane to
acrylonitrile (ACH: acetone cyanohydrins). Adapted from [18a] .
20.3 Propane Ammoxidation to Acrylonitrile 779
780 20 Catalytic Ammoxidation of Hydrocarbons on Mixed Oxides
combustion to N
2
, in order to avoid a lack of ammonia in the fi nal part of the
reactor, which would favor combustion of the hydrocarbons and the formation of
propene.
More complex process concepts have also been proposed which attempt to
optimize the processes. One option is the use of multiple reaction steps operating
at different reaction conditions [18a] . Three sequential process steps are used, each
operated with increasing conversion of propane. Product is separated after each
step and the unreacted propane is sent to the next reactor with fresh ammonia
and air/oxygen. An overall propane conversion of 95% with overall acrylonitrile
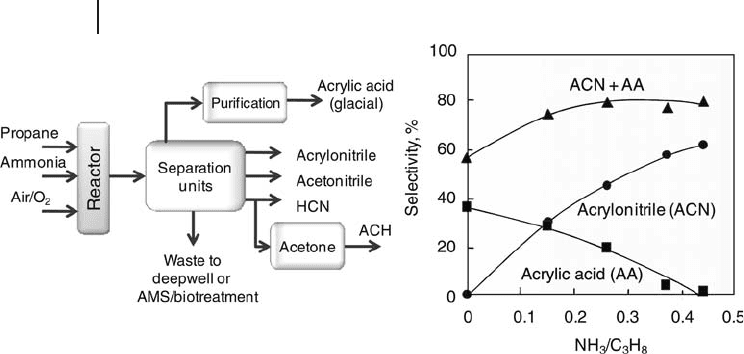
yield of 63% is achieved in three steps. Another approach is to increase the process
fl exibility in synthesizing multiple products. By changing the ratio of propane/
ammonia in the feed to the reactor, acrylonitrile and acrylic acid could be simul-
taneously produced with variable yields, depending on NH
3
/C
3
H
8
ratio (Figure
20.6 ). Even if the complexity of product purifi cation and recovery sections increases
the process costs, this option provides the opportunity to optimize the operation
of the plant based on changes in feedstock and product price and demand.
A number of reviews have discussed the catalyst and catalytic reaction chemistry
in propane ammoxidation [21 – 25] . So far, two main catalytic systems have been
proposed in the literature. They are based on V - antimonates with rutile structure
or multi - component molybdates (Mo/V/Nb/Te/O). Among the antimonates are
compositions of the Al/Sb/V/W/O system which give the highest acrylonitrile
yields reported (about 39%) [26] . The most promising catalyst discovered thus far
is the Mo/V/Nb/Te/O system, which has been shown to be both active and selec-
tive, giving acrylonitrile yields up to 62% [6b, 27] . Mitsubishi Kasei [27] originally
developed this catalyst composition which gives the highest yield of acrylonitrile
to date, although the long - term stability is still unclear. The optimal catalyst com-
position is MoV
0.3
Te
0.23
Nb
0
.
12
O
x
, which gives a yield of acrylonitrile of about 50%;
this can be further increased to nearly 60% by the addition of Sb, B or Ce. Key
characteristics of the catalyst are not only its composition, but also the procedure
Figure 20.6 Multiple product option with propane feedstock
(US Patent 6,166,241 (2000)). Adapted from [18a] .
