Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.


21.1 Introduction 821
morphology and/or particle size. This aspect has seldom been emphasized,
although a study of structure sensitivity in a base - catalyzed reaction is given by
Chizallet and coworkers [13] . In this work, a detailed study of the effect of surface
morphology upon the conversion of 2 - methylbut - 3 - yn - 2 - ol is presented and struc-
ture sensitivity is reported. Mg
3C
O
4C
and Mg
4C
O
3C
ion pairs, combining a strongly
basic O
2 −
site with a Lewis acidic site able to stabilize the resultant anion, were
shown to be active for the deprotonation of various probe molecules. High surface
area forms of MgO have been applied to Michael addition of malonates to enones
and a range of Michael addition and Knoevenagel condensation reactions by
Kantam and coworkers [14] and Xu and coworkers [15] respectively.
In considering their application, it is, of course, crucial to be able to characterise
a base catalyst both in terms of the strength of sites and their number. Unlike
acids, the distinction between Br ø nsted and Lewis sites does not have to be made
for bases. Historically, as for acid catalysts, base site strengths have been measured
by application of indicator methods. This procedure relies upon the observation
of color changes of indicators of known strength. Considering the general Br ø nsted
acidic indicator A
–
H, and the base catalyst B, when the strength of the base is
high enough the following process can occur:
AHB A BH−−+→ +
−+
A Hammett base scale, H
−
, can then be defi ned:
HK
−
−
=+
[]
[]
()
pAAH
a
log
where p K
a
refers to the acid strength of the indicator. Consequently, by applying
a range of indicators of varying strength, the base site strength characteristics
of a solid can be determined. A selection of indicators of different acid strength is
given in Table 21.1 . Indicators are generally applied as solutions in non - polar sol-
vents such as benzene and isooctane. As for the case of acidity determination, the
determination of base strengths by indicator methods is subject to severe limita-
tions. Using the naked eye, color changes only become perceptible when circa 10%
Table 21.1 A selection of indicators used to probe base strength. (Adapted from [3] ).
Indicators Acid form color Base form color p K
a
Bromothymol blue yellow green 7.2
2,4,6 - Trinitroaniline yellow reddish - orange 12.2
2,4 - Dinitroaniline yellow violet 15.0
4 - Chloro - 2 - nitroaniline yellow orange 17.2
4 - Nitroaniline yellow orange 18.4
4 - Chloroaniline colorless pink 26.5
a)
a Estimated value.

822 21 Base Catalysis with Metal Oxides
of the adsorbed layer of indicator is adsorbed, giving a limiting accuracy, and hence
UV - Visible spectroscopic methods would be preferred. Questions can also be
raised concerning equilibration time effects, solvent effects, and color changes
induced simply by adsorption. Consequently, although the application of indicator
methods is still common in the literature, it is generally backed up by the use
of additional methods, many of which also give site strength distributions, as
described below.
Despite the fact that the additional methods do not generate H
0
values directly
as such, base strength is most generally described and classifi ed in terms of H
0
value. For example, “ superbases ” are defi ned as systems which contain sites with
H
0
≥ 26. Recently, Kelly and King [16] have defi ned the minimum base strength
requirements for different classes of reaction. The scale is reproduced in Table
21.2 . It can be seen that the minimum site strength requirement for the base -
catalyzed heterolytic activation of propane would have a p K
a
of 42. To date, few
solid bases have been identifi ed as possessing such high strength, although Na/
NaOH/Al
2
O
3
is reported to be the strongest known superbase applied commer-
cially, with a value of 37 [16] . Such materials would, of course, be extremely sus-
ceptible to poisoning.
If indicator methods are used, site distributions can be determined by UV -
Visible spectroscopic procedures. Historically, a common way of determining
distributions was via titration methods, although these are now less widespread.
Examination of the reaction pathways of probe molecules has also been applied
to the characterization of basicity. In this method, a suitable probe molecule is
selected with the intention that base sites will convert it uniquely to a given
product. The conversion of isopropanol has been widely applied in this context,
Table 21.2 Base strength required to remove a proton from a
R
1
–
CH
2
–
R
2
reactant molecule. (Adapted from [16] ).
R
1
R
2
p K
a
Base required
CH
3
C H
3
42 Superbase
CH
3
CH
=
CH
2
35.5 Superbase
C
6
H
5
H 35 Superbase
C
6
H
5
C
6
H
5
33 Superbase
CH
3
CN 25 Strong base
CH
3
COOR 24.5 Strong base
CH
3
COCH
3
20 Strong base
CH
3
COH 19.7 Strong base
COOR COOR 11.5 Medium base
CN CN 11.2 Medium base
CH
3
N O
2
10.6 Medium base
COR COR 9 Medium base
COH COH 5 Mild base
NO
2
N O
2
3.6 Mild base
21.1 Introduction 823
for example Reference [17] and references therein, and it is generally considered
that base sites will yield acetone via catalyzed dehydrogenation, whereas acid sites
would yield propene via catalyzed dehydration. Whilst this type of test is normally
used in a qualitative way – that is, the production of acetone demonstrating the
presence of base sites – in principle careful determination of the kinetics may yield
parameters that relate to base site strength (activation energies) and site density
(pre - exponential factors.) Within the literature, a whole range of different probe
molecules have been applied. Among the most popular has been methanol [18] .
A potential pitfall with this method is the possible existence of alternative non -
base - catalyzed pathways to yield the signature product, and consideration must
also be given to the accessibility of surface sites to the probe molecule. In the
former context, attention is drawn to the case of solid superacids, where sugges-
tions of bimolecular and also one - electron oxidation pathways, as opposed to acid -
catalyzed unimolecular isomerization pathways, have been raised, throwing into
doubt the possibility that some solids are superacidic [19] .
Providing the mode of adsorption is known, namely that it is truly an acid – base
interaction, thermal methods are useful in quantifying the number and base
strength of sites. Perhaps the simplest method is the thermal desorption of a probe
molecule. Most commonly CO
2
has been used in this respect and a sample tem-
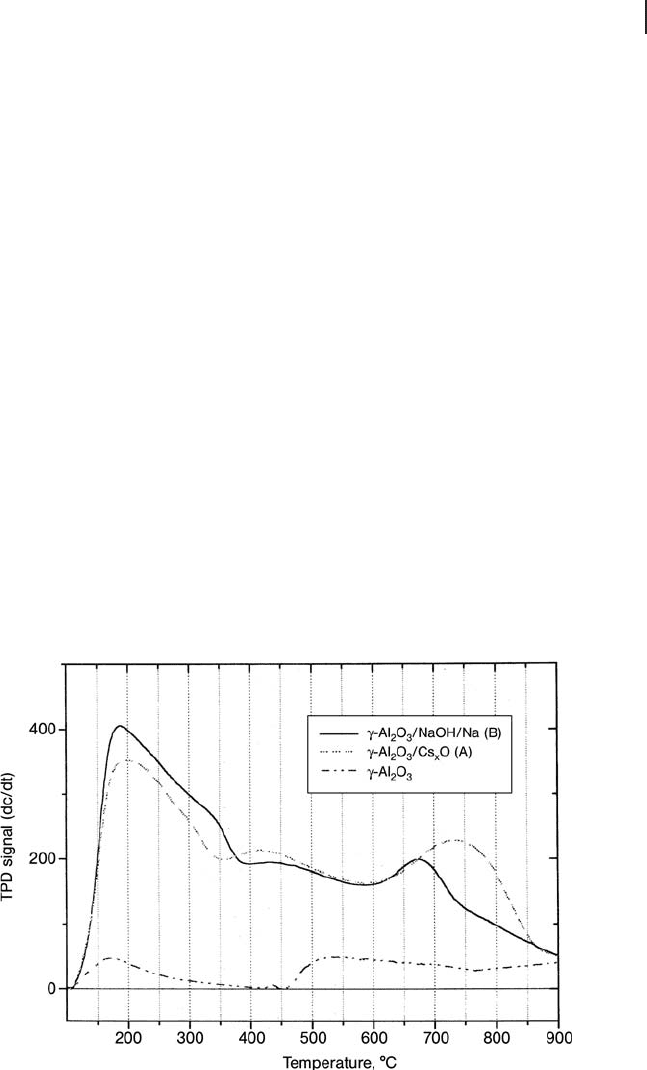
perature - programmed desorption ( TPD ) profi le is shown in Figure 21.3 [20] .
Again, it may be possible to derive information concerning the number and
strength of sites from this method, although appropriate caution must be applied.
For example, in one study [21] , it was shown that the CO
2
desorption temperature
Figure 21.3 C O
2
TPD profi les on selected base catalysts.
(Reproduced from [20] with permission).
824 21 Base Catalysis with Metal Oxides
matched the decomposition temperature of the bulk metal hydrogencarbonate.
The activation energy of desorption can be determined by investigation of the
infl uence of temperature ramp rate on the temperature of the desorption maxima
of profi les. In this method it is important that the adsorbate molecules do not
modify the surface, and it is prudent to check that they desorb intact (i.e. that
the true surface acid - based interaction is being probed and not a temperature -
programmed reaction.) Again, in this respect, parallels can be drawn with the lit-
erature relating to the determination of acid site strength. Even with a molecule
as simple as CO
2
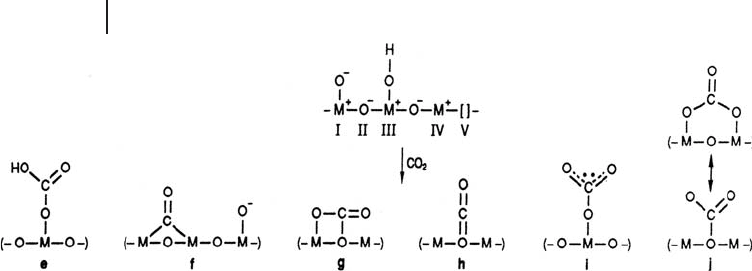
, a variety of different interactions could be envisaged as shown
in Figure 21.4 [22] .
For a selected range of heterogeneous bases, Martin and Duprez [23] have
demonstrated that there is generally a good level of agreement between the
determination of base strength via the cyclohexanol probe reaction and CO
2
chemisorption.
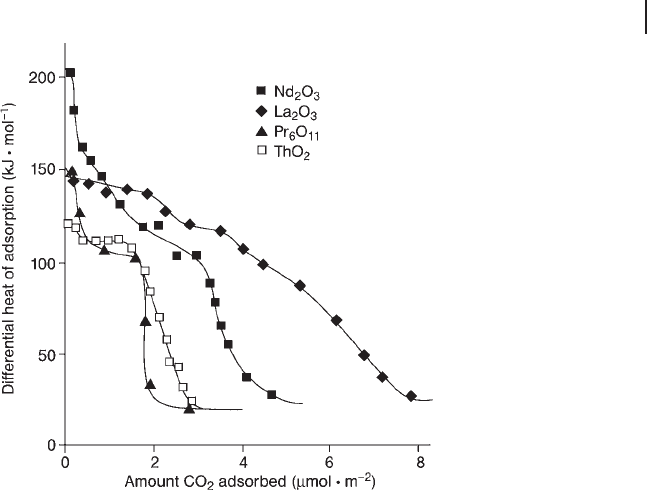
Although more complex experimentally, the application of microcalorimetry
allows the determination of both base site strength and site distribution. In this
method, the heat of adsorption of an acidic probe molecule, often CO
2
, is measured
directly as a function of surface coverage. An example of this type of measurement
is given in Figure 21.5 , which has been taken from the work of Auroux and
Gervasini [22] . Again, it is prudent to verify the nature of the interaction via spec-
troscopic observation and, as for TPD, attention must be paid to aspects such as
sample pre - treatment which can, for example, dramatically alter the degree of
surface hydration and therefore the nature of interaction with the probe molecule.
In general, the combination of thermal methods and spectroscopy, in particular
Fourier - transform infrared FTIR spectroscopy, represents a very powerful tool for
the characterization of surface acid - base characteristics.
Spectroscopic methods have been used in isolation for the determination of base
characteristics. A number of studies, for example Reference [24] , have correlated
the O 1s binding energy measured by X - ray photoelectron spectroscopy ( XPS ) to
the determination of base site strength. It has been proposed that decreasing
binding energy relates to an increased ability for electron pair donation and hence
Figure 21.4 Different interactions of CO
2
with a metal oxide
surface. (Reproduced from [22] with permission).
stronger basicity. However, whilst such relationships have been established
between materials of similar type, application to materials of different type must
be treated with caution. In addition, it is generally the case that catalysts exhibit a
range of differing site strengths and therefore spectral deconvolution is required.
An example of the application of this type of method to solid “ superbase ” catalysts
prepared from γ - Al
2
O
3
is given in work by Tanaka and coworkers [25] . Table 21.3
presents the corresponding binding energies for the materials studied. N 1s XPS
spectra of adsorbed pyrrole, a probe molecule, have also been applied to the mea-
surement of basicity with a degree of success [2] .
Pyrrole has also been applied as a probe molecule in FTIR specroscopic studies.
Upon interaction with a base site, the N
–
H stretching vibration is found to shift
to lower wavenumber and in alkali metal - exchanged zeolites this behavior has
been found to correlate with both N 1s XPS data and the negative charge calculated
from Sanderson electronegativities [4, 26] .
21.2
Catalysts and Catalytic Processes
In this section, we briefl y discuss some of the salient points concerning various
different types of solid base catalyst. This section is not exhaustive and is designed
Figure 21.5 Differential heats of adsorption as a function of
CO
2
coverage on various rare earth metal oxides.
(Reproduced from [22] with permission).
21.2 Catalysts and Catalytic Processes 825

826 21 Base Catalysis with Metal Oxides
to draw attention to some aspects of various classes of base, rather than providing
detailed discussion of individual materials.
21.2.1
Alkali Metal Oxides
Alkali metal oxides have principally been studied in their supported form, most
notably as catalyst modifi ers. In these cases, the basic properties are often the key
to the modifi er action yet the potential for a direct base catalysis role is rarely
considered. The literature concerning the use of alkali metal oxides as base cata-
lysts is less extensive. The base strength increases down the group with Li
2
O <
Na
2
O < K
2
O < Cs
2
O, hence many of the processes use cesium as the preferred base
to optimize the basic strength of the catalyst. Production of supported alkali metal
oxides usually involves impregnation with a simple alkali metal salt, typically the
nitrate, followed by calcination at high temperatures, for example Reference [27] .
It is rare for any characterization to be performed that confi rms that it is indeed
the oxide that is formed. In the presence of a hydroxylated support surface it is
likely that more than a single species is formed, namely a combination of oxide
and hydroxide. Indeed, from the study by Canning and coworkers [21] , in which
the alkali metal hydrogencarbonate was formed from carbon dioxide adsorption,
one can postulate that a surface hydroxide was present. However, for this review
we will consider that, unless there is specifi c evidence to the contrary, the material
produced after high temperature calcination will be principally oxide and hence
will fall within the scope of this discussion. This need for high temperature activa-
tion to produce the oxide, and the sensitivity of the oxide to poisons such as water
and carbon dioxide, could potentially limit the industrial applicability of these
materials. Nevertheless, supported alkali metal oxide catalysts are being actively
Table 21.3 Observed O 1s binding energies of a selection base
catalysts. (Adapted from [25] ).
Sample O 1s (eV)
γ - Al
2
O
3
531.1
NaOH/ γ - Al
2
O
3
530.3
Na/NaOH/ γ - Al
2
O
3
529.5
Na/NaOH/ γ - Al
2
O
3
exposed to air
531.5
α - Al
2
O
3
530.1
NaOH/ α - Al
2
O
3
530.0
Na/NaOH/ α - Al
2
O
3
529.9
KOH/ γ - Al
2
O
3
530.4
K/KOH/ γ - Al
2
O
3
529.1
RbOH/ γ - Al
2
O
3
530.6
Rb/RbOH/ γ - Al
2
O
3
529.7
Rb/RbOH/ α - Al
2
O
3
530.2
researched by industry, as witnessed by reports in the patent literature. The syn-
thesis of aziridine from N - methylethanolamine is reported in BASF [28] and
Nippon Shokubai patents [29] using cesium and potassium oxides in conjunction
with phosphoric acid supported on silica or glass fabric. Merck have patented a
zeolite - supported cesium catalyst [30] for the production of 4 - methylthiazole
(Scheme 21.1 ).
4 - Methylthiazole is a fungicide and a pharmaceutical intermediate. The catalyst
gives a selectivity of > 60% at a conversion of > 85% but deactivates with time on
stream such that half the activity is lost within two weeks.
The Lucite ALPHA process [31] for the production of methyl methacrylate via
an aldol reaction between methyl propionate and formaldehyde, also uses a sup-
ported cesium oxide catalyst, which requires various additives to minimize catalyst
deactivation. An issue that is rarely discussed in the academic literature, possibly
because of the nature of catalyst testing in academia, is that of deactivation due to
volatilization. Alkali metal oxides may exhibit appreciable volatility under reaction
temperatures > 400 ° C and so there can be a loss of activity over time through
volatilization. To overcome this deactivation mechanism, an AMOCO patent [32]
describes adding an alkali metal compound into the process gas stream so that
the alkali metal compound is deposited on the catalyst during operation to com-
pensate for any loss. It was also noted in the Lucite patent [31] that alkali metal
oxides under some reaction conditions (those where water is in the feedstream)
might enhance the loss in surface area of the silica support. To counteract such
effects the incorporation of modifi ers, such as boron, aluminum, magnesium,
zirconium or hafnium may be added to the catalysts to retard the rate of surface
area decrease. In other studies [33] , cesium oxide was thought to sinter under a
water - containing feed but could re - distribute when the water was removed.
The use of alkali metal oxide catalysts for aldol condensation reactions has been
examined for the production of 2 - ethylhexenal from butanal [34] . When coupled
to a hydrogenation catalyst the system can produce the plasticizer alcohol 2 - ethyl-
hexanol directly. When isobutyraldehyde was used as the feed to a silica - supported
sodium oxide catalyst, no products were formed but a signifi cant amount of carbon
was deposited on the catalyst and in the reactor (Scheme 21.2 ).
These results indicated that an aldehyde with a methyl branch α - to the carbonyl
group passed over a Na
2
O/SiO
2
catalyst was unable to undergo an aldol reaction
but did lay down carbon. This has implications for a combined Pd/Na
2
O/SiO
2
system, as the 2 - ethylhexanal that is formed as an intermediate has an ethyl branch
α - to the carbonyl group and may lead to product poisoning of the base sites of
the catalyst, in a manner similar to that found with isobutyraldehyde over a Na
2
O/
SiO
2
catalyst.
Scheme 21.1
21.2 Catalysts and Catalytic Processes 827

828 21 Base Catalysis with Metal Oxides
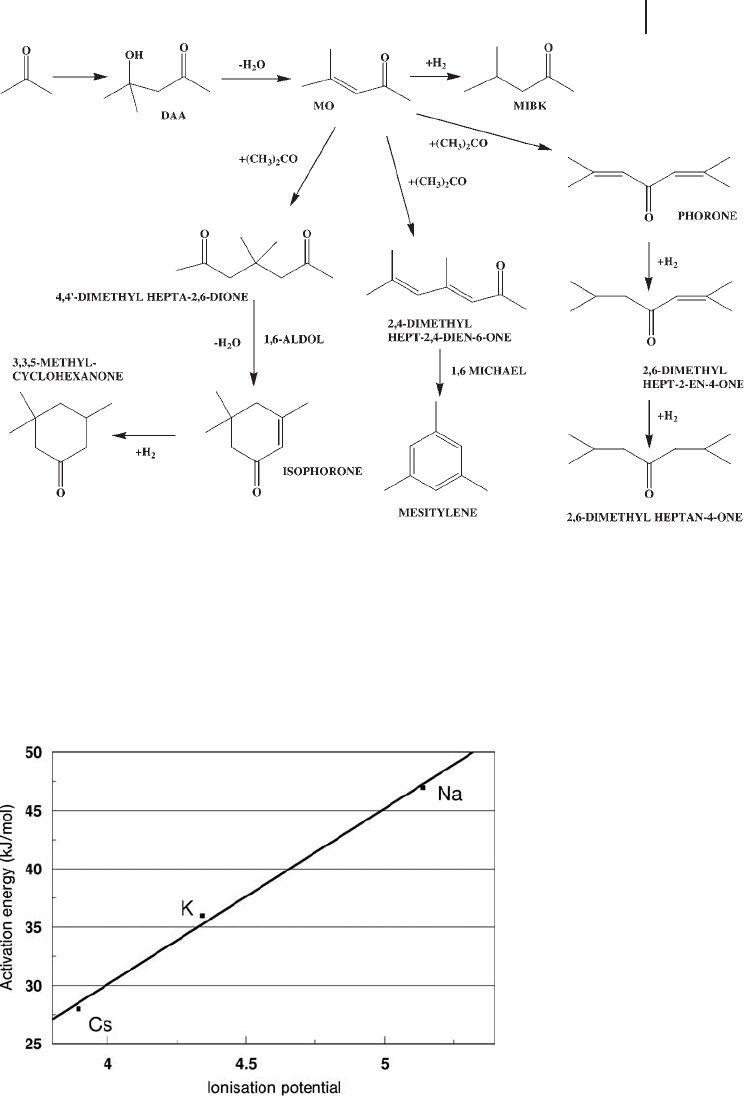
The conversion of acetone to methyl isobutyl ketone ( MIBK ) also uses a combi-
nation of base catalysis with a hydrogenation catalyst [35] . The base component
converts the acetone to diacetone alcohol ( DAA ) via an aldol reaction, which is
then dehydrated by the silica to give mesityl oxide ( MO ). The fi nal step is the
hydrogenation of the MO to MIBK over the metal component. The action of
the base catalyst in the absence of the hydrogenating metal has been studied [36] .
As well as the aldol condensation reactions shown below, the cesium oxide also
hydrogenated MO to MIBK, albeit at a low level (Scheme 21.3 ).
Deuterium studies showed that the hydrogenation was not affected by gas - phase
deuterium but used protium left on the basic site from the exchange reaction of
acetone and deuterium. Further studies [37] revealed that activation energies cal-
culated for MO and MIBK showed a trend following the notional base strength,
with the Na
2
O/silica catalyst having the highest activation energy and the Cs
2
O/
silica catalyst having the lowest activation energy, as shown in Table 21.4 .
The variation in the MO values, although following the same trend as MIBK,
is, however, within the error limits of the measurement and so this variation is
not statistically signifi cant. The variation for the MIBK activation energies is sig-
nifi cant. Also, these values are higher than those for MO formation and so can be
related to the addition of hydrogen rather than the aldol condensation.
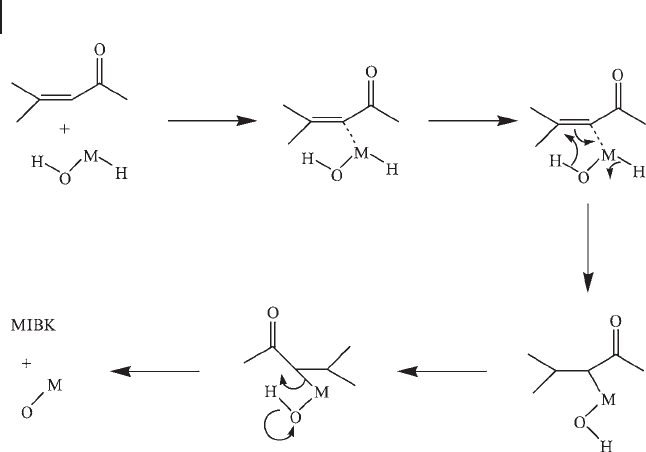
In Figure 21.6 it can be seen that there is a direct relationship between the
activation energy and the ionization potential of the alkali metal. An outline
mechanism for the hydrogenation of MO is shown in Scheme 21.4 .
Scheme 21.2
Table 21.4 Activation energies for MO and MIBK (kJ mol
− 1
).
NaOH/SiO
2
KOH/SiO
2
CsOH/SiO
2
MO 33 25 23
MIBK 47 36 28
Scheme 21.3
Figure 21.6 The relationship between ionization potential and
hydrogenation activation energy.
21.2 Catalysts and Catalytic Processes 829
Gorzawski and Holderich [38] examined the generation of a Cs
x
O/ γ - Al
2
O
3
super-
base from the decomposition of CsOAc/ γ - Al
2
O
3
. After heating in vacuum to 700 –
750 ° C the resulting material had an H
0
value ≥ 37. The catalyst was used for the
transesterifi cation of methyl benzoate and dimethyl terephthalate with ethylene
glycol. A selectivity of 90% at a conversion of 87% was obtained for the methyl
830 21 Base Catalysis with Metal Oxides
benzoate reaction. In the transesterifi cation of dimethyl terephthalate, a selectivity
to bis(2 - hydroxyethyl) terephthalate of 88% at a 100% conversion of dimethyl
terephthalate was obtained. Once again the catalyst was not characterized to
confi rm the presence of Cs
2
O, although the superbasic nature of the material sug-
gests that the oxide was formed. Note that the support hydroxyl population will
have been dramatically reduced and, as has been mentioned before, it is likely that
the hydroxyl population will infl uence the strength and nature of the alkali metal
basic site.
21.2.2
Alkaline Earth Metal Oxides
The alkaline earth metal oxides have been extensively studied in terms of their
base behavior, for example Reference [39] , with perhaps most attention centering
upon MgO. In general, base strength increases down the group as anticipated,
with the order being BaO > SrO > CaO > MgO, for example, in their surface -
area - normalized reaction rates in the aldol condensation of acetone [40] . High -
temperature pre - treatment is generally required in order to activate the oxides
and they are particularly sensitive to rehydration and recarbonation. Figure 21.7 ,
taken from Hattori ’ s review [4] , shows the equilibrium pressure for the decom-
position of the carbonates where the increasing severity of necessary pre - treat-
ment is evident, with decomposition equilibria being negligible at ambient
temperature. Similar considerations apply to the metal hydroxides. Figure 21.8
shows the evolution of sites of varying strength as a function of the temperature
applied in the thermal pre - treatment of Mg(OH)
2
.
Scheme 21.4
