Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.

274 6 Photoelectron Spectroscopy of Catalytic Oxide Materials
(Figure 6.7 ) makes the V 2p core levels of VPOs analogous to binary oxides! Fur-
thermore, applying Coulston ’ s equation (Equation 6.7 ), calculated average valences
nicely agree with the result of the curve fi tting. According to the electron attenua-
tion in the solid, the applied excitation energies correspond to approximately 1 and
3 nm information depth, respectively the notifi cation “ surface ” and “ bulk ” . At
1254 eV excitation energy (which is typically also applied in laboratory experiments
with Mg K α excitation) the two samples are clearly different, mainly because of
the relatively high V
5+
contribution in VPO
P9
. However, surface - sensitive spectra
indicate many more similarities. Undoubtedly, VPO
P9
is inhomogeneous in the
top 1 – 3 nm: some V
5+
species (phase?) sit in sub - surface positions, while mainly
V
4+
occupies the top surface position. As opposed to VPO
P9
, VPO
P4
is fairly homo-
geneous in XPS sampling depth. However, in line with the fi tting results, the
opposite asymmetry of the peaks for surface - and bulk - sensitive modes clearly
indicates a small V
5+
contribution at the surface. Whether or not VPO
P9
also con-
tains V
5+
in the top layer is not easily concluded simply on the basis of the spectra.
(Note that, theoretically, it is possible to answer this question, if a precise geo-
metrical model of the terminating few layers of VPO material is available. However,
the characteristics of the top few layers are widely disputed in the VPO community,
and furthermore the V
5+
phase in the sub - surface position is not compatible with
the (VO)
2
P
2
O
7
structure. We thus decided not to model our depth distribution.)
Based on similar catalytic performance, however, it is likely that both materials
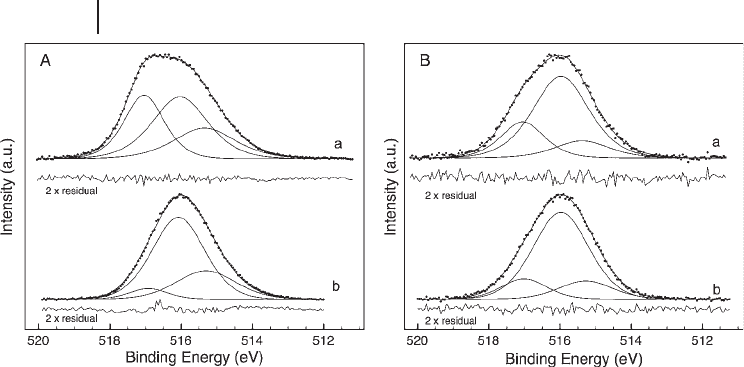
Figure 6.8 Vanadium 2p
3/2
region of two
VPO catalysts (a: VPO
P9
, b: VPO
P4
) in situ
under butane selective oxidation conditions
(T: 673 K) at two excitation energies.
(A) 1254 eV ( “ bulk ” sensitive), as typically
applied in laboratory XPS experiments.
(B) 730 eV ( “ surface ” sensitive). As the
spectral function of V
4+
in VPO is not known,
formally three Gauss - Lorenz curves were
used to approximate the obviously visible
V
5+
contribution in VPO
P9
and to account
for the asymmetry seen on the low E
B
side
of the peaks. Although this latter peak,
according to its E
B
, might be assigned to V
3+
,
its identifi cation purely on this basis is not
straightforward. Its location however seems
to be more sub - surface than directly on the
surface.
6.3 Case Studies 275
contain small V
5+
centers in a V
4+
matrix at the surface. This result is in line with
numerous literature reports [79, 137, 140 – 143, 149, 157, 167] derived from con-
ventional XPS experiments, in which, however, precaution is necessary. It is
important to note that different conclusions would have been drawn on the sole
basis of the spectra at typical laboratory XPS excitation! The similarity of the two
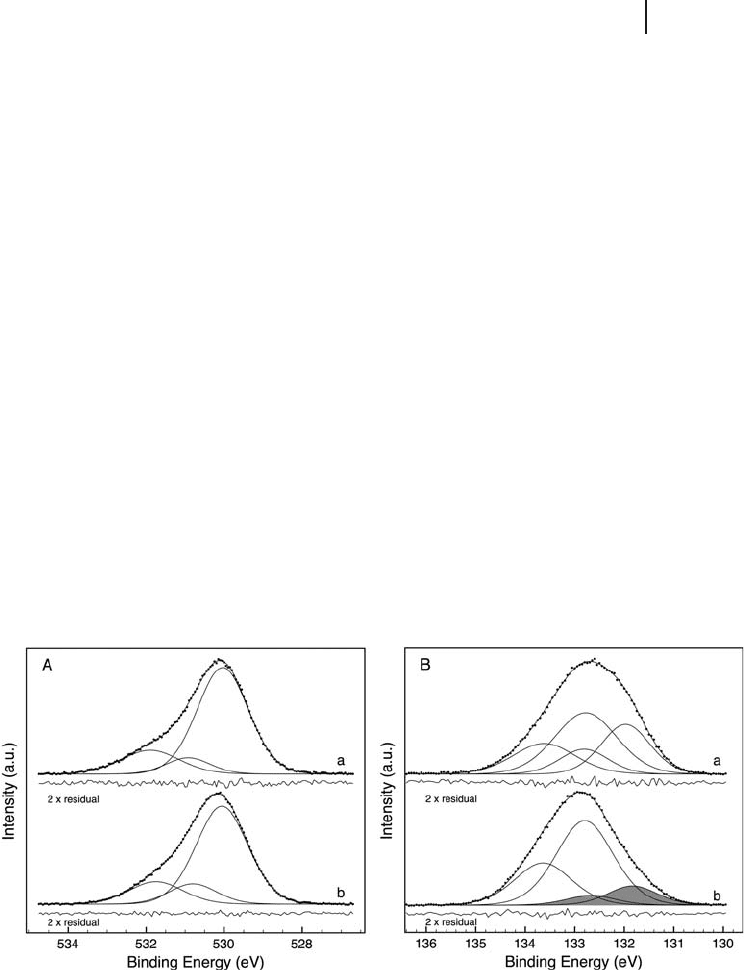
VPO materials is further manifested in the surface - sensitive O 1s spectra (Figure
6.9 A), indicating nearly identical oxygen species. The main line at ∼ 530 eV is in
accordance with the lattice oxygen in binary vanadium oxide systems, while the
higher E
B
components might, as a fi rst approximation, correspond to more elec-
trophilic surface oxygen species, for example OH groups or various oxygen species
bonded to phosphorus or carbon [149, 152, 166, 170] . (It is assumed in the litera-
ture that phosphate and pyrophosphate oxygen is more electrophilic and should
appear at signifi cantly higher binding energy than oxygen bonding to vanadium.)
Comparison of these spectra with binary vanadium oxides has serious conse-
quences in data interpretation: in both cases the main line is found at the same
E
B
. Therefore, either (i) the surface consists mainly of VO
x
in VPO, and there is a
considerable excess of vanadium on the surface or (ii) phosphorus linkage does
not modify the binding energy of lattice oxygen (at least within the resolution of
our and previous XPS studies). In either case, most XPS interpretations and their
consequences related to mechanistic considerations in the literature would need
substantial revision! To be able to further comment on this issue, the surface P/V
ratio has to be taken into consideration.
Figure 6.9 (A) Oxygen 1s region of two VPO catalysts
(a: VPO
P9
, b: VPO
P4
) in situ under butane selective oxidation
condition (T: 673 K) recorded at 730 eV ( “ surface ” sensitive)
excitation energy. (B) Phosphorus 2p region of two VPO
catalysts (a: VPO
P9
, b: VPO
P4
) in situ under butane selective
oxidation conditions (T: 400 ° C) recorded at 854 eV
( “ bulk ” sensitive) excitation energy.

276 6 Photoelectron Spectroscopy of Catalytic Oxide Materials
The second motivation for doing an XPS analysis of materials is to learn about
the elemental stoichiometry of the near - surface region, which brings us to the
widely disputed issue of the P/V ratio of VPOs. The P/V ratio is often considered
to be an important factor in the catalytic properties of VPO [126, 128, 140, 171,
172] . This is mainly based on conventional XPS experiments, in which phosphorus
enrichment in the top few nanometers was found [140 – 142, 150, 156, 157, 159 –
162, 164, 168, 172, 173] . According to a literature survey, the P/V ratio varied
mainly in the range of 0.8 – 3 (Table 6.3 ). Owing to the different sampling depth
of V 2p and P 2p in typical ESCA experiments (as a result of the different kinetic
energies of the photoelectrons), surface impurities (e.g. carbon deposits) will
attenuate the lower KE electrons from vanadium more strongly and hence slightly
overestimate the P/V ratios. Furthermore, the calculation relies on the often -
questioned accuracy of photoemission cross - sections or sensitivity factors. To cir-
cumvent this problem, several attempts were made to apply reference substances
in recalibrating possible incorrect sensitivity factors [158, 165, 168, 174] . Okuhara
and coworkers [165, 174] prepared VPO glasses with different stoichiometry, and
found that the Scofi eld [175] sensitivity factors overestimate the P/V ratio by a
factor of 4.1/2.75 (i.e. by approximately +50%), decreasing the P/V ratio of their
VPO samples to values close to the nominal bulk composition. In the same vein,
using VPO glass Richter and coworkers [168] found a much smaller discrepancy
of only ∼ 14%, and their estimation gave a surface P/V ratio of 1.3 – 1.5. Considering
the sampling depth as 3 nm and assuming a profi le of P segregation, the phos-
phorus concentration in the top layer was assumed to be even higher. Ion - scatter-
ing spectroscopy experiments, showing a P/V ratio of 2 – 3, confi rmed this
hypothesis. Coulston and coworkers [158] used organometallic complexes contain-
ing vanadium and phosphorus for XPS calibration. The observed deviation was as
high as ∼ +75%, overestimating the theoretically calculated composition of the
phosphorus content. In this way, the surface composition of different VPO phases
nicely approached the nominal bulk values. Interestingly, the Scofi eld sensitivity
factors gave a fairly reasonable V
2
O
5.18
stoichiometry for vanadium pentoxide,
indicating that the problem of high P/V ratios is manifested in the phosphorus
calculation. As Richter [168] pointed out, the VPOs (even VPO glasses) are sensi-
tive to water/moisture, giving rise to hydrolysis of V
−
O
−
P bonds and hydration
of phosphate groups (see below). Therefore, if moisture - driven segregation of P
occurs in both VPOs and VPO glasses, the recalibration of cross - sections will
simply cancel out the P segregation, resulting in a stoichiometry resembling that
of the bulk. Unfortunately, even for organometallic complexes it has not been
proven that the ligands do not undergo X - ray - induced damage and are stable under
UHV conditions, which may hamper determination of the real P/V ratio. Hence
the calculated P/V ratios (whether recalibrated or not), should be approached, in
most cases, with caution.
There might, however, be some exceptions. Once the calculated ratios exceed 2,
even using Coulston ’ s recalibration, the P/V ratio (2/1.75) will be high enough to
account for a surface terminated by pyrophosphate groups. Sometimes, values
even higher than 2 were reported (see Table 6.3 ), which would clearly indicate

6.3 Case Studies 277
phosphorus segregation to the surface. In other cases, P enrichment is strongly
indicated when, using the same calculations, a series of samples shows a clear
variation/tendency in the P/V ratio. Such a situation was described in [150, 160,
162, 164] . Phosphorus enrichment was further supported by low - energy ion scat-
tering ( LEIS ) experiments by two independent research groups [142, 176] . This
technique, as opposed to XPS, is sensitive to the topmost surface layer. Delich è re
and coworkers [142] observed an initial P/V ratio of 2.35, which slowly decreased
to below 1.2 by the end of the measurement owing to the sputtering nature of the
experiment. From the sputtering profi le of the individual elements, they concluded
that, in fact, it is not an enrichment of phosphorus, but rather a defi ciency of
vanadium, that is responsible for the observations. Jansen and coworkers [176]
detected a P/V ratio of 2 ± 0.2 and they argued that VPO catalysts could be termi-
nated by a distorted vanadyl pyrophosphate structure, where the excess phospho-
rus is positioned between the vanadyl units and the phosphate groups. To sum
up, there are many cases in which no conclusion on surface enrichment can be
drawn; however, in many others phosphorus does indeed seem to segregate to the
surface.
Phosphorus spectra were collected during in situ experiments as well; hence the
P/V ratios could be calculated. Applying theoretical cross - sections by Yeh and
Lindau [177] , the P/V ratios were as high as 2.5. When using measurements on
reference VOPO
4
phases, the ratio drops to ∼ 1.2 – 1.3, as calculated by Kleimenov
[100] . Repeated experiments on VPO
P4
confi rmed the small phosphorus excess in
the topmost layers ( ∼ 1.2) while with higher ( ∼ 3 nm) sampling depth the ratio
approached the nominal stoichiometry of 1. Note, however, that possible phos-
phate segregation in the reference material might give rise to underestimation of
the P/V ratio of our VPOs.
As opposed to the P/V ratios, the phosphorus core levels are rarely depicted in
the literature. In situ P 2p spectra are show in Figure 6.9 B. In general, phosphorus
in the non - elemental form is represented by an unresolved doublet with energy
separation of ∼ 0.85 eV and area ratio of 2 : 1. The shape of our P 2p spectra can be
best described by the presence of at least two doublets, indicating a minimum
number of two distinct phosphorus species. Two doublets were used in the curve
fi tting procedure. The 3/2 spin orbit component of the more abundant species is
close to the maximum of the 2p envelope at 132.8 eV, which is again lower than
most of the reported energies. Morgan and coworkers [20] investigated alkali
phosphates and pyrophosphates, and cesium and potassium pyrophosphate would
match perfectly to this energy. All the other (Rb, Na, Li) pyrophosphates would
also fi t, assuming small differential charging in Morgan ’ s work, relative to the
reference C1s line, as the separation of O 1s – P 2p was mainly independent of the
type of cation. Morgan observed the corresponding phosphate 2p values at 0.5 –
0.7 eV lower energy, which is not far away from the 0.8 – 0.9 eV in our case. There-
fore, we suppose the presence of mixed pyrophosphate/phosphate species in
the surface - near region. Recalling the V 2p spectra, VPO
P9
contained signifi cant
amounts of V
5+
, which is most likely one of the phosphate phases/species,
while the other sample contained much less. The relative concentration of

278 6 Photoelectron Spectroscopy of Catalytic Oxide Materials
V
5+
/V
4+
in both samples fi ts quite well to the phosphate/pyrophosphate ratio,
indicating the correspondence of the two core levels. Obviously, owing to lack of
resolution in the 2p core level, the presence of additional P species cannot be
excluded.
The entirely neglected, but extremely important, region of XPS investigations
of VPO material is the valence band. The high - lying levels correspond to bonding,
anti - bonding and delocalized orbitals, which hold essential information about the
catalytic properties of the material. As the valence region is more sensitive to the
chemical environment (temperature, presence of reactive gases; i.e. reaction condi-
tions) than the core levels, in situ instrumentation is highly benefi cial, and is
expected to unravel important pieces in the puzzle of VPO chemistry. Fortunately,
recent advances in theoretical calculations can facilitate the interpretation of the
complex overlapping structures observed in the valence band. Considering fi rst a
naive ionic view, VPOs with fully oxidized vanadium and phosphorus (V
5+
, P
5+
)
will show only an O 2p contribution in the valence band. From the more simple
V
2
O
5
system, it can be easily concluded that it is most likely incorrect, as all -
electron DFT calculations by Eyert and H ö ck [64] have clearly shown strong
hybridization of the crystal fi eld split V 3d and O 2p states. Therefore in the valence
band of VPOs a strong mixing of V 3d, O 2p and P 3p/3s orbitals is expected.
Using cluster and DFT calculations, Hermann, Witko and coworkers [69, 70]
investigated the electronic structure of the perfect VPP surface and model clusters.
The calculations show that vanadyl pyrophosphate forms a material with mixed
ionic – covalent character. According to DFT results, the vanadium atoms are
described by atomic charges ∼ +1.0 and phosphorus atoms by ∼ +1.3 [69] . Major
covalent contributions participate in the V
−
O as well as in the P
−
O bonding.
Triply coordinated oxygen sites are found to be the most negatively charged
( − 0.74). Furthermore, the calculations indicated that doubly coordinated oxygen
sites and oxygen atoms singly coordinated to phosphorus are characterized by a
similar local susceptibility with respect to electrophilic attack. Figure 6.10 depicts
the valence band region of VPO
P9
in situ (as shown previously for the low - lying
core levels). Since vanadium is mainly in the reduced formal V
4+
state, its 3d orbital
contribution at ∼ 1 eV represents the highest occupied molecular orbital ( HOMO )
state of VPO. The 2 – 7 eV region is typically occupied by non - bonding O 2p and
mixed bonding orbitals in vanadium oxides, while the features between 8 and
12 eV are characteristic of P
−
O mixed orbitals [170] . XPS analysis by Sherwood
and coworkers [152 – 155] of different (pyro)phosphate materials indicate a non -
negligible contribution of the (pyro)phosphate orbitals in the higher lying 3 – 8 eV
region, as well. Thompson and coworkers [178, 179] calculated local and total
density of states ( DOS ) of vanadyl pyrophosphate. Their calculation included DOS
of bulk, relaxed and non - relaxed (100) surface, pyrophosphate termination and the
hydrated surface. Our data adequately reproduced the hydrated surface (the calcu-
lation is included in Figure 6.10 ), while it did not match the other DOS curves.
Some of the intensity marked by “
䊊
” might be described by additional contribu-
tions from phosphate orbitals [170] , and the arrow indicates a tiny contribution
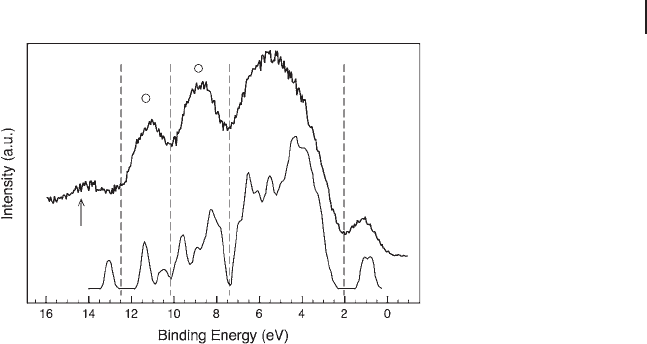
6.3 Case Studies 279
from gas - phase O
2
. (Some tiny contribution of O
2
is blurred at ∼ 6.5, 11 and 12.5 eV,
as well.) Recalling the problem with the O 1s spectra, they seem to be the most
compatible with the interpretation of O 1s being composed of a main unresolved
peak of lattice oxygen of all types (V
−
O, P
−
O, V
−
O
−
PV, V
−
O
−
P; remember the
relatively small difference in their electron population [69] ) and a higher binding
energy hydroxyl peak (P
−
OH, V
−
OH). As most of the VPO material contains a
signifi cant amount of carbon from the preparation steps, it will also give a small
contribution to the O 1s envelope. The weak photon energy dependence of the O
1s envelopes (not shown) and the relatively high information depth of the valence
band (1 – 2 nm) indicate that not only the top, but likely more layers (2 – 3) are
hydrolyzed and hydrated. The enrichment of phosphorus at the surface was clearly
linked to this process [168] . Nevertheless, the mismatch of the hydrated and all
the other surfaces calculated by Thompson clearly indicates that mechanistic con-
siderations have to start with a highly hydrated (and likely defect - rich [180, 181] )
surface, which will obviously modify the acid – base character of the material.
Further, the DOS calculations have shown that the lowest unoccupied molecular
orbital ( LUMO ) of VPP was composed of V 3d unoccupied orbitals, strongly sug-
gesting that both Lewis acid and base character reside in reduced vanadium
centers.
Armed with these results, in what follows we attempt to construct a model best
describing the observations and the essential literature knowledge on VPO chem-
istry. Vanadium phosphates are fascinating materials, capable of abstracting
hydrogen and transferring many electrons and oxygen during the selective oxida-
Figure 6.10 Synchrotron based valence band
spectrum of VPO
P9
in situ under butane
selective oxidation conditions (T: 673 K). In
comparison, the total density of states
according to DFT cluster calculation [178] of
hydrated VPP is shown. The DFT result is
reproduced with kind permission of D.
Thompson. The theoretical curve is aligned
with the experiment to coincide with the band
gap transition at ∼ 1 eV. (The peak marked by
the arrow corresponds to gas phase signal,
while intensity in the position labeled with a
circle “ 䊊 ” is partly due to phosphate orbitals.)

280 6 Photoelectron Spectroscopy of Catalytic Oxide Materials
tion of butane. In spite of the proposed correlation between the unique bulk
structure of VPP and the oxidation process, the overwhelming evidence (effective-
ness of different bulk structures, amorphous VPO, signifi cant P excess at the
surface) suggest that the active site of the reaction cannot be represented as a
simplifi ed termination of the VPP phase. However, the surface seems to have
some similarities with VPP: it is a strongly hydrolyzed and hydrated VPP [182] ,
and likely containing additional minority of V
5+
(and possibly V
3+
sub - surface)
sites, as well. The high P/V ratio is apparently the consequence of the local struc-
ture decomposing, slowly releasing phosphoric acid [168] , which segregates to the
surface. This process is in line with the regenerative treatment of VPO catalysts
using phosphorus compounds and/or steam, according to patent literature (for
example refs [183, 184] ). The destruction of the top few layers is compatible with
the 1 nm non - crystalline adlayer found on equilibrated catalysts after back extrapo-
lation to zero time (to remove beam damage) in high - resolution transmission
electron microscopy ( TEM ) experiments [185] . The local nucleophilicity in the
surface is manifested through occupied vanadium 3d orbitals (see valence band),
but empty vanadium 3d levels predominate in low - lying conduction band regions,
indicating that vanadium is also the surface acid site [178, 186] . The kinetic isotope
effect in butane oxidation indicated that the rate - determining step is the activation
of a methylene C
−
H bond [146] . Nucleophilic activation of the methylene position
was deduced by comparing the HOMO – LUMO structure of butane [185] . Quantum
chemical calculations [186] of butane adsorption on VPP clusters have shown that
butane is adsorbed via nucleophilic attack of methylene carbon on vanadium,
donating electron density toward it. Concomitantly, transfer of electron density
from vanadium to methylene hydrogen occurs, with rupture of the C
−
H bond.
Since the vanadium sites, being the most nucleophilic centers, are not modifi ed
upon hydration, the calculation gives a highly likely scenario on the decomposed
VPP surface as well. Furthermore, in the post - activation steps the participation of
mono - coordinated terminal P
−
O oxygen (as the most basic O) was suggested by
the calculations. However, hydration clearly changed that part of the surface DOS
[178] thus eliminating the possibility of this scenario. It was shown that butadiene,
and to a lesser extent furan, can be produced on reduced VPP and the formation
of MA was strongly hindered [144] . Lattice oxygen ions located in the top surface
(and to a lesser extent in the sub - surface) layer are suggested to be responsible for
the oxidation of butane [146] . Hence it is reasonable to assume, as suggested in
many publications [137, 140 – 145, 187] , that V
5+
sites are necessary in the last
oxygen insertion steps. This is compatible with the XPS observations. Although,
up to now VPO is the only relevant material for this process, MA can be formed
on supported binary V
x
O
y
, but with lower selectivity [188 – 190] . That said, the role
of phosphorus in VPO seems to increase MA selectivity by isolating individual
V
x
O
y
active ensembles in two dimensions, controlling the over - oxidation of the
vanadium ensemble [128, 191] (i.e. hampering the formation of V
2
O
5
). Conse-
quently, the total oxidation is rendered a minority path. A similar model was
constructed and proposed recently by Schl ö gl [185] .
6.3 Case Studies 281
6.3.3.2 Direct Oxidation of Propane to Acrylic Acid Over M o V - Based
Mixed Metal Oxides
In recent years, the direct (amm)oxidation of propane into acrylic acid (or to acry-
lonitrile) has received considerable interest from both an industrial [192 – 194] and
an academic point of view [127, 195, 196] , owing to its possible high economic
benefi t in future technology. Although many different types of materials were
investigated for this process, by far the most promising is the orthorhombic phase
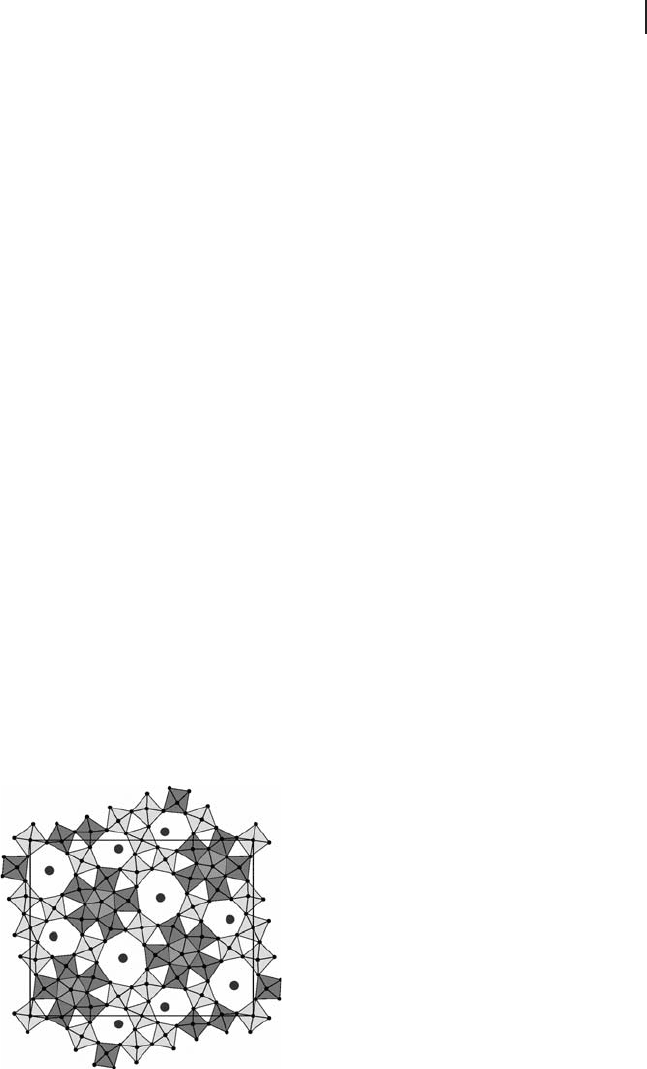
of a mixed Mo/V/(Te or Sb)/Nb oxide, called M1 [197] . Its structure, shown in
Figure 6.11 , was refi ned by DeSanto and coworkers [198] and Murayama and
coworkers [199] , and consists of fi ve - , six - and seven - membered rings of corner -
linking octahedra. The octahedra are proposed to be occupied by molybdenum
and vanadium, the pentagonal ring (pentagonal bipyramid) by niobium, and the
larger channels with tellurium (or antimony). Another phase often found in
MoVTeNb mixed oxides is the so - called M2 phase, which, however, was shown to
be inactive for propane activation, but revealed good activity and selectivity in the
subsequent reaction steps [197, 200, 201] . M1 crystallizes in the form of needles,
and its basal (001) plane, exposing the multi - membered rings, was suggested to
contain the active sites of the selective oxidation reaction [202 – 204] . In numerous
communications, Grasselli and coworkers [204 – 206] proposed a detailed reaction
mechanism at the molecular level assuming perfect termination of the basal plane
on the basis of “ chemical reasoning ” . The few XPS investigations (Table 6.4 ) on
the M1 phase, however, indicated that the surface is typically enriched by Te (or
Sb) and is defi cient in V [202, 208 – 212] . The enrichment of Te was as high as
+233%, compared to the bulk composition. Phase - pure M1 samples prepared by
Celaya [218] were investigated by in situ XPS under propane oxidation conditions
in the sub - millibar pressure range. In line with the ex situ XPS experiments, the
top few layers were signifi cantly different from the bulk (Figure 6.12 ), that is, Te
was strongly enriched while V and Nb were depleted. Even more importantly, the
surface seems to possess a certain fl exibility, since the addition of water to the feed
Figure 6.11 Schematic representation of the structure of M1
phase along the (001) direction as determined in [198] .

282 6 Photoelectron Spectroscopy of Catalytic Oxide Materials
Ref V2p (3/2) Mo 3d (5/2) Nb 3d (5/2) Te 3d (5/2) [Sb 3d 5/2] Note
Table 6.4 XPS characteristics of the MoVTeNb related materials according to the literature survey.
Ref. V2p (3/2) Mo 3d (5/2) Nb 3d (5/2) Te 3d (5/2) [Sb 3d 5/2] Note
E
B
(eV)
[identifi ed as]
Relative
change to
the bulk (%)
E
B
(eV)
[identifi ed as]
Relative change
to the bulk (%)
E
B
(eV)
[identifi ed
as]
Relative
change to
the bulk (%)
E
B
(eV)
[identifi ed
as]
Relative
change to
the bulk (%)
[207] 516.7 – 516.5
[5+ and 4+]
232.5 – 232.8
[6+ and 5+]
206.3 – 206.6
[mainly 5+]
576.2 – 576.3
[4+]
MoVTeNb
[208] 516.7
[4+]
− 39%
235.7/234.6
( 3/2! ) [6+/5+]
norm. to Mo
a)
206.8
[5+]
− 7%
576.7
[4+ and 6+]
+233% M1 (Te)
before ammoxidation
[208] 516.7
[4+]
− 50%
235.5 ( 3/2! )
[6+]
norm. to Mo 206.7 +33% 576.7
[4+ and 6+]
+167% M1 (Te)
after ammoxidation
[208] 516.3
[4+]
− 50%
235.9/234.8
( 3/2! ) [6+/5+]
norm. to Mo 206.3
[5+]
+33% 540.1
[4+ and 6+]
+123% M1 (Sb)
before ammoxidation
[208] 516.1
[4+]
− 43%
235.9/234.8
( 3/2! ) [6+/5+]
norm. to Mo 206.3
[5+]
+27% 540.1
[4+ and 6+]
+92% M1 (Sb)
after ammoxidation
[209] 516.2/517.3
[82% 4+]
0% 232.7/231.7
[56% 6+]
norm. to Mo ?
b)
[5+]
+46% 576.2
[4+]
+32% T6 - 2 sample (M1)
[210] 516.2/517.3
[84% 4+]
0% 232.7/231.7
[57% 6+]
norm. to Mo ?
[5+]
− 9%
576.2
[4+]
+91% NC - 5 (M1)

6.3 Case Studies 283
Ref
.
V2p
(3/2)
Mo
3d
(5/2)
Nb
3d
(5/2)
Te
3d
(5/2)
[Sb
3d
5/2]
Note
E
B
(eV)
[identifi ed as]
Relative
change to
the bulk (%)
E
B
(eV)
[identifi ed as]
Relative change
to the bulk (%)
E
B
(eV)
[identifi ed
as]
Relative
change to
the bulk (%)
E
B
(eV)
[identifi ed
as]
Relative
change to
the bulk (%)
[211] 516.7
− 45%
232.8/231.9
[81% 6+]
norm. to Mo 207.2
[5+]
+27% 576.7
[6+]
+64% M1 before ammoxidation
[211] 516.3
− 55%
232.8/231.9
[72% 6+]
norm. to Mo 207.2
[5+]
+18% 576.4
[6+]
+73% M1 after ammoxidation
[202] 516.1
[4+]
− 49%
232.4
[6+]
+22% – – 575.9
[4+]
+13% Mo
6
V
3
Te
1
O
x
(M1)
(Air/N2 grinding)
[212] ?
[4+]
− 28%
?
[93% 6+]
norm. to Mo ?
[5+]
+42% +73% M1
[213] ?
− 21%
? norm. to Mo ?
− 41%
? 0% HT - 5 (MoVTeNb)
[214] 516.4/517.5
[85% 4+]
− 14
232.8/231.8
[57% 6+]
norm. to Mo 206.5
[5+]
+12 576.3
[4+]
+12 MoVTeNb - 5
[215] 517.0
[5+]
+130 232.0
[6+]
– – 576.5
[4+]
− 20
MoV
0.2
Te
0.1
/SiO
2
(I)
(Not M1)
[216] 516.7 - 517.1
[5+]
− 48%
232.8/231.5
[mainly 6+]
− 6%
207.3
[5+]
− 14%
576.3 - 576.8
[4+ and 6+]
+57% MN - 30: after ammoxidation
(M2 phase)
[205] 516.9 - 515.6
[5+ and 4+]
232.7/231.6
[6+ and 5+]
207.1
[5+]
576.1/572.9
[4+ and 0]
M 1
[217] 516.3/517.3
[mainly 4+]
− 20% ∼ 232.6
[mainly 6+]
norm. to Mo ?
− 12%
? +83% #678 (M1andM2)
a Changes in surface concentration relative to bulk were normalized to Mo concentration.
b Binding energy is not given in the publication.
