Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.

264 6 Photoelectron Spectroscopy of Catalytic Oxide Materials
catalysts towards butene and butadiene increased slightly, in agreement with the
fi ndings of Jackson and coworkers [109] with 1 bar n - butane dehydrogenation at
873 K over the 3.5% V/alumina catalyst. Furthermore, the same group found that
V
x
O
y
/alumina catalysts containing polyvanadates were more effective at dehydro-
genation than isolated species [97] .
An additional feature of the XPS analysis was the formation of surface carbon
during the reaction. Figure 6.4 B shows that during the reaction the gas - phase peak
shifts to higher binding energy, revealing a surface carbon species which increases
as the reaction time increases. The binding energy of the surface carbon species
( ∼ 284.2 eV) is typical of graphene or aromatic carbon [110] . The formation of a
surface carbon species correlates with the mass spectra; surface carbon appears at
the point of maximum in benzene formation. It then increases as benzene forma-
tion decreases. Hence, it appears that the deposition of carbon on the surface is
linked to the retention of benzene. The percentage of surface carbon did not reach
more than 3% of the total surface (XPS measured at a kinetic energy of 290 eV,
which corresponds to an escape depth of ∼ 1 nm owing to the “ universal curve ”
[111] ), during the sub - millibar in situ reaction.
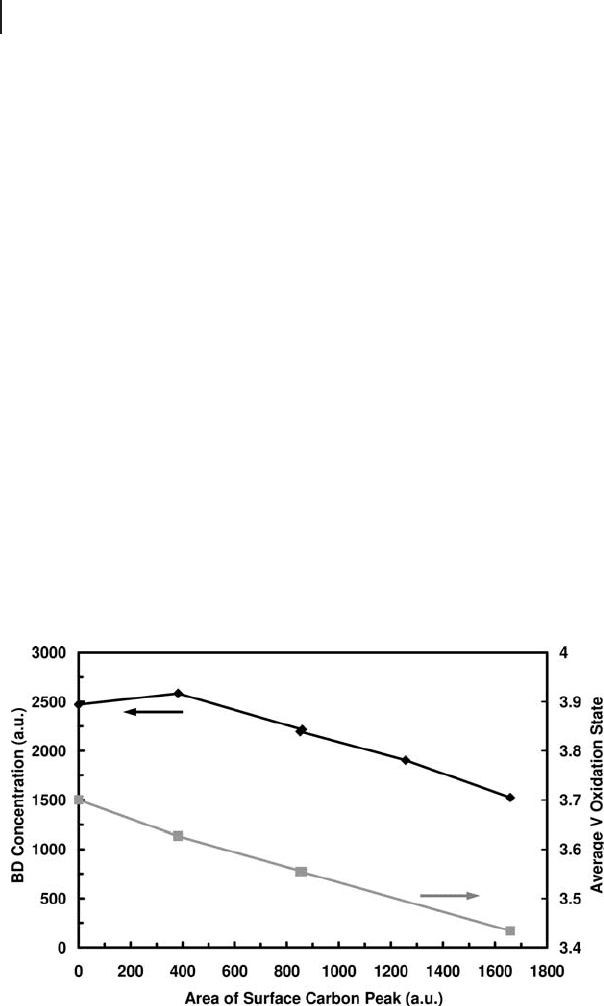
Links between changes in activity and surface structure can be clearly observed
in Figure 6.5 . The oxidation state of vanadium fi rst decreases before carbon begins
to form on the surface. The fi gure shows that deactivation only becomes more
signifi cant after formation of carbon, and the vanadium continues to be reduced.
Figure 6.5 Structure - activity link between growth of surface
carbon, formation of butadiene (BD) and formal oxidation
state of vanadium (reaction conditions: 0.4 mbar n - butane
and 723 K).

6.3 Case Studies 265
Therefore, it can be postulated that by increasing the number of reduced vanadium
sites (as observed by XPS), the number of adjacent sites available for strong hydro-
carbon chemisorption increase. Thus XPS indicates that multiple reduced sites
may be required for coke formation. Although reduced sites may be active for
dehydrogenation, the benefi t of maintaining mixed oxidation states may be to
separate reduced vanadium sites. Literature suggests that the loading and the type
of site (e.g. isolated VO
4
units) infl uences the reducibility of supported vanadium
catalysts [96, 103] . In situ Auger electron yield XAS at the carbon K - edge (not
shown) have a strong feature at ∼ 285 eV during the dehydrogenation reaction, due
to a π * resonance from unsaturated hydrocarbon(s), which agrees well with the C
1s binding energy. In the mechanism of dehydrogenation, hydrogen is initially
removed to form an alkene; if the reaction is selective it may stop here. In the case
that the catalyst is unselective, the alkene can be dehydrogenated further to form
multiple unsaturation and coke precursors. Studies of coke formation suggest
that the dehydrogenation process is followed fi rst by polymerization, then by
aromatization of surface species. The formation of benzene can be explained
by hydrogenolysis of the secondary – secondary C
−
C bond of butane followed by
oligomerization to benzene.
In addition to our in situ study, catalysts deactivated at 100 mbar pressure were
examined after treatment in an adjoining high - pressure reaction cell ( n - butane,
723 K). The samples were transferred without exposure to air and, as expected, the
concentration of surface carbon was much greater ( ∼ 45%). Reduction of the V/Al
ratio suggests that either particle agglomeration or preferential deposition of
carbon on vanadium occurs, resulting in a reduction of the vanadium signal. It
appears that this process is irreversible, as in situ reactivation measurement in
oxygen (after two activation/reaction cycles) does not show the same composition
as the “ fresh ” sample.
Although carbon XAS is rarely applied in catalysis, several studies have looked
at the carbon K - edge of post - reaction carbonaceous species using electron energy
loss spectroscopy [91, 112] . This can contain valuable information about the
nature of carbon deposited on the catalyst during deactivation. A range of cata-
lysts (1 – 8% V/alumina) were examined after deactivation in 1 bar of n - butane
at 873 K. The main feature for all of the catalysts was a strong π * ( ∼ 285 eV) reso-
nance, which is due to the presence of unsaturated hydrocarbons. At higher
energies in the region associated with σ - related features, the spectra were rela-
tively featureless and suggested the presence of disordered carbon [113] .
Although the higher loading catalysts were similar, the 1% V/alumina catalyst
showed a shoulder on the low - energy side of the main feature which suggested
the formation of a styrene - like compound, in agreement with fi ndings of Wu
and coworkers [99] .
By combining the techniques of XPS and XAS, insights into the electronic
structure of the dehydrogenation catalysts V
x
O
y
/alumina have been observed, and,
by correlation with activity data, a greater understanding of the dehydrogenation
mechanism and subsequent coke formation, was possible.
266 6 Photoelectron Spectroscopy of Catalytic Oxide Materials
6.3.2.2 Oxidative Dehydrogenation of n - Butane
As mentioned previously, the main advantages of oxidative dehydrogenation are
enhanced thermodynamics together with a reduction in coking. Oxidative dehy-
drogenation of alkanes has been investigated by a number of authors [93 – 96, 114,
115] . Oxidative dehydrogenation catalysts are normally based on mixed - metal
oxides, and vanadium oxide - based catalysts are among the most successful of those
considered. Thus, the V
x
O
y
/alumina catalysts used for in situ butane dehydrogena-
tion (Section 6.3.2.1 ) were also examined under the conditions of in situ oxidative
dehydrogenation.
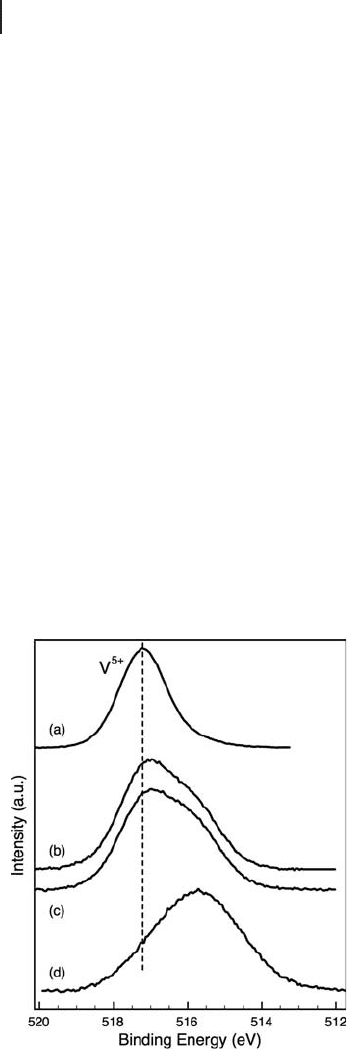
The addition of oxygen (1 – 2 vol%) to an n - butane feed resulted in strong changes
in the vanadium 2p
3/2
spectra and to a lesser extent in the vanadium L
3
edge (not
shown). Figure 6.6 indicates that on addition of oxygen, a signifi cant amount of
V
5+
species were maintained, compared with direct dehydrogenation conditions.
This is not completely unexpected, as in the absence of oxygen in the reaction
feed any oxygen removed from the catalyst during reaction is not replenished.
Unfortunately, keeping V
5+
as the main species was accompanied by an increase
in the formation of oxygenated side - products such as furan, dihydrofuran and
CO
x
, which have been observed during n - butane oxidation and to a lesser
extent in the initial stage of dehydrogenation [83] . However, surface carbon
was not detected and only the gas - phase peak from butane was observed in the C
1s region. This is likely due to the automatic removal of surface carbon species by
the oxygen in the feed (or prevention of irreversible adsorption), thus reducing
Figure 6.6 Comparison of V 2p
3/2
XPS of 3.5% V/alumina at
723 K in (a) 0.5 mbar oxygen, (b) 0.4 mbar 2% O
2
in n - butane,
(c) 0.4 mbar 1% O
2
in n - butane and (d) 0.4 mbar n - butane.

6.3 Case Studies 267
the need for catalyst regeneration; however, this is at the expense of reduced
selectivity.
Lopez - Nieto and coworkers [93, 95] proposed that tetrahedral V
5+
is the active
and selective site for oxidative dehydrogenation of short chain alkanes (C
2
−
C
4
)
and isolated sites prevent the occurrence of consecutive reactions, that is, to total
oxidation products. Others suggested that neither terminal V
=
O nor bridging
V
−
O
−
V bonds are essential to the catalytic activity of alumina supported vana-
dium oxide catalysts, but rather that V
−
O
−
Al bonds play a crucial role [96] . On
the other hand, Iglesia and coworkers [116] measured the extent of reduction of
active VO
x
surface on γ - Al
2
O
3
during oxidative dehydrogenation ( ODH ) of propane
by in situ UV - Vis spectroscopy, and observed a positive correlation with the reac-
tion rate. Transient observations during changes in C
3
H
8
and O
2
concentrations
clearly indicated that only a fraction of the prevalent reduced centers ( ∼ 30 – 40%)
are responsible for the catalytic turnover. Blasco and coworkers [117] identifi ed V
4+
species in post - reaction (ODH of ethane and n - butane) catalysts, the contribution
of which increased with increased conversion of reactants. Additionally, the
highest formaldehyde signal was observed in temperature programmed desorp-
tion experiment from adsorbed methanol when the vanadyls were partially
removed from the V
2
O
5
surface [118] . Furthermore no formaldehyde formed from
perfectly vanadyl - terminated surface. Our results are in line with these latter fi nd-
ings, and suggest that careful control of the surface oxidation state by addition of
oxidizing agents can be useful in stabilizing the active catalytic site as well as in
preventing coke deposition. Steps taken to reduce coke formation include control
of feed gas composition (for example inclusion of hydrogen, steam, carbon dioxide
or oxygen to prevent chemisorption of hydrocarbons [119] ) and addition of pro-
moters to modify the surface properties [119 – 123] . However, addition of oxidizing
gases should be minimized to prevent reduction in selectivity through formation
of side - products.
6.3.3
Selective Oxidation
Selective oxidation of hydrocarbons is of key importance in functionalization of
hydrocarbon molecules. It is always a multi - step process with consecutive abstrac-
tion of hydrogen and addition of oxygen atoms. The diffi culty of this reaction is,
undoubtedly, that the process should go through these many steps, but also should
stop at the desired product. Such requirements can be met by complex
mixed - metal oxides, and the XPS characterization of two selected examples is
briefl y reviewed here.
6.3.3.1 n - Butane Selective Oxidation to Maleic Anhydride Over VPO
Maleic anhydride ( MA ) is an important intermediate in the production of chemi-
cals such as unsaturated polyester resins, lube oil additives, maleic copolymers
and others [124] . Currently, MA is predominantly produced by the oxidation of
butane, and all industrial catalysts for this reaction are based on vanadium phos-

268 6 Photoelectron Spectroscopy of Catalytic Oxide Materials
phorus oxide s ( VPO ). Different types of technology were developed (fi xed, fl uid-
ized or transported bed reactors), and an important aspect of the following
discussion is that the catalyst is able to operate in both oxidizing and reducing
environments.
Several review papers have been published on VPO materials, covering
various aspects, from preparation and characterization to the catalytic properties,
with focus on the reaction mechanism [125 – 129] . In brief, VPO as a complex
catalytic system can comprise many distinct and known phases ( α - , β - , γ - , δ -
VOPO
4
, (VO)
2
P
2
O
7
, VO(PO
3
)
2
, VPO
4
, etc.) but also less well characterized amor-
phous material. It is prepared by different (e.g. aqueous, alcoholic) routes with
the intermediate formation of VO(HPO
4
) · 0.5H
2
O, the hemihydrate, which is
the precursor of the active catalyst. Depending on the P : V ratio in the precipi-
tation and the conditions of the heat treatment of the precursor material, the
prepared “ non - equilibrated ” catalyst can comprise different phases with various
degrees of crystallinity and defect structure. However, after long - term operation
the catalysts prepared in the conventional VPD route exhibited high crystallinity
and the only phase identifi ed by XRD was (VO)
2
P
2
O
7
(VPP) [128, 130 – 134] .
This led several research groups to conclude that (VO)
2
P
2
O
7
(i.e. V
4+
) is the
active material and that its (100) crystal plane (which is supposed to be the
most exposed) contains the active sites. Different preparation routes, however,
resulted in materials with non - uniform bulk structure and different Brunauer –
Emmett – Teller surface area, but comparable intrinsic activity [129, 135] . This
led Hutchings to conclude that the surfaces exposed on these different materials
must all be the same, even though their bulk structures are completely differ-
ent. Furthermore, the necessity of crystallinity becomes dubious considering
that the amorphous vanadium phosphate catalyst prepared by the same group
(Hutchings and coworkers) [136] in supercritical CO
2
conditions was more
active (MA formation rate per surface unit) than the comparable crystalline
VPO catalysts, although with worse selectivity. Previously, combined XRD,
31
P
NMR and Raman studies [137 – 139] indicated that XRD alone is not suffi cient
for identifying the presence of minority phases, and that not only V
4+
but some
V
5+
species exist in almost all materials. Many studies [137, 140 – 144] provided
indirect evidence that vanadium in both oxidation states is needed for the whole
catalytic process. Furthermore, Coulston and coworkers [145] argued about the
central role of V
5+
, as its reduction showed a good quantitative correlation with
the MA formation on supported VPO. Thus there is no clear agreement in the
literature about the active sites of the reaction. Even the reaction mechanism,
the participation of lattice or various types of surface oxygen species (
O
2
−
,
O
2
2−
,
O
−
) and the role of phosphorus enrichment remain under discussion [126, 128,
146 – 150] .
Being relatively surface sensitive, XPS can contribute to an understanding of
this catalytic system, and has already done so. It gives valuable information on the
formal oxidation state and the stoichiometry of the constituents in the sampling
depth of the technique. However, XPS investigations reported in the literature are

6.3 Case Studies 269
not without problems. VPOs are insulators at room temperature and in vacuo
(under conventional XPS conditions); hence the emission of electrons from the
samples leaves the surface charged making the exact binding energy identifi cation
diffi cult. Charge compensation using an electron fl ood gun reduces charging
effects, and referencing the binding - energy scale to “ adventitious ” carbon contami-
nation is widely applied. In fact, in the reviewed research papers on VPO - related
materials, the vast majority calibrate the binding - energy scale according to the
observed C 1s energy. A few others use arbitrarily taken P 2p energies. As not the
same C 1s binding energy is used as reference (C 1s: 284.5 – 285 eV), and because
this peak can be relatively broad (because of charging or overlapping components),
the uncertainty of position will be high, and the reported E
B
s of V/P/O core levels,
even in very similar materials, strongly diverge (Table 6.3 ). A further complication
arises if the main VPO material is differentially charged compared to the carbon
taken as reference. This can frequently happen considering the different conduc-
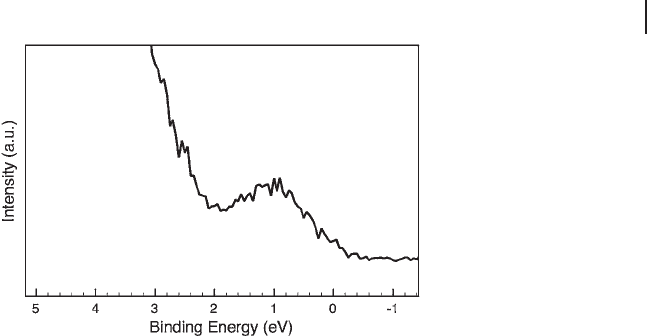
tivity of carbon and the oxide material. Most VPOs have open valence orbitals with
V 3d electrons at ∼ 1 ± 0.2 eV E
B
(Figure 6.7 ), similar to binary vanadium oxides in
non - V
5+
states [63] . Since, except for charging, no signifi cant spectral difference
between the insulating and metallic phases of VO
2
has been observed, a more
appropriate referencing of VPOs can be achieved, circumventing differential
charging, by means of the V 3d transition. In the reviewers ’ opinion, differential
charging is the main reason that most of the reported core level binding energies
in the VPO - related literature are markedly higher than for example, in the V
x
O
y
system. For example, the V 2p energy of binary oxides is found at 517.1 ± 0.1 eV
for V
2
O
5
and 516.0 ± 0.2 eV for VO
2
. On the contrary, most of the E
B
values of V
2p in VPOs corresponding to the V
4+
state are reported in the range 516.8 – 518.2 eV,
which is 1 – 2 eV higher than in the binary VO
2
. This binding - energy offset is
usually conserved in O 1s and P 2p core levels, as well. Fortunately, Garbassi and
coworkers [160] noticed that the binding - energy difference (O 1s – V 2p) can be
related to the oxidation state of vanadium. Later, Coulston [158] proposed a quan-
titative correlation between the average oxidation state of vanadium and the previ-
ously mentioned splitting of the transition centroids, according to the following
equation:
VOsVp
ox
=− −
()
[]
13 82 0 68 1 2 3 2..
(6.7)
This equation has been extensively accepted and applied, and thus comparison
of various VPO materials in different publications has been possible (see Table
6.3 ). Although calculating the mean oxidation state of vanadium is exceptionally
valuable (especially with spectra lacking resolution), it allows determination of the
fraction of different contributions if only two oxidation states are present in the
sample (i.e. it is not useful if V
5+
/ V
4+
/ V
3+
are simultaneously present). Further-
more, with a signifi cantly high E
B
contribution in the O 1s line (from adsorbed
water, OH groups and carbon - related O) the equation will overestimate the degree
of reduction of the formal vanadium valence.

270 6 Photoelectron Spectroscopy of Catalytic Oxide Materials
Table 6.3 XPS binding energies of VPO and related materials according to the literature survey.
Reference E
B
V2p (3/2) E
B
O1s
DE
B
(O1s - V2p
3/2)
E
B
P2p E
B
reference V
5+
/V
4+
P/V Note
[137] 518 and 516.9 530.5 12.5 and 13.6 131.7 C1s: 284.5 0.21
Co:0.3; Fe:0.14
∼ 2%
VPO
Co - VPO
Fe - VPO
[151] 518 and 516.9 C1s: 284.5 15% V
5+
VPO
[138] 518 and 516.9 C1s: 284.5
V5+: 47 ↓ to 37%
VPOs with different
activation time on
stream
[134] 518.2 and 516.9
518.2 and 516.9
517
531.3 (532.6)
531.3 (532.6)
531.3 (532.5)
13.1 and 14.4
13.1 and 14.4
14.3
133.6
133.7
133.7
C1s: 284.9 50:50?
50:50?
Only V
4+
Calcined sample
Non - equilibrated VPO
Equilibrated VPO
[152] 532.35 135 P2p: 135 Na
4
P
2
O
7
· 10H
2
O
[153] 517.7
( ∼ 532.0) 533.51 ∼ 14.3
135 P2p: 135 (V
4+
) VO(H
2
PO
4
)
2
V3d: ∼ 3 eV!
[154] 518.25 532.62 14.37 135 P2p: 135 (V
4+
) VOHPO
4
* 0.5H
2
O
V3d: ∼ 3.5 eV!
C1s:285 eV diff.
charging
[155] 519.84 533.07 13.23 135 P2p: 135 (V
5+
) VOPO
4
· 2H
2
O
C1s:286.1 eV!
[156] 517.7 – 517.9 532.2 14.3 – 14.5 C1s: 284.6 2.8
2.6
Eq.VPO
Addition of Co(2+)

6.3 Case Studies 271
Reference E
B
V2p (3/2) E
B
O1s
D E
B
(O1s - V2p
3/2)
E
B
P2p E
B
reference V
5+
/V
4+
P/V Note
[140] 517.9 532.3 14.4 134.3 C1s: 284.6 1.8 (up to 7.8) (Ref compounds)
and VPOs
After H
2
or inert:
P/V ↑ ↑ ↑
a)
[157] 517.7 – 517.9 532.2 14.3 – 14.5 C1s: 284.6 2.8
2.6 – 3.5
Un - promoted VPO
Co - promoted VPO
Surface enrichment of
Co
[158]
∼ stoichiometry ∆ E
B
(O1s - V2p 3/2)
Formula
[141] 518
516.9
C1s: 284.5 30% – 70%
50% – 50%
1.6
1.55
VPO
Nb
5+
doped VPO
[142] 517.5 – 517.2
Fit: 518,516.9
∼ 531.4
13.9 – 14.2 C1s: 284.5 47% – 53%
37% – 63%
1.6 VPO (0.1 h TOS)
VPO (132 h TOS)
[159] 517.0
517.1
531.3
531.5
14.3
14.4
134.0
134.1
C1s: 284.6 1.4
2.4
Bulk VPO
VPO/ZrO
2
(H
3
PO
4
)
[160] 516.0 and 517.2 530.2 13.0 and14.2
∼ 133.3 as
overall max.
C1s: 284.6 1.2 - 2.3 Depending on the
nominal P/V, but
surface P enrichment
[161] 517.7/517.6
517.5/517.5
532.2/532.4
531.9/532.1
14.5/14.8
14.4/14.6
133.9/134.1
134/134
C1s: 284.8 1.23/1.54
1.6/1.82
VPO1/VPO2
VPBiO1/VPBiO2
[162] 518 532.3 14.3 133.9 – 134.3 C1s: 284.6 2.5 - 2.9
Refs: 1.2!!
b)
VPO, Mo - VPO
β - VOPO
4
and
(VO)
2
P
2
O
7
a After treatment with H
2
or inert, the P/V ratio strongly increased (up to 7.8).
b Reference VPO phases gave P/V ratio 1.2, while catalysts up to 2.9.

272 6 Photoelectron Spectroscopy of Catalytic Oxide Materials
Reference E
B
V2p (3/2) E
B
O1s
DE
B
(O1s - V2p
3/2)
E
B
P2p E
B
reference V
5+
/V
4+
P/V Note
[163] 517.3 – 517.1
517.1 – 517.4
531.4 – 531.6
532.6 – 9 (SiO2)
14.1 – 14.5 133.6 – 7
133.7 – 134
C1s: 284.6 VPO bulk
VPO/SBA - 15
[143]
518 and 516
C1s: 284.5 28/72 In
fuel rich (O2/C4:0.6)
[164] 516.8
517
C1s: 284.6 1.5
After r: 1.3
c)
5% VPO/MCM - 41
5% VPO after reaction
[165] 517.5 134.1 C1s: 285 VPO glasses
for cross section
[166] 516.6 531.2 14.6 C1s: 284.8 VOHPO
4
· 0.5H
2
O
[167] 518, 516.9
∼ 532 ∼ 14, 15.1
C1s: 284.5? 82/18 fresh VPO.
[79]
∼ 517.2 ∼ 531.3
14.1
14.4
C1s: 284.5 VPO
UHV effect on V
5+
[149] 517.4 – 517.1 531.6 – 531.2 14.5 – 13.9 133.6 – 133.9 C1s: 285 1.37 – 1.68 (Bi) - VPOs
[150] 517.6 – 517.8 532 – 532.6 133.7 – 134.1 C1s: 285 1.32 – 2.05 (Co) - VPOs
[168]
∼ 517.2
531.2 C1s: 284.6 1.3 – 1.4 (Cs) - VPOs
(Ref. VPO glasses)
[20] 530.3 – 531.7
530.4 – 531.9
132.3 – 133.8
132.8 – 134.5
C1s: 285 Alkali phosphate,
Pyrophosphate
∆ E
B
O - P ∼ const.
c The P/V ratio decreased after reaction to 1.3.
Table 6.3
Continued
6.3 Case Studies 273
In addition, the existence of different oxidation states can be determined by
curve fi tting of the recorded spectrum. In the case of vanadium, however, the situ-
ation is a bit more complex, as fi nal state effects can complicate the spectral
interpretation. As all the lower valence vanadium cations have open valence shells,
multiple splitting, which is typically not resolved in the V 2p region, will signifi -
cantly broaden (sometimes asymmetrically) the lower valence components. There-
fore one has to use known, but widely different, FWHM values to fi t the individual
components. Spectral resolution depends strongly on the instrumentation; hence
adequate fi tting results can be obtained after carrying out XPS experiments on
known reference compounds, and applying the observed (or at least similar) spec-
tral function in the fi tting procedure of VPOs. Unfortunately such data treatment
has almost never been carried out, and only arbitrarily taken reference positions
were fi xed in fi tting VPO spectra and forcing the algorithm to observe different
valences in an unresolved spectral envelope. Considering the strong scattering and
shift of the core level E
B
s (Table 6.3 ), the reliability of such data treatment is highly
questionable.
Another widely neglected fact in the VPO literature when interpreting XPS data
is that information arises from a certain depth. This, using Mg or Al K α excitation,
is as high as ∼ 3 nm, the technique being thus a priori not sensitive to the top
surface layer on which the catalytic reaction proceeds. Furthermore, the sample
can be inhomogeneous in the top few layers, allowing no conclusions to be drawn
as to whether a certain state coexists on the surface or not. This problem can be
circumvented by carrying out XPS investigations with a tunable X - ray source at a
synchrotron as detailed below. Figure 6.8 compares the in situ V 2p core levels of
two, approximately equally active, VPO materials (VPO
P4
, VPO
P9
: prepared in
Hutchings ’ laboratory [169] ), under fuel - lean n - butane oxidation conditions, at two
different excitation energies. The spectra were published in a preliminary form
[100] . Note that referencing the binding - energy scale to the V 3d valence transition
Figure 6.7 Top of the valence band of a VPO catalyst showing
the band gap transition of V 3d.
