Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.

304 7 X-ray Absorption Spectroscopy of Oxides and Oxidation Catalysts
goes through pathways that are either evacuated or fi lled with He to reduce absorp-
tion of the less hard X - rays. For softer X - rays, such as the transition metal L - edges,
Al, Si, S, P or As, a vacuum system has to be used to reduce the non - specifi c
absorption of the X - rays. However, even in these cases gas atmospheres can be
used as long as pathlengths are kept short. One of the major advantages of X - ray
absorption spectroscopy is the comparably undemanding experimental setup. It
has been used for in situ measurements of heterogeneous catalytic reactions,
electrochemistry and fast reaction kinetics.
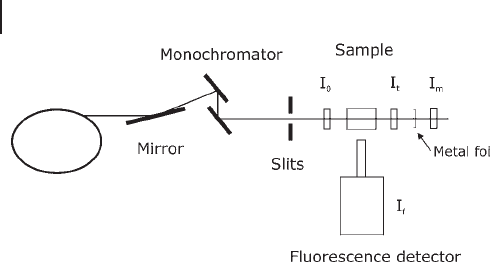
Figure 7.4 shows the experimental set - up of a typical XAS experiment. For most
EXAFS experiments the source is a synchrotron. Laboratory sources are rare and
usually do not exhibit enough intensity to get usable EXAFS spectra.
The photon sources from synchrotrons range from bending magnets, to wig-
glers and undulators. The specifi c device used depends on the frequency range
and brightness required for the specifi c application.
Often the white beam is refl ected by a mirror, which can be used for harmonic
rejection and focusing on the sample.
The mirrors normally have to be cooled (often using gallium) since they are
exposed to more or less the full intensity of the white beam. The monochromators
for harder X - rays are often silicon single crystals exposing (220) or (111) planes.
For lower energy photons such as the Mg, Si and Al edges, Y66B single crystals
can be used. Below 1000 eV (e.g. fi rst row transition metal L - edges, the O K - edge
and the C K - edge) the technology changes signifi cantly and gratings are used.
In transmission methods, after the monochromator, the optical path traverses
through the I
0
detector, the sample chamber, the I
t
detector and, for energy calibra-
tion, through a foil and the I
m
detector.
7.1.3
Detection Methods
Absorption is the simplest method for measuring an X - ray spectrum. The incom-
ing radiation, I
0
is measured and related it to the transmitted radiation ( I
t
).
Figure 7.4 A typical XAS experimental set - up.
Absorption is then given by:
µt
I
I
=
⎛
⎝
⎞
⎠
ln
0
t
(7.4)
where t is the sample thickness. and µ is the linear mass absorption coeffi cient.
This method makes use of all the photons that are incident on the sample.
To measure the absorption signal, a monochromator is stepped through the
energy range and the variation of the mass absorption coeffi cient measured as a
function of the Bragg angles (i.e. energy.) For signifi cantly faster scanning, the
full EXAFS energy range of radiation is shone on the sample and the emitted
radiation is detected spatially using X - ray detector plates [5 – 9] . The detectors most
commonly used for absorption are ion chambers, which are fi lled with gases with
compositions dependent on the X - ray energy. The absorption of the gas should be
in the range of 20% for the I
0
detector and 80% for the I
t
detector.
Sometimes it is of advantage to measure absorption not directly, but by associ-
ated processes. This is mainly used when the X - ray absorption is only a small
fraction of the total absorption process. Problems with absorption are often
observed with highly diluted samples and also for low - energy EXAFS. In these
cases the transmission results from the difference of either two nearly identical
signals or two vastly different signals, both needing very accurate data.
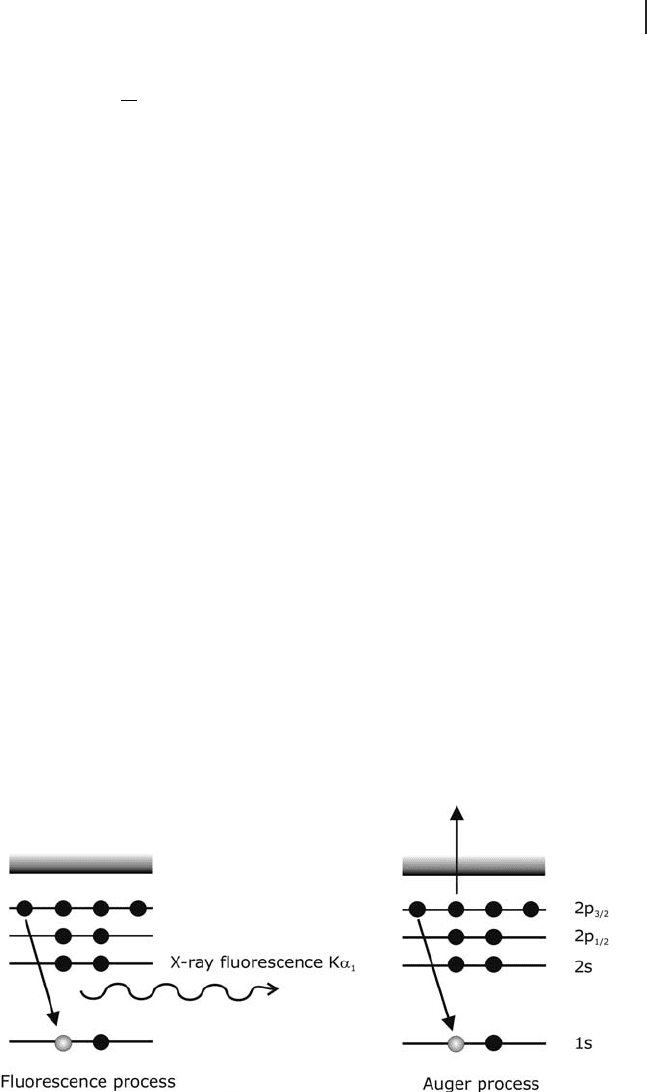
Two of the techniques applied in this category are Auger electron process and
X - ray fl uorescence process. A schematic in Figure 7.5 shows the processes for
these two techniques. These two processes compete with one another, and their
relative abundance is dependent on the atomic number of the absorber. In general
fl uorescence is more pronounced for heavier elements, whereas the Auger process
is more prominent for lighter elements.
In addition to the removal of one core electron by the incident X - rays (photo-
electron process), the Auger process is taking place approximately 10
− 14
seconds
after the photoelectron event. In this process an outer electron falls into the inner
orbital vacancy from the photoelectron process and a second electron is emitted
Figure 7.5 Processes resulting from the removal of a photoelectron by X - rays.
7.1 Principles of EXAFS 305

306 7 X-ray Absorption Spectroscopy of Oxides and Oxidation Catalysts
carrying the excess energy. The Auger electron carries and energy equal to the
difference between the energy of the initial ion and the double charged fi nal state.
The Auger process is competing with the fl uorescence process.
Fluorescence radiation is a result of the fi lling of a core hole (created by the
absorption process) by outer electrons. For K - edges K
α 1
radiation is emitted, which
is characteristic of the absorbing element. There is signifi cant background created
by Compton radiation and elastic scattering. This radiation is at higher energy
than the fl uorescence line. Thus, detectors need to be energy discriminative. The
scattering contributions can be removed using as a fi lter a metal foil with an atomic
number ( Z ) one lower than the element of interest. Compton and elastic scattering
frequencies are often above the absorption edge of the Z − 1 element and so they
are removed.
Modern solid - state detectors have windowing functions that can fi lter the fl uo-
rescence radiation.
7.1.4
Data Reduction
As mentioned, EXAFS is defi ned by the variation of the mass absorption
coeffi cient:
χ
µµ
µ
k
EE
E
()
=
()
−
()
()
0
00
(7.5)
Equation 7.5 describes the energy - dependent EXAFS oscillations that can be
interpreted using the EXAFS equation (as given in Equation 7.1 or Equation 7.2 ).
χ ( k ) EXAFS as a function of electron wave vector.
The mass absorption coeffi cient is determined experimentally from the curves
as illustrated in Figure 7.2 µ
0
( E ) is the smooth atomic background, whereas µ
0
( E
0
)
is the edge step. The edge step is the mass absorption coeffi cient at the edge posi-
tion. This can be determined by extrapolating the post - edge baseline or from the
edge position. The edge position can be defi ned as the fi rst point of infl ection at
the absorption edge.
EXAFS analysis is performed by a number of data treatment steps:
1. Deglitch the individual spectra.
2. Add the experimental spectra and individual contributions of the detectors
(for, e.g., fl uorescence).
3. Convert intensities to µ .
4. Subtract a background function pre - edge.
5. Normalize the spectra to an absorption of 1.
6. Subtract a background function post - edge to obtain χ ( E ).
7. Obtain E
0
and convert to χ ( k ), that is, convert from energy to k - space.
8. Defi ne a model and calculate the sum of the model function from the EXAFS
equation as, for example, from Equation 7.3 .
9. Compare the calculated function with experimental χ ( k ) and minimize the
differences.
Steps 8 and 9 are specifi c to the SRS Daresbury Laboratory suite of programs,
which will be the focus of this discussion. Other programs use Fourier fi ltering
techniques to isolate the contribution of the various shells. A number of other
software programs use this technique. Fourier fi ltering can be problematic as
outlined in refs [10, 11] .
Deglitching is often necessary depending on the type and spectral region of the
monochromator. The easiest way of deglitching is removal of the datapoints. A more
detailed guide to deglitching can be found on: http://srs.dl.ac.uk/xrs/index.html
The Daresbury suite of programs used to add and convert data are Excalib and
Exspline, and the EXAFS fi tting is called Excurv98.
There are a number of other programs available (often free of charge or with
nominal costs) such as FEFF and viper (which uses FEFF code).
Exspline is an X - window - based program that uses spline fi rst for pre - and post -
edge fi tting baselines. It has the advantage that the Fourier transform, the EXAFS
and the pre - and post - baseline fi t can be observed interactively. This is a great
improvement on Exback, which used non - interactive baseline correction.
Owing to the various processes detailed above, the EXAFS oscillations decay
quickly with the photoelectron wavevector ( k ). The EXAFS is often weighed as
k , k
2
or k
3
to increase the intensity of the oscillations at higher k values.
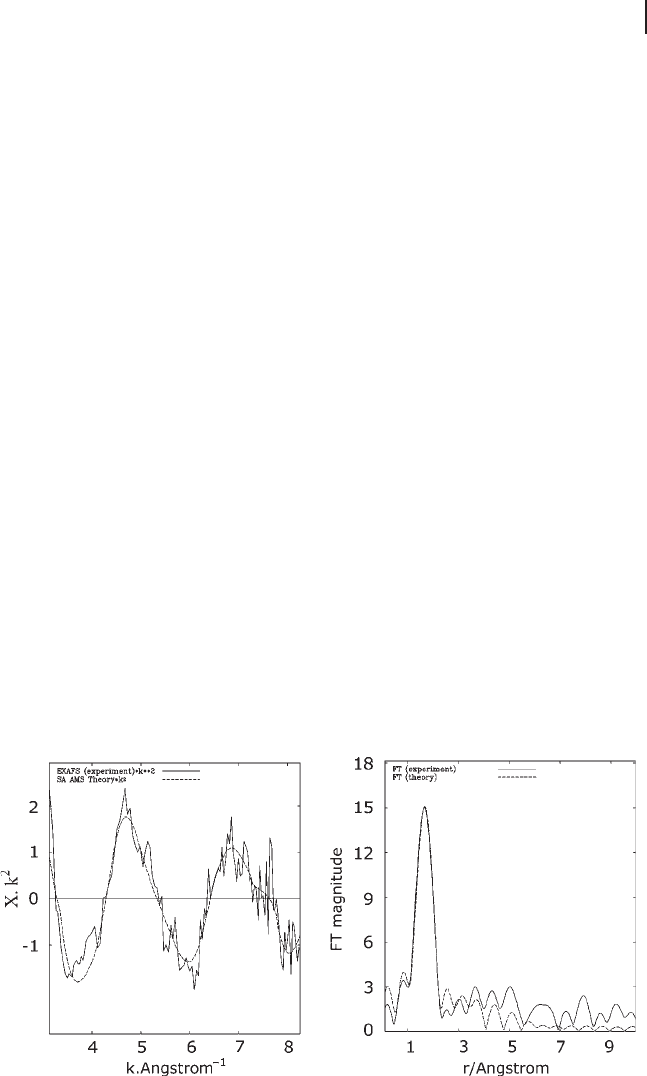
Figure 7.6 shows the EXAFS oscillation together with the amplitude of the
Fourier transform of the Al K - edge of the zeolite Na - FAU. The spectrum and its
Fourier transform ( FT ) are shown together with fi tted data. The fi rst peak in the
Fourier transform can be found at 1.67 Å . However the real position of the peak
is at 1.74 Å .
The discrepancy between the two values is a result of the phase - shift function,
which is included in Equation 7.3 . Phase shifts are dependent on the absorber
atom and can be calculated by ab initio methods. The model building for phase - shift
fi les include a model of the most abundant atomic neighbor for a specifi c atom.
7.1 Principles of EXAFS 307
Figure 7.6 EXAFS and FT transformed spectra of Na - FAU.
The dashed line represents fi tted and the solid line
experimental data.

308 7 X-ray Absorption Spectroscopy of Oxides and Oxidation Catalysts
Fourier transformation of EXAFS makes χ ( k ) a complex number, but often the
FT is only represented by its amplitude, as shown in Figure 7.6 .
Often the FT (as seen in Figure 7.6 ) shows well separated peaks and is then
used to perform the fi tting procedure in Fourier space or to fi lter the data. Of
course, in this case a windowing function has to be applied with all its associated
problems. The fi ltering is often performed at higher r values to remove some of
the noise.
The fi ltering process itself introduces an arbitrary function, which can cause
artifacts. For instance, the statistical signifi cance of shells is altered [10] . Unless
Eigen functions are used for the Fourier fi ltering [11] the introduction of the arbi-
trary function to perform Fourier fi ltering should be omitted. If absolutely neces-
sary (for example if there are problems with baseline correction) very wide fi ltering
functions can help in reducing the problem.
The fi tting procedure is normally carried out consecutively, that is, shells are
added to the fi tting process as seems appropriate. Every shell should be checked
for statistical signifi cance, that is, if the addition of, normally, three parameters
( σ , R,N ) is statistically viable. The check can be made by comparison of the change
in the Fit index with F - test values, as outlined in ref. [12] . The oscillations are often
very broad and with few features for oxides. In this case good statistical parameters
are especially important.
Interpretation of XANES is signifi cantly more diffi cult than for EXAFS. The
EXAFS equation breaks down in the low - k XANES region and the mean free path
goes up. The XANES region is even more complicated by the signifi cant multiple
scattering than EXAFS. However, the electronic transitions can be handled as a
fi ngerprint and the XANES, the white line intensity and edge position are a
measure of the electron density. XANES can provide us with important informa-
tion of the chemistry of the probed solid. For instance for transition metals the
pre - edge peak can distinguish between octahedral, square planar, tetrahedral or
distorted tetrahedral coordination. The oxidation state of transition metal species
can also be determined from the XANES.
Figure 7.7 Differential XANES spectra of iron containing
compounds. Dotted line Fe metal, full line FeO dashed line
Fe
2
O
3
. Adapted from ref. [13] .

Figure 7.7 shows typical derivative XANES spectra of some iron oxides and iron
metal. The edge position is in the maximum of the second peak for the oxides. It
can be seen that the edge position changes signifi cantly depending on the oxida-
tion state. It also shows that the variation of the XANES is signifi cant, and can be
used as a fi ngerprint to determine the species present in the oxide.
Pre - edges as observed in Figure 7.7 , which are found in transition metals with
partially fi lled d - shells, can be indicative of the coordination environment of the
transition metal. The pre - edge can be interpreted as an s – d transition, which is
forbidden in octahedral symmetry. Only small pre - edges are found for transition
metal compounds having octahedral or distorted tetrahedral symmetry. On the
other hand, tetrahedral compounds can have big pre - edges which can be used as
an indicator for the coordination chemistry. For example tetrahedral FePO
4
and
Fe - MFI zeolites exhibit signifi cant pre - edges, whereas most transition metal
exchanged zeolites, the iron oxides and iron salts only show smallish s – d
transitions.
It is interesting to note that oxygen XANES exhibits pre - edges if bound to a
transition metal (e.g. Co), as well.
XANES probes the transition of core electrons into unoccupied electronic states.
The fi nal states may be mixed with other orbitals and can be used to determine
the coordination environment of an element in a compound, its electron density
and oxidation state. The intensity can be used to accurately determine relative
oxidation state ratios. Furthermore, XANES can be used to determine relative
amounts of species by linear combination of individual compounds. In recent
years, the codes for XANES calculation (especially John Rehr ’ s FEFF code), have
signifi cantly improved and it can be expected that theoretical models of com-
pounds will be accurately determined by XANES measurement and calculation in
the future.
XANES and EXAFS complement each other very well and are ideal to determine
the site structure of even mixtures of catalysts or oxides.
7.2
Applications of EXAFS
XAS is in general less accurate than XRD techniques. The accuracy depends on
the edge energy, the resolution of the monochromator and, last but not least,
the quality of model building. As a general rule distances can be determined up
to 5 × 10
− 3
Å and coordination numbers at maximum to 10%. This is clearly in -
ferior to XRD.
However, as mentioned in the introduction, the main advantage of XAS com-
pared to other structural techniques is its element specifi city, its potential opera-
tion under more or less any experimental conditions, its applicability for amorphous
and X - ray amorphous materials and its straightforward sample preparation. In the
following, some applications of XAS in catalytic systems will be discussed.
7.2 Applications of EXAFS 309

310 7 X-ray Absorption Spectroscopy of Oxides and Oxidation Catalysts
XAS was originally used mainly for supported metal catalysts. XRD, despite
being applicable under reaction conditions of high temperatures and pressures,
is not normally able to detect small clusters of noble and other metals in supported
catalysts. A signifi cant number of systems are too small to be detected by conven-
tional XRD techniques because they become X - ray amorphous due to line broaden-
ing. Group VIII noble metals were among the fi rst catalysts investigated and the
work by Lytle and also Sinfelt is of particular note in this area [14, 15] . These classic
investigations, and especially in situ investigation [16] , paved the way for modern
XAS in catalysis.
7.2.1
Acid Zeolites
Zeolites are crystalline alumosilicates with a regular pore structure. These materi-
als have been used in major catalytic processes for a number of years. A signifi cant
number of processes use zeolites as catalysts either as supports or as catalysts
themselves. The application using the largest quantities of zeolites is Fluid Cata-
lytic Cracking ( FCC ). The fi rst zeolites used as catalysts for FCC were reported in
a 1961 patent by Exxon [17] and very quickly replaced many of the amorphous
silica alumina catalysts which dominated the process up to that time. The zeolites
with signifi cant cracking activity are ultrastable Faujasite ( USY ), ZSM - 5 (alterna-
tively known as MFI), mordenite, offretite and erionite. By far the highest volume
of zeolite type used for this application are USY zeolites.
The catalytic activity of zeolites has its origin in the fact that some of the silicon
atoms in the crystalline framework of the solids are replaced by an aluminum
atom. Since aluminum is trivalent, the replacement of the tetravalent silicon
results in the introduction of a negative charge into the zeolite lattice. This negative
charge has to be compensated by a cation. When synthesized, most zeolite materi-
als have sodium as a counter - cation, resulting in very little catalytic activity for
acid - catalyzed reactions. Therefore zeolites are normally exchanged by rare earth
or ammonium cations to create catalytically active acidic sites. The ammonium
cations are normally decomposed at higher temperatures leaving residual H
+
species, forming the Br ø nsted acidic site. Mixed rare earth and ammonium zeo-
lites are among the most widely used zeolite formulations for FCC.
Despite the enormous commercial success and widespread use of acidic zeolite
catalysts, important questions on the local structure of zeolitic materials remain
unanswered. The local structure of aluminum in the zeolites has proven rather
elusive to investigation.
It has proved diffi cult to establish the local structure surrounding these alumi-
num sites by diffraction methods because the similarity of the backscattering
powers of aluminum and silicon result in the elements being virtually indistin-
guishable using these methods. Although aluminum is slightly larger than silicon,
most crystallographic data on zeolites does not report any distinction between
silicon and aluminum atoms in the lattice. Neutron diffraction [18] is one of
the few techniques able to provide direct structural data on the position of the

zeolitic proton. Neutron diffraction has been used both to characterize the average
proton site structure and to investigate the interaction of the sites with reactant
molecules.
Magic Angle Spinning ( MAS ) Nuclear Magnetic Resonance ( NMR ) also had
some success in determining proton positions. Side - band analysis from solid - state
1
H NMR was used to determine proton positions [19, 20] in ZSM - 5 type zeolites.
In addition to the limited experimental techniques, some theoretical studies on
the local structure of aluminum sites in the zeolites were published in the early
1990s [21] . More recently, some systematic studies of the acid – base properties of
zeolites were determined using theoretical approaches. It was suggested that the
local structure of the zeolites has some infl uence on the acid strength of the indi-
vidual sites [22, 23] . It has long been recognized, for example, that the concentration
of aluminum has signifi cant infl uence on the acid strength of individual sites.
Variations in the acid strength were suggested to depend on the structure type and
concentration of lattice aluminum, which result in a variation of the local site struc-
ture. In contrast to earlier studies [24] , the authors suggested no direct correlation
of acid strength with bond angles. The authors also reported that the Al
−
O bond
that carries the acidic proton is longer than the other Al
−
O bonds [22, 25, 26] .
The fi rst EXAFS study of aluminum in zeolites was a study of Na - and H - FAU
(Faujasite) by Koningsberger and coworkers [27]. The authors observed slight
variations in the Al
−
O bond lengths when comparing Na - , H - and NH
4
- form zeo-
lites. However, they did not fi nd any signifi cant variations between the four dif-
ferent aluminum – oxygen bond distances. This is in contrast to the theoretical
calculations, which suggested an increased Al
−
O distance for one of the Al
−
O
bonds. Similarly, more recently Bell and coworkers [28] did not fi nd an increased
distance Al
−
O distance for acidic and Cu - exchanged ZSM - 5 . Bell suggested that
his data does not support a distinction between Al
−
O bonds but did not give any
statistical support for this conclusion.
In line with the theoretical predictions [4, 29] an increased Al
−
O distance was
observed by some workers. Furthermore multiple scattering calculations enabled
calculation of the detailed local structure, including bond angles. The group that
initially reported very similar Al
−
O distances [27, 30] later observed a variation
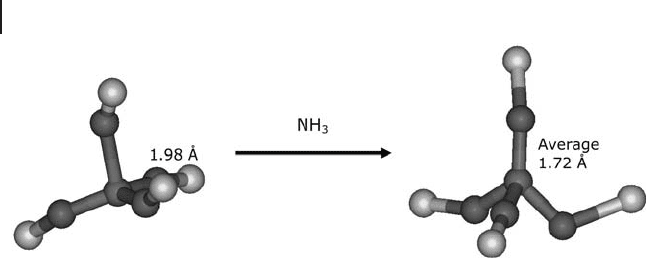
under in situ conditions [31] . This is not too surprising, since other cations were
found to relax the zeolite local structure to a more regular tetrahedron [32] (Figure
7.8 ). Sorption of water and the presence of alkali metal or ammonium counter -
cations result in the more regular tetrahedral structure that had been reported in
the earlier papers [27, 30] .
Weaker bases such as toluene or isopropanol induced some relaxation of the
aluminum in acidic zeolites. Water also exhibited a similar effect [32] , R.W. Joyner,
A.D. Smith, and M. Stockenhuber 2007, unpublished].
One of the disadvantages of EXAFS in general is that it is an averaging tech-
nique. Thus it is very important to make sure the zeolites are very well defi ned.
Other techniques such as
27
Al MAS NMR spectroscopy and in situ infrared spec-
troscopy are needed to ensure single aluminum sites. Depending on the data
quality it is also possible to defi ne more than one type of site; however, in this case
7.2 Applications of EXAFS 311
312 7 X-ray Absorption Spectroscopy of Oxides and Oxidation Catalysts
statistical analysis is absolutely crucial for the determination of the number of
statistically relevant shells [12] . XAS can also be used to characterize site variations.
It has been suggested that XANES can be used to distinguish between tetrahedral,
octahedral, distorted octahedral and square planar geometry [30, 33] .
However, the accuracy of the ab initio calculations for the large number of mul-
tiple scattering pathways present in the XANES region is limited and thus diffi cul-
ties arise in the interpretation of the data (see above).
XANES, particularly the edge position and white line intensity, can, however,
give a good indication of the electron density of the aluminum. The electron
density on the aluminum atom then in turn infl uences the electron density on the
oxygen, which can have an infl uence on acid strength of the solid.
A more direct approach to assess this has been made very recently [R.W. Joyner,
J.A. Purten, C. France and M. Stockenhuber, 2007, unpublished]. Oxygen EXAFS
was recorded for the fi rst time in zeolites and it was possible to get accurate data
for Co
−
O and K
−
O structures from oxygen K - edge data. The XANES for oxygen
also exhibited very rich features which can easily distinguish between the various
counter - cations. It was also possible to separate oxygen interacting with cations
from “ spectator oxygen ” atoms. Oxygen X - ray absorption is relatively diffi cult
experimentally because a good vacuum has to be achieved. Furthermore, detector
technology is diffi cult for the low energies involved. This initial study used electron
yield detection, which is rather noisy and needs mixing of the zeolite with graphite
to get better conductivity. However, solid - state and gas microstrip detectors exist
that can be used for oxygen K - edge detection at 530 eV.
7.2.2
Transition Metal Exchanged Zeolites
Exchanging the proton forms of zeolites with transition metals leads to redox active
catalysts. Great interest in this technology was triggered at the beginning of the
1990s, when Iwamoto [34 – 36] reported the use of copper exchanged ZSM - 5 material
for the selective catalytic reduction of NO
x
. The incentive for this reaction came from
the potential use of the NO reduction process in diesel and lean - burn engines.
Figure 7.8 The relaxation of the aluminum tetrahedron upon
adsorption of ammonia on H - ZSM - 5. Adapted from ref. [33] .

EXAFS of transition metal exchanged zeolites is the best technique for
direct structure analysis of these materials. Under ideal conditions, the transition
metal forms single atoms or very small clusters, which are X - ray amorphous.
EXAFS, on the other hand, in particular for the harder edges, can determine the
local structure of exchanged zeolites very well. It is not surprising that EXAFS
spectroscopy has been widely used to characterize Cu - ZSM - 5 materials. Among
the fi rst to characterize copper species in zeolites was Hamada as early as 1990
[37] . It was suggested that there are signifi cant differences in the copper species
present in the ZSM - 5 zeolite as compared to bulk copper oxides, and a number
of authors proposed that the copper species present in zeolites depend on the
loading. At lower loadings single copper ions were thought to be present in cat-
ionic zeolite positions, whereas at higher loadings small copper clusters were
thought to be the active species in the NO
x
reduction process [37, 38] . The nucle-
arity of the clusters can be determined from fi tting the coordination numbers and
should generally be treated with some caution. However, a combination of the
use of good standards and cautious data treatment generally allows determination
of the cluster sizes involved, even for oxidic materials, which are more diffi cult
to analyze than metal clusters. The advantage of in situ determination of EXAFS
and XANES is very well demonstrated in a number of studies, both of variation
of the local structure and of the oxidation state under reactive conditions [38, 39] .
Deactivation of the catalyst under reaction conditions is one of the biggest prob-
lems of Cu - zeolite catalysts. It is therefore not surprising that a number of studies
have attempted to understand the origin of the problem using XAS. Sulfur and
water tolerance have been investigated in detail [40, 41] .
It was suggested by a number of authors that sintering similar to metal catalysts
leads to deactivation of copper - modifi ed zeolites.
Sulfate formation and coke deposition were often excluded as reasons for deac-
tivation, using EXAFS spectroscopy. The formation of larger clusters in the zeolite,
which can also lead to destruction of the host structure, can be observed by an
increase in the coordination number of the Cu
−
Cu scattering contribution to the
EXAFS.
The formation of extended oxidic species was deemed detrimental to the cata-
lytic performance by most authors. XAS has been instrumental in the detection
of these species.
Deactivation of the copper zeolites under de - NO
x
conditions was one of the
major reasons why the catalyst was never used in a commercial application. Recent
environmental legislation intensifi ed the hunt for a water - and sulfur - stable active
catalyst. One of the most successful preparative methods was reported by Hall and
Feng [42, 43] . They reported excellent de - NO
x
performance based on an iron
exchanged ZSM - 5 zeolite. The activity was reported to remain constant for extended
times, even under high water and sulfur content conditions. The initial catalytic
study initiated a whole raft of characterization studies by a number of groups. The
interest was signifi cantly increased when it became obvious that there are issues
with catalyst preparation reproducibility [44, 45] . XAS was crucial in the discussion
of the structure of active sites for de - NO
x
and the site responsible for the high
7.2 Applications of EXAFS 313
