Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.


3.6 The IR Spectroscopy of Adsorbed Probe Molecules for Surface Chemistry Characterization 133
pounds which fi nd a number of applications in several fi elds. Its thermal decom-
postion gives rise to a mixed oxide whose virtual composition is 5 MgO · MgAl
2
O
4
,
although these phases give rise to partial solid solutions depending on decomposi-
tion temperature. Such materials fi nd several applications in today ’ s industrial
heterogeneous catalysis. Similar materials are obtained using other divalent (Ni,
Co, Zn, Cu) and trivalent (Ga, Fe, Cr) elements with different anions (nitrate,
chloride, etc.) Hydrotalcite - type solids crystallize in the
Rm D
d
3 166
3
5
≡≡n. space
group. The full rhombohedral unit cell contains three cations. However, owing to
the presence of three lattice points in the overall rhombohedral unit cell, the small-
est Bravais cell only contains one cation, that is, one of the above formula units.
Cation distribution in the 3a Wyckoff sites may be considered random. The struc-
ture is formed by brucite - type layers with the formula M(OH)
2
with carbonate ions
in the interlayer region to balance the charge excess due to the substitution of the
trivalent cation for the divalent one. Obviously, there are two trivalent cations per
carbonate ion. The water content is also related to the proportion of trivalent
cations. For x = 0.33 (for every three cations, one is trivalent) there is half a water
molecule and one sixth of a carbonate ion per smallest Bravais cell. The brucite -
type layers are made of fl attened octahedral MO
6
and triply bridging hydroxyl
groups. Both water molecules and carbonate ions stay planar centrally between
the layers and parallel with respect to them. The distance between the protons of
both hydroxyls and water and the oxygen atoms of the carbonate ions and water
are such that hydrogen bonding should be considered to be negligible. The IR
spectra show the features of hydroxyl groups and of the anions [78] .
3.5.4
Impure Metal Oxides
Commercial metal oxide powders may be contaminated by molecules from the
atmosphere as well as those arising from the preparation procedure. Among
typical contaminants, water may produce hydroxides while carbon dioxide can
produce surface or bulk carbonates. This is the case of the “ MgO ” sample whose
spectrum is reported in Figure 3.13 a, which shows the OH stretching band near
3700 cm
− 1
of Mg(OH)
2
and the strong bands in the region 1500 – 1400 cm
− 1
due to
the C
=
O stretching of carbonate species. Both features disappear on heating under
vacuum, owing to decomposition to MgO. Other frequent contaminants are sul-
fates and hydrocarbon species.
3.6
The IR Spectroscopy of Adsorbed Probe Molecules for Surface
Chemistry Characterization
IR spectroscopy of adsorbed probe molecules is mostly performed with either the
transmission/absorption technique or with the DRIFT technique. In the transmis-
sion/absorption technique, self - supporting pressed disks of the pure oxide powders

134 3 The Use of Infrared Spectroscopic Methods
are prepared and put into the IR beam, in an appropriate cell that allows heating,
cooling and gas/vapor manipulation. Activation is mostly performed by outgassing
at relatively high temperature. In the case of DRIFT experiments the catalyst is
deposited on the sample holder, with gentle pressure, and activation is mostly
performed by fl owing in inert dry gas.
The transmission/absorption IR spectrum of the oxide disk has a baseline slope
increasing towards higher frequency, refl ecting the scattering of the beam, which
increases with increasing frequency (wavenumber), as shown in Figure 3.13 b and
c, where the spectra of pressed discs of a “ MgO ” commercial powder are reported.
The slope depends on the particle size and may be very steep for powders that are
constituted by quite large particles (near 1 µ m). Thus, transmission for large particle
size oxides may be zero at 4000 cm
− 1
or even lower frequencies. In the case of
DRIFT, the refl ectance actually increases by increasing scattering, so that the base-
line is fl at or even decreasing. The absorptions due to the surface hydroxyl groups
are superimposed on this line, in the region 3800 – 3000 cm
− 1
. In some cases, absorp-
tions due to overtones of bulk or surface metal oxide stretching vibrations are also
superimposed on the scattering line. On the low - frequency side, below 1300 –
600 cm
− 1
depending on the skeletal spectrum of the solid, the absorptions due to
bulk skeletal fundamental vibrations cut off the spectra of the pure powder disks.
The advantages of the DR technique over the transmission/absorption tech-
nique in the fi eld of the surface chemistry of oxides are:
1) easier sampling ;
2) applicability to powders that scatter too much for the transmission/absorption
technique, assuming the surface area is suffi ciently high to detect surface
vibrations with a suffi ciently high signal - to - noise ratio ;
3) slightly lower sensitivity to bulk conduction phenomena, because of a higher
surface - to - bulk sensitivity ratio.
In effect, for general purposes (adsorption studies on relatively small - crystal - size
powders) the main advantage of the DR technique is the fi rst one, with the disad-
vantages of: (i) less easy optical setting up; (ii) the requirement to work in fl ow
rather than in vacuum (mainly because the sample is not pressed and powders
are highly mobile in vacuum, and because of a more diffi cult evacuation of the
cell, due to their design and size) with a consequently more diffi cult sample
activation.
The spectra of adsorbed and surface species obtained using transmission and
DR techniques are very similar in quality, in regard to resolution, signal - to - noise
ratio and sensitivity.
3.6.1
Infrared Characterization of Surface Element – Oxygen Bonds
3.6.1.1 Surface M – O – M Bridges
The adsorption of probe molecules may provide evidence of the existence of
metal – oxygen bonds at the surface of a metal oxide. In Figure 3.14 , the subtraction
3.6 The IR Spectroscopy of Adsorbed Probe Molecules for Surface Chemistry Characterization 135
spectra relative to the adsorption of pyridine on β - Ga
2
O
3
are reported. Besides the
sharp “ positive ” peaks due to adsorbed pyridine, a broad “ negative ” band is
observed in the range 850 – 880 cm
− 1
, with a tail towards higher frequencies. A
similar absorption is found in several other cases, such as on aluminas [79] and
aluminates [80] just above the cut - off due to the skeletal modes, and is attributed
to the relaxation of surface metal – oxygen bonds by adsorption of molecular
probes.
The existence of surface metal – oxygen bonds can also be indirectly deduced by
the reactivity of these bonds with molecules from the gas phase, such as the
surface hydration producing new hydroxyls, the surface carbonation producing
carbonates, and also some more complex reactivity, such as the reactivity with
alkoxysilanes producing surface alkoxides that may later be converted to surface
hydroxides by elimination [81] .
3.6.1.2 Surface M = O “ Double ” Bonds on Binary Oxides
Metals and other elements in very high oxidation states can give rise to element –
oxygen double bonds in their oxides. This is the case for vanadyl, niobyl,
molybdenyl, chromyl and tungstyl groups, as well as of P
=
O bonds present in
oxo - compounds of the corresponding elements. The location at the surface of
Figure 3.14 FTIR spectra of the surface species produced by
adsorption of pyridine on β - Ga
2
O
3
activated at 673 K: (a) in
the presence of 1 Torr of the vapor and after outgassing at
(b) room temperature, (c) 373 K, (d) 523 K. The spectrum of
the activated sample has been subtracted from those
recorded after adsorption.
136 3 The Use of Infrared Spectroscopic Methods
such bonds has been observed in the cases of V
=
O bonds (1038 cm
− 1
) at the
surface of V
2
O
5
[82] , Nb
=
O bonds at the surface of niobic acid (Nb
2
O
5
.
n H
2
O)
[83] and W
=
O bonds at the surface of WO
3
(950 or 1040 cm
− 1
) [84] . These fea-
tures are more clearly observed as negative bands in the subtraction spectra
after adsorption of probe molecules (spectrum recorded after adsorption from
which the spectrum of the “ clean ” sample has been subtracted), showing that
such surface species are perturbed upon adsorption. Note that double bonds
also exist in the bulk but adsorb at lower frequencies in vanadia and niobia,
while they do not exist in the bulk of tungsta (a distorted ReO
3
structure with
octahedral coordination for the cations) but are formed at the surface as in the
structure of several tungstate species.
3.6.1.3 Surface Oxo - Anions
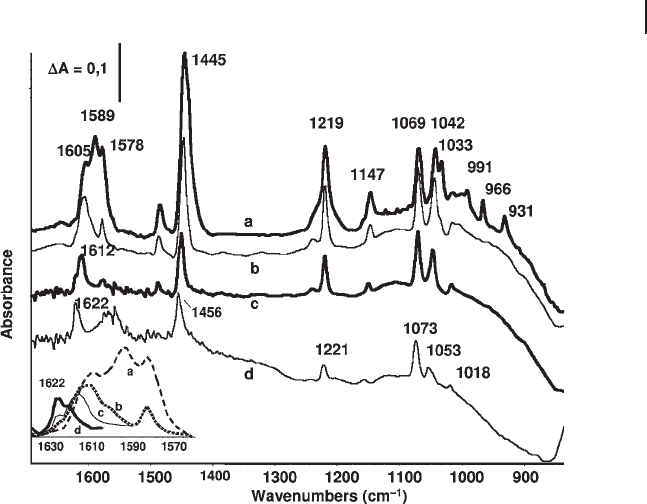
Surface Carbonate, Nitrate and Borate Species As already reported (Table 3.5 ), free
carbonate, nitrate and borate ions, as a result of their trigonal D
3 h
symmetry, have
one characteristic strong IR vibration ( ν
3
; asymmetric XO stretch) found near
1480 cm
− 1
for borates, 1415 cm
− 1
for carbonates and near 1380 cm
− 1
for nitrates,
together with two lower frequency IR - active deformation modes.
The lowering of the symmetry connected with coordination causes the splitting
of the doubly degenerate ν
3
vibration, as well as the IR activation of ν
1
, the Raman -
active symmetric deformation mode. Four different types of carbonate ions are
usually considered as possible surface species. They can be represented by the sim-
plifi ed models I – IV (Scheme 3.1 ). Type I (symmetrical) refers to a surface species
whose spectroscopic features correspond to those of a non - coordinated ion. Mono-
dentate, bridging, chelating and polydentate structures may also be formed. The
structures may be distinguished considering in parallel the stability (polydentate >
bidentate > chelating > monodentate) and the extent of the splitting of ∆ ν
3
(bidentate
≥ chelating > monodentate ≥ polydentate) [85] . Some carbonate species, character-
ized by very large ∆ ν
3
and very weak stability have been denoted as “ covalent ” or
“ organic - like ” , but are most likely due to bent very strongly perturbed CO
2
molecules
[86] . Bicarbonate ions [HCO
3
]
−
can also be formed by adsorption of or contamination
by CO
2
. These species are characterized by ν OH modes near 3620 cm
− 1
, δ OH modes
near 1300 – 1200 cm
− 1
, as well as by two ν C
=
O modes ( ∼ 1600 and ∼ 1450 cm
− 1
).
Surface Silicate and Hydrogen - Silicate Species The addition of silica to oxides such as
alumina and titania is sometimes due to the need to enhance surface area and thermal
Scheme 3.1 Posible coordinations of carbonate ions and
other trigonal anions (nitrates, borates): I: trigonal symmetric;
II: monodentate; III bidentate (bridging); IV chelating;
V: polydentate.
3.6 The IR Spectroscopy of Adsorbed Probe Molecules for Surface Chemistry Characterization 137
stability and retard phase transitions [87] . Surface silication using silicon alkoxides is
also sometimes performed in order to modify the surface acidity [88, 89] . These treat-
ments generally give rise mostly to surface hydrogen - silicate species [HOSiO
3
]
3 −
, well
characterized by the characteristic OH stretching of surface silanol groups near
3735 cm
− 1
, and strong Si
–
O stretching modes in the region around 1000 cm
− 1
. In the
case of basic oxides, such as MgO and CaO, bulk orthosilicate species form easily
[90] . This also occurs by the reactive adsorption of methylsiloxanes [91] .
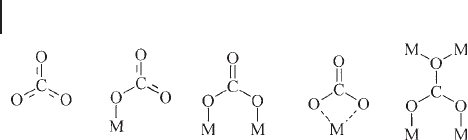
Surface Sulfates Tetrahedral oxo - anions give rise to nine internal vibrations plus
six external vibrations contributing to lattice vibrations and to acoustic modes of
the solid. When the ion takes its highest symmetry (T
d
point group) the symmetric
stretching and the symmetric deformation are Raman active, the last being doubly
degenerate. The asymmetric stretching and deformation are both IR and Raman
active and are triply degenerate. By lowering of the symmetry, as occurs on sur-
faces (Scheme 3.2 ), the degeneracies are broken and all modes can become IR and
Raman active. Scheme 3.2 shows possible geometries of these species, when they
are located on surfaces.
Spectroscopic studies showed that the sulfate ions [92] on ionic oxides in dry
conditions at low coverage, are tetracoordinated with one short S
=
O bond (mono -
oxo structure), characterized by very strong S
=
O bands in the range 1420 –
1350 cm
− 1
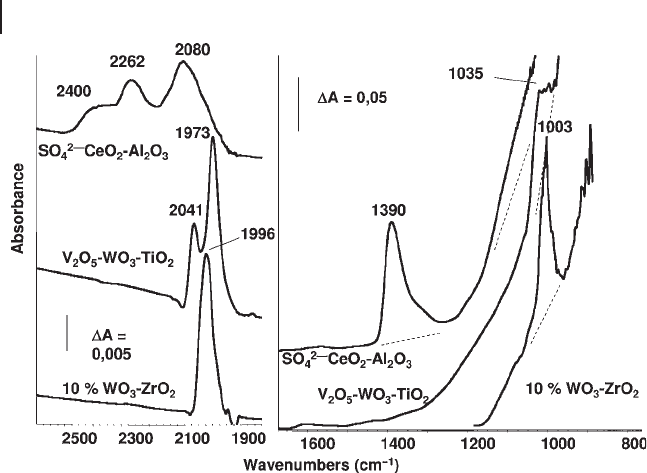
. In Figure 3.15 the spectrum of a sulfated ceria - alumina is presented;
the band at 1380 cm
− 1
is in fact due to this S
=
O stretching mode. S
–
O single - bond
stretching modes are observed below 1250 cm
− 1
, with the corresponding overtone
modes in the region 2500 – 1900 cm
− 1
. At higher coverage, disulfate species are
assumed to exist [93, 94] , although real proof of this probably does not exist.
However, surface sulfate species are strongly sensitive to hydration. The spectrum
changes extensively and the species probably convert into dioxo species.
Surface Vanadate, Molybdate and Tungstate Species The pure and mixed oxides
and the salts of vanadium, molybdenum and tungsten in their higher oxidation
states are used widely as heterogeneous catalysts, for selective oxidation as well as
for acid catalysis. Similarly, supported chromia and rhenium oxides fi nd wide
application in different catalytic processes.
For most of the oxide - supported “ monolayer ” oxides (e.g. vanadia, molybdena
and tungsta supported on alumina), titania, zirconia and silica surface species are
Scheme 3.2 Left: isolated truly tetrahedral metallate species:
the four M
–
O bonds are equally long and the O
–
M
–
O angle
is near 109 ° 28 ′ ; the point group is T
d
; middle, “ deformed ”
fourfold coordinated metallate species, i.e. di - oxo species, the
point group is C
2v
; right, “ deformed ” fourfold coordinated
metallate species, i.e. mono - oxo species, the point group is C
3v
.
138 3 The Use of Infrared Spectroscopic Methods
observed (at least at low loadings, i.e. well below the loading corresponding to the
“ theoretical geometric monolayer ” ) which are spectroscopically characterized after
treatments which allow the desorption of water [42, 47] . Features include:
(i) a very high position of the very intense band observed in IR and Raman
activity, similar to that of gaseous mono - oxo species;
(ii) the coincidence of the position of the band detected in Raman and IR spectra
and its non - complexity in both cases;
(iii) the detection in the IR spectrum of a single overtone band.
Partial
18
O/
16
O exchange experiments showed a simple splitting like that found,
for example, in the cases of WO
3
/Al
2
O
3
[95] and V
2
O
5
/TiO
2
catalysts [96] .
These data are a strong demonstration that the species are isolated and mono -
oxo. In Figure 3.15 , the fundamental (right) and fi rst overtone region (left) for a
WO
3
/ZrO
2
acid catalyst and of a V
2
O
5
- WO
3
/TiO
2
SCR. DeNOx catalyst are shown.
The strong band at 1003 cm
− 1
and the weaker one at 1996 cm
− 1
are due to the
stretching mode (fundamental and fi rst overtone, respectively) of W
=
O “ double
bonds ” of mono - oxo tungstyl species of the WO
3
/ZrO
2
catalyst. The same modes
are found at ∼ 990 and 1973 cm
− 1
for the V
2
O
5
- WO
3
/TiO
2
catalyst where the bands
at 1035 and 2041 cm
− 1
are due to the fundamental and the fi rst overtone, respec-
tively, of the V
=
O double bonds.
On the other hand, a further demonstration of the “ isolated ” nature of these
species is expected from the absence of modes assignable to M
–
O
–
M asymmetric
Figure 3.15 FTIR spectra of pure powder pressed disks of
sulfated ceria - alumina, V
2
O
5
- WO
3
- TiO
2
catalyst and WO
3
- ZrO
2
catalyst.
3.6 The IR Spectroscopy of Adsorbed Probe Molecules for Surface Chemistry Characterization 139
stretchings. These modes are expected to be very strong in the IR spectra, but
unfortunately the region of these modes is not available for alumina and titania
supported catalysts because of the opacity of the pressed disks in this region. In
the case of zirconia supported catalysts, where this region is in part available, this
band is not present, at least for low loaded molybdena [97] and tungsta [98]
samples. This topic has been discussed in detail previously [99] . At high loadings,
most authors agree that polymeric anions form.
3.6.2
Spectroscopic Detection of Surface Br ø nsted Acid Sites
The fragments arising from the dissociative adsorption of water on the surface of
metal oxides give rise to hydroxyl groups that are potentially active Br ø nsted acid
sites [100] or basic sites. Such surface hydroxyl groups can be detected directly,
recording the IR spectra of the oxide catalyst powders after treatments that allow
the desorption of molecularly adsorbed water, in the region 3800 – 3000 cm
− 1
, where
the O
–
H stretching modes ( ν OH
s
) fall. The position and shape of the ν OH bands
of the surface hydroxyl groups is informative on their coordination. Covalent oxide
components [101, 102] usually give rise to very typical strong sharp peaks due to
covalently bonded terminal OH groups. For example, silica - containing oxides (e.g.
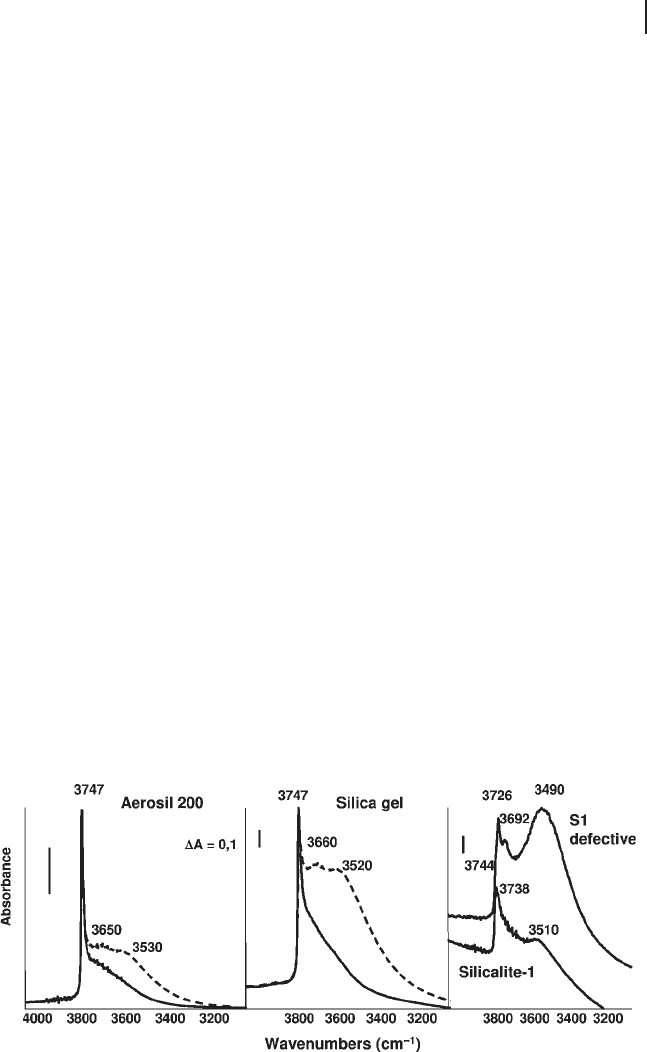
any silicas and silicated oxides, see Figure 3.16 ) almost invariably show a peak in
the range 3750 – 3730 cm
− 1
while P
2
O
5
- containing samples contain a sharp band at
3670 – 3650 cm
− 1
observed in supported phosphoric acid samples [103 – 105] and in
several bulk phosphates [69, 83, 106 – 108] . Similarly, B
–
OH bonds give a sharp
band at 3710 – 3690 cm
− 1
[109, 110] , Ge
–
OH bonds at 3685 – 3670 cm
− 1
.
Conversely, in ionic oxides bridging and triply bridging hydroxyl groups are also
formed at the surface. Thus, the OH spectrum is frequently more complex (see,
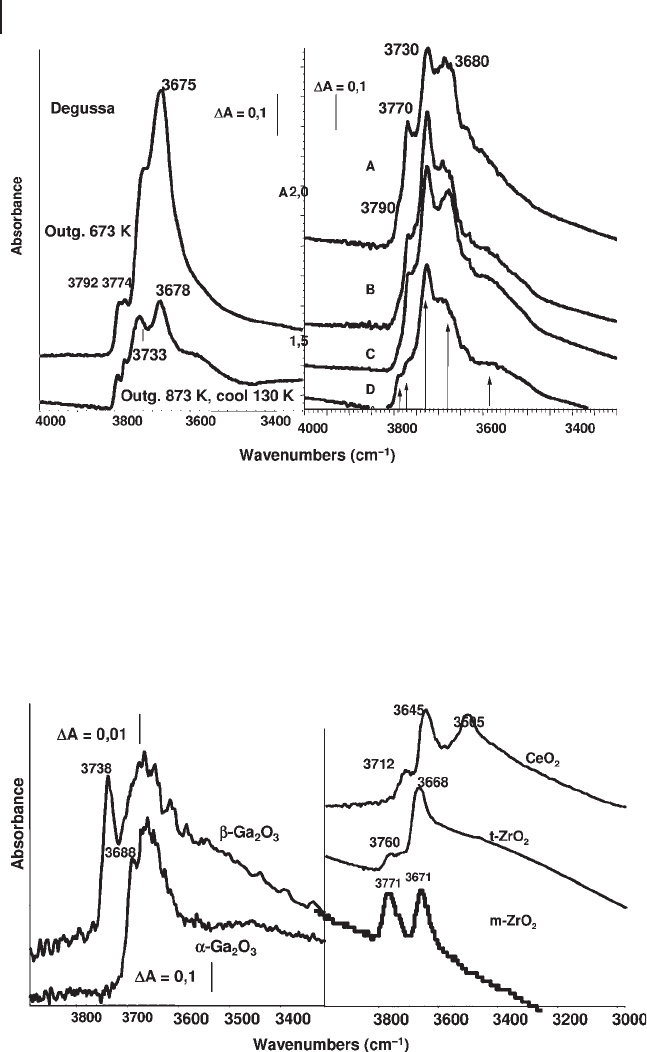
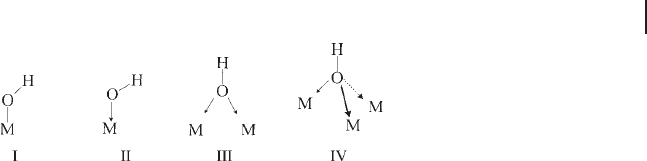
for example, Figure 3.17 for alumina and Figure 3.18 for gallias, zirconias and
ceria). It is usually agreed that the OH stretching is highest for terminal OH
Figure 3.16 FTIR spectra of the surface hydroxyl groups of
SiO
2
samples after outgassing at 473 K (broken lines) and 673 K
(full lines).
140 3 The Use of Infrared Spectroscopic Methods
Figure 3.17 FTIR spectra of the surface hydroxyl groups of
γ - Al
2
O
3
samples: left aluminum oxide C from Degussa after
outgassing at 673 K and 873 K; right, samples from Akzo (A),
Sud Chemie (B), Engelhardt (C) and Condea (D), all having
surface area in the range 180 – 200 m
2
g
− 1
.
Figure 3.18 FTIR spectra of the surface hydroxyl groups of
α - Ga
2
O
3
, β - Ga
2
O
3
, monoclinic ZrO
2
, tetragonal ZrO
2
and
CeO
2
, all after outgassing at 773 K.
3.6 The IR Spectroscopy of Adsorbed Probe Molecules for Surface Chemistry Characterization 141
groups, intermediate for bridging and lowest for triply bridging OH groups (see
Scheme 3.3 ) This approach was fi rst systematically proposed by Tsyganenko and
Filimonov [111] . Interesting support for this approach was given more recently by
the group of Lavalley [112] who showed a relationship between the OH stretching
bands of the surface hydroxyl groups and the C
–
O stretching bands of the surface
methoxy groups of methoxylated oxides, namely zirconia, ceria and thoria.
3.6.2.1 Hydroxyl Groups in Silica
Structurally, amorphous silica is quite a covalent material [101, 102] whose surface
behavior is dominated by the chemistry of the terminal silanol groups, characterized
by a sharp and strong IR OH stretching band at 3748 – 3730 cm
− 1
(Figure 3.16 ). The
IR band of silanol groups always presents a tail to lower frequencies, which has
been in part attributed to geminal silanols. Near - lying terminal silanol groups also
make hydrogen bonds with each other, giving rise to chain - bonded hydroxyls. These
groups, responsible for broad OH stretching bands near 3650 and 3530 cm
− 1
, con-
dense upon outgassing at high temperature, to produce gas - phase water and surface
siloxane bridges. The relative amount of isolated and H - bonded silanols depends
on porosity [113] , as shown in Figure 3.16 where the spectra of a highly porous silica
gel are compared with those of an almost non - porous material (Aerosil). The silanols
make the surface of highly hydroxylated silicas strongly hydrophilic, and their wet
surfaces are even more active in adsorption. It is well known that hydrogen bonds
also occur between the silanol groups and non - polar molecules such as hydrocar-
bons, allowing the use of silicas for adsorption of these compounds.
Silicalite - 1 is a fully siliceous zeolite, with the MFI structure. Its crystalline
framework, composed of SiO
4
tetrahedra, has an essentially covalent and hydro-
phobic character. When well crystalline, hydrophilic silanols, whose acidity is
comparable with that of silica [113, 114] , are present essentially at the external
surface (Figure 3.16 ). However, when prepared in a “ defective ” form, nests of H -
bonded silanol exist, giving rise to a quite complex IR spectrum in the OH stretch-
ing region with a prominent band at 3500 cm
− 1
and several sharp maxima in the
range 3745 – 3650 cm
− 1
; they are at least in part located in the channels [115] and
make the structure more hydrophilic.
3.6.2.2 Hydroxyl Groups in Alumina
Many studies have been devoted to the multiplicity of the surface hydroxyl groups
of aluminas. After the work of Peri [116] , and of Tsyganenko and Filimonov [111] ,
Scheme 3.3 Structure of hydroxyl groups on metal oxides:
I: covalent, terminal; II: ionic, terminal, III: ionic, bridging;
IV: ionic, triply bridging.
142 3 The Use of Infrared Spectroscopic Methods
Kn ö zinger and Ratnasamy reported a very popular model of the different exposed
planes of spinel type aluminas [117] . This model has been later modifi ed by Busca
and coworkers [80, 118] and reviewed by Morterra and Magnacca [119] . More
recently, additional investigations have been published by Tsyganenko and Mar-
dilovich [120] and, on the basis of theoretical calculations by Fripiat and coworkers
[121], by Digne and coworkers [122] , who also attempted to model the interaction
of probe molecules. Lambert and Che [123] further reviewed these models and
demonstrated that the problem is still not solved. At least fi ve components are
usually present in the IR spectrum of the hydroxyl groups of aluminas (Figure
3.17 ), at about 3790, 3770, 3740 – 3720, 3700 – 3670 and 3580 cm
− 1
, although in many
cases the observed peaks are multiple. The available data show that the hydroxyl
groups absorbing at higher frequency (above 3700 cm
− 1
) are available for interac-
tion with molecular probes, while those absorbing below 3700 cm
− 1
appear to be
substantially inactive [124, 125] . Liu and Truitt [126] emphasized the close proxim-
ity of surface OH groups of aluminas to Lewis acid sites. The bands below 3700 cm
− 1
are more sensitive to outgassing than those above 3700 cm
− 1
(Figure 3.13 , left)
suggesting that they can be hydrogen bonded. To our knowledge, a complete
investigation of the accessibility of these sites has not yet been published.
3.6.2.3 Surface Hydroxyl Groups of Other Sesquioxides and of Spinel - Type
Mixed Oxides
The spectra of the surface hydroxyl groups of oxides with similar structures show
some parallelism [118, 119] . In Figure 3.18 , left, the spectra of the surface hydroxyl
groups of gallia polymorphs α - Ga
2
O
3
and β - Ga
2
O
3
are shown. In Table 3.7 the
observed bands for these solids are compared with those observed for the corre-
sponding alumina polymorphs α - Al
2
O
3
and θ - and γ - Al
2
O
3
, as well as for the poly-
morphs of ferric oxide α - Fe
2
O
3
and γ - Fe
2
O
3
. α - Al
2
O
3
, α - Fe
2
O
3
and α - Ga
2
O
3
are
isostructural as is α - Cr
2
O
3
(corundum – haematite structure) with only octahedral
coordination for the cations, while β - Ga
2
O
3
, θ - and γ - Al
2
O
3
and γ - Fe
2
O
3
are all
structures derived from non - stoichiometric spinels with both tetrahedral and octa-
hedral coordination for the cations. The IR spectra of the surface hydroxyl groups
show some similarity, supporting the assignments of the higher frequency band
to terminal OH groups on cations in a tetrahedral - like environment. These bands
are always split, and are present in the spectra of the spinel - type sequioxides, but
Table 3.7 Surface OH groups on sesquioxides.
γ - Al
2
O
3
α - Al
2
O
3
γ - Fe
2
O
3
α - Fe
2
O
3
β - Ga
2
O
3
α - Ga
2
O
3
IV
M
3+
I 3790 – 3740 – 3745
IV
M
3+
II 3770 – 3725 – 3738
VI
M
3+
I 3730 3730 3675 3680, 3660 3685 3688
Bridging 3680 3690 3640 3640 3670 3660
Triply bridging 3580 3550 3450 3480, 3440 3550
