Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.

higher refraction index than the other, and the incidence angle is greater than the
limit for refraction.
In the fi rst case, it has been recognized that if molecules are adsorbed on the
surface of a metal, only part of the grazing incident radiation is refl ected, part
being absorbed by the adsorbed species. This absorption is greatly increased if the
incident radiation is polarized perpendicular to the metal surface. This is the basis
of so - called Infrared Refl ection Absorption Spectroscopy ( IRRAS ) [27], which is
applied widely to surface studies on metal surfaces. In these conditions, an addi-
tional selection rule applies:
∂
∂
⎛
⎝
⎜
⎞
⎠
⎟
≠
⊥
µ
Q
0
0
(3.17)
which means that only the adsorbate vibrations associated with a change in the
dipole moment perpendicular to the crystal surface are detectable. The total refl ec-
tion technique can be applied to detect the growth of oxidic layers over metal sur-
faces (i.e. the skeletal bands of the oxide layers) and this is a widely applied
technique in corrosion and electrochemical research, colloid chemistry and coating
technology.
The second case refers to the so - called Internal Refl ection Spectroscopy, that is
used in the so - called Attenuated Multiple Total Internal Refl ection technique
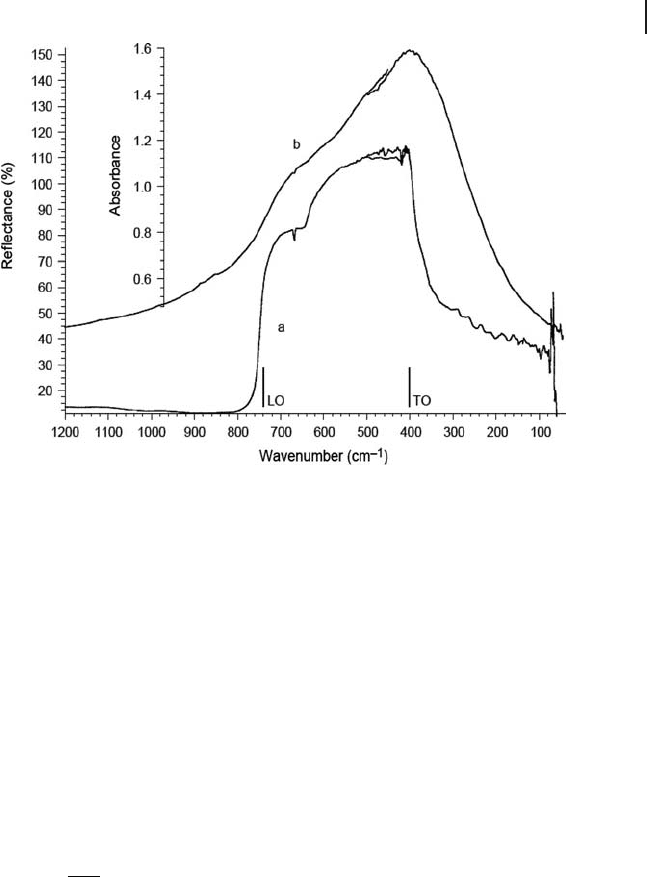
Figure 3.1 (a) FTIR refl ectance spectrum of a
MgO monocrystal (incidence angle 26.5 ° ,
face [001]). (b) FTIR and FTFIR absorption /
transmission spectra of MgO powder
(reprinted with permission from G. Busca
and C. Resini, “ Vibrational spectroscopy
for the analysis of geological and inorganic
materials ” , in Encyclopedia of Analytical
Chemistry , Robert A. Meyers ed., Wiley,
Chichester, 2000, pp. 10984 – 11020).
3.2 Experimental Techniques 103

104 3 The Use of Infrared Spectroscopic Methods
( ATR ) [28], where an absorbing layer is deposited on one or two external surfaces
of a prism. The light penetrates the prism from a free surface and is multiply
refl ected by the other faces. During the refl ection, part of the light penetrates the
external layer and is absorbed by it. Thus, in the case of the internal refl ection
techniques the refl ectance is given by the following relation:
RA cd=− =−11ε
(3.18)
where d is the thickness of the layer penetrated by the radiation, c the molar con-
centration of the absorbing species in that layer and ε the molar absorption coef-
fi cient of those species. An interesting variation of this technique is the so - called
Cylindrical Internal Refl ection technique ( CIR ), invented by Wilks [29] . In this
case, for example, a hydrogel slurry can be analyzed using cylindrical crystals
immersed in the medium so that IR radiation is multiply transmitted and refl ected
at the internal surface. This technique could be successfully applied to heteroge-
neous catalytic systems at the water – solid interface, or in stages of catalyst prepara-
tion (for example zeolite syntheses).
Another interesting variant of the total refl ection technique is the so - called
Surface Electromagnetic Wave Spectroscopy ( SEWS ), which consists of the genera-
tion of a surface plasmon on a substrate by frustrated total internal refl ection in
a prism located a few microns from the surface. This plasmon is decoupled by a
second prism. Some interesting data relating to surface modes on alumina have
been reported with this technique [30] .
3.2.4
The Diffuse Refl ectance Technique
In recent years, the use of IR spectroscopy of powders in the diffuse refl ectance
( DR ) mode has grown strongly, assisted by the commercialization of appropriate
attachments and cells. This technique is particularly attractive because it does not
require much effort in sample preparation (the powder is simply deposited in a
sample holder). Moreover, this technique, in contrast to the above transmission
technique, takes advantage of light scattering, and hence is very useful for studies
of surface chemistry.
This technique requires the collection, with appropriate collecting mirrors (such
as an integrating sphere), of the radiation scattered by the sample. Obviously, most
photons are essentially simply scattered but those corresponding to the energies
of vibrational transitions are potentially absorbed. The interpretation of the DR
spectra is based on the phenomenological theory of Kubelka and Munk [31, 32]
who defi ned the so - called Kubelka – Munk ( KM ) function as follows:
fR R R ks
∞∞∞
()
=−
()
=12
2
(3.19)
where R
∞
is the refl ectance of an “ infi nitely thick ” layer and may in practice be
substituted by RR
∞∞
−
′
(i.e. by the refl ectance spectrum from which the refl ectance

of a reference transparent material such as KBr has been subtracted). The KM
function depends linearly on the absorption coeffi cient k (which can be expressed
as k = 2.303 ε c , where ε is the molar absorption coeffi cient and c is the molar con-
centration) and inversely on the scattering factor s . Obviously, the greater the
absorption coeffi cient and the smaller the scattering, the higher the KM function.
An approximation of the KM function is that k is treated as a variable but s is
assumed constant. This is not true in the vicinity of strong absorptions.
More realistic and complex theories consistent with the quite complex nature
of the phenomena involved in the DR of light were developed later. Experimental
studies have shown that this technique is affected by particle size, granulometric
distribution and the refractive index of the particles, which has an important role
when the particle size is near the wavelength of the IR radiation. Diffuse refl ec-
tance Fourier transform ( DRIFT ) studies in the FIR region allow detection of the
skeletal spectra of materials, such as mixed oxide catalysts, pigments and metal
halides.
One important practical problem in DR measurement is the need to cancel the
specular refl ectance from the front surface of the sample, which generates “ nega-
tive ” bands in the DRIFT spectrum, so causing apparent shifts in the true absorp-
tion bands. This is achieved by using appropriate cell designs in commercial
DRIFT attachments.
3.2.5
The Emission Technique
According to Kirchoff ’ s law:
εα==II
em b
(3.20)
the emittance, ε , (i.e. the ratio of the light emitted by the sample I
em
with respect
to that emitted by a black - body at the same temperature, I
b
) is equal to the absor-
bance, α , of the sample. The emissivity is proportional to the fourth power of the
temperature difference between the emitting sample and the detector (Stefan ’ s
law). This implies that emissivity is suffi ciently strong at relatively high tempera-
tures to give a good signal - to - noise ratio in a large part of the IR spectrum. In fact,
the blackbody emission at T < 1000 K shows its maximum in the medium IR
region.
A number of problems arise in connection with the use of emission IR spec-
troscopy ( IRES ). One of them arises from the existence of temperature gradients,
which can cause self - absorption of the emitted radiation by the colder outer parts
of the sample itself; another is concerned with the selective refl ection that occurs
in the vicinity of strong absorption bands. This reduces the absorptance and hence
the emittance. Moreover, perturbations can be created by refl ections and emission
by the cell elements. These problems, however, can in part be overcome so that
IR emission spectra can be successfully recorded and are widely used, for example,
in the fi elds of polymer and corrosion science and mineralogy. Some uses of IRES
3.2 Experimental Techniques 105

106 3 The Use of Infrared Spectroscopic Methods
of metal oxides and in the fi eld of surface chemistry and catalysis were reviewed
some years ago by Sullivan and coworkers [33] . These authors reported several
examples of emission spectra of oxide catalysts and of adsorbates on supported
metals, and their review cites at least 11 papers concerning metal oxide surfaces.
Emission studies on minerals and catalytic materials [34] and on adsorption on
metal oxide gas sensors [35] have been published more recently. Some of the
advantages of this technique are the very easy sample preparation and its easy
applicability at high temperatures (150 – 400 ° C). It can be applied to investigate the
temperature - dependence of the radiative properties of materials including, for
example, glasses.
3.2.6
Photoacoustic and Photothermal Techniques
When an IR beam is incident on a solid surface it can be absorbed in part, and
this leads to its conversion into heat. If the beam is modulated (as in interferom-
eters like those of FTIR instruments) and the solid is in contact with a gas (air,
He, Ar, etc.), its conversion to heat gives rise to an acoustic signal. In fact, the
periodic temperature rise so obtained causes a periodic modulation of a gas pres-
sure in the cell, and this can be detected by a sensitive microphone. This acoustic
signal will be the more intense the stronger is the absorption at a particular wave-
length. Refl ected and scattered light are not absorbed and hence do not cause a
photoacoustic signal. However, light absorbed by the gas over the sample causes
signals. This makes necessary the use of monatomic non - absorbing gases (He, Ar,
etc.) The photoacoustic effect, discovered as early as in 1880 by Bell [36] , could be
successfully applied only after the work of Rosencwaig and Gersho [37] in 1976.
The main limits of this technique are that (i) it requires a gaseous atmosphere;
(ii) the cell needs a microphone close to the sample, so the sample cannot be
heated and otherwise activated conveniently; (iii) the technique has an intrinsically
low signal - to - noise ratio. On the other hand, the photothermal effect is much more
effi cient for species in the vapor phase than for bulk and surface species. Photo-
acoustic spectrometry ( PAS ) is not exactly a surface spectroscopy, because the
penetration of the thermal effect is always signifi cant. The extent of this depends
on the modulation frequency, which varies with wavenumber in a FT spectrum.
The PAS technique has found successful application in several fi elds including
heterogeneous catalysis, as reviewed in ref. [38] .
An alternative technique is the so - called Photothermal Beam Defl ection Spec-
troscopy [ PBDS ], based on the so - called “ mirage ” effect fi rst reported by Boccarra
and coworkers [39, 40] . In this case, the periodic temperature rise caused by the
absorption of the modulated IR radiation (i.e. the photothermal effect) is detected
optically because it causes periodic defl ections of a laser beam passing close to the
surface of the solid sample. The PBDS technique has some advantages over the
PAS technique, because of its lower limits of sample dimensions, but it has dis-
advantages because of the critical geometric setup. Like PAS, PBDS can have
advantages with respect to traditional IR technique for the detection of surface

vibrations in very opaque materials. This has resulted in its application to carbons
and coals.
3.3
The Vibrational Modes of Molecular Species and of Inorganic Solids
3.3.1
“ Isolated ” Molecular Species
The total degrees of freedom associated with a chemical species containing N
atoms are 3 N . If this chemical species is a molecule in the gaseous state, three of
these degrees of freedom are associated with its translations and another three
with its rotations, so that, in the most general case, six modes are associated with
“ external ” motions (rotations and translations). The remaining 3 N − 6 degrees of
freedom are associated with “ internal ” vibrational modes. However, if the mole-
cule is a linear one, only two degrees of freedom are associated with rotations,
because no rotational freedom exists around the molecular axis. Thus, in this
particular case, the degrees of freedom associated to vibrations are 3 N − 5. A
complete treatment of the principles of vibrational spectroscopy is beyond the
scope of the present chapter. The methods for the determination of the number
and the optical activity of the vibrational modes of molecular species can be found
in several books [41 – 43] .
The 3 N − 6 (or 3 N − 5) degrees of vibrational freedom give rise to vibrational
modes that differ in their symmetry with respect to the symmetry elements of the
molecular point group and in their multiplicity. The non - degenerate modes are
denoted as A or B in relation to their symmetry or antisymmetry with respect to
the rotation about the principal symmetry axis. Doubly degenerate modes and
triply degenerate modes are denoted E and F respectively. The subscripts g and u
denote modes that are symmetric or antisymmetric, respectively, with respect to
the center of symmetry (if any), the superscript symbols ′ and ″ distinguish modes
that are symmetric or antisymmetric, respectively, with respect to symmetry planes
not containing the principal axis ( σ ≠ σ
v
), while 1,2,3 subscripts are related to the
symmetry with respect to other symmetry axes.
Character tables, which can be found in several vibrational spectroscopy books,
allow the determination, for any molecular point group, of the species (or irreduc-
ible representations) in relation to the symmetry elements typical of that group.
As further cited below, the classifi cation in terms of a particular symmetry species
determines the activity (IR activity, Raman activity, both IR and Raman activity or
inactivity) of any mode.
In the gas phase, the vibrational transitions couple with the rotational ones,
giving rise to rotovibrational spectra . The different rotovibrational contours depend
on the symmetry of the vibration in relation to the symmetry of the molecule, and
on the resolution of the rotational components. In some cases, the energy of the
“ pure ” vibrational transition corresponds to the minimum of the absorption band:
3.3 The Vibrational Modes of Molecular Species and of Inorganic Solids 107
108 3 The Use of Infrared Spectroscopic Methods
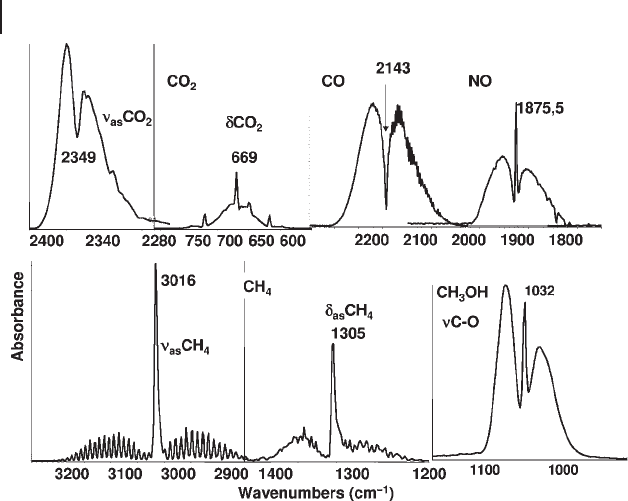
in fact the “ pure ” vibrational transition is forbidden because the rotational quantum
number does not change. This is the case for gaseous CO
2
and CO, as shown in
Figure 3.2 . In other cases the energy of the pure vibrational transitions must be
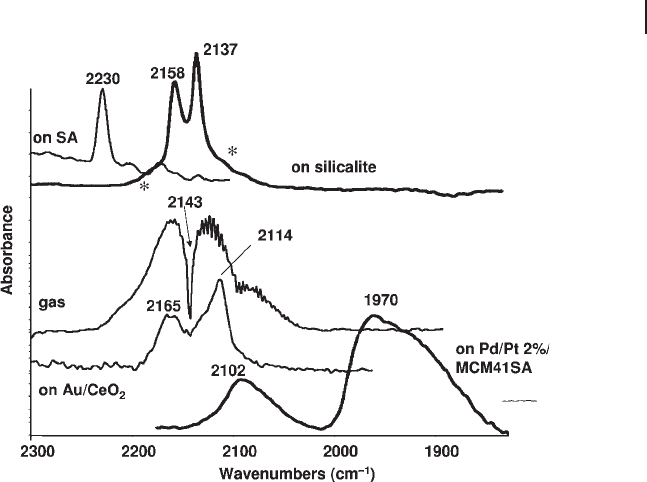
determined using the sharp maximum in the middle. In Figure 3.3 the spectrum
of gaseous CO is compared with that of CO adsorbed on different solids. The
complex structure due to the rotovibrational contour present in the gas phase
spectrum is essentially lost upon adsorption. When weakly adsorbed, such as on
the OH group of silicalite, some rotational features may still be present (see aster-
isks in the fi gure) because the molecule can still rotate around its main axis. The
CO stretching frequency for gaseous CO (2143 cm
− 1
) shifts a little down in the liquid
phase (2137 cm
− 1
) where the rotational structure has disappeared. Liquid CO is
frequently observed upon adsorption on solids at low temperature (such as on sili-
calite in the fi gure). Relevant shifts upwards and downwards in the adsorbed state
are relative to the different interaction on cationic sites and hydroxyl groups, or on
reduced metal centers, on which the use of CO as a probe is based (see below).
3.3.2
Crystalline Solids
When a crystalline solid is considered, the N atoms present in the smallest (primi-
tive) Bravais cell must be taken into consideration to count the fundamental
vibrational modes. They give rise to 3 N total degrees of freedom, three of which
Figure 3.2 FTIR spectra of gaseous CO
2
, CO, NO and CH
4
(IR active fundamental modes) and of CH
3
OH (C
–
O stretching
mode only).
give rise to translational modes of the cell as a whole, which are the acoustic
modes. Thus, the vibrational degrees of freedom (optical modes) are, in this case,
3 N − 3 .
If the solid is molecular, the molecules (considered to be formed by M atoms,
where M = N / r and r is the number of molecules in the smallest Bravais cell) can
be treated as for the gas phase, so giving rise to 3 M − 6 (or 3 M − 5 if linear) vibra-
tions for each molecule. The degrees of freedom associated with the “ external ”
modes of every molecular unit (6 r for non - linear molecules and 5 r for linear mol-
ecules) give rise to lattice vibrations ( “ frustrated translations and rotations ” ) and
to three acoustic modes. On the other hand, the “ internal ” vibrations of each
molecules should in principle give rise to r - fold splitting, owing to the coupling of
the vibrations within its primitive unit cell as a whole.
Analysis of the symmetry of the chemical species under study (i.e. the point
group for a “ free ” molecule, the space and factor groups for a crystal [44] ), accord-
ing to the site symmetry of every atom, allows the determination of the irreducible
representation of the total modes and, after the subtraction of the translational
and rotational modes (the acoustic modes for the crystals), the irreducible repre-
sentation of the vibrational (or “ optical ” ) modes can be obtained. This means that
the number of vibrational modes belonging to the symmetry species associated
with the molecular or crystal symmetry can be counted. Consequently, the number
of active modes can be counted, according to the symmetry selection rules of the
different techniques (in particular IR and Raman).
Figure 3.3 FTIR spectra of gaseous CO and of CO adsorbed
on silica - alumina, on silicalite (150 K), on Au/CeO
2
and on
Pt - Pd alloy nanoparticles on MCM41 mesoporous
silica - alumina.
3.3 The Vibrational Modes of Molecular Species and of Inorganic Solids 109

110 3 The Use of Infrared Spectroscopic Methods
Every vibrational mode is due to motions of the entire chemical species (the
molecule or the primitive unit cell) as a whole. In principle, molecular and crystal
dynamics calculations should defi ne rigorously the motions of every atom of the
chemical species upon a vibrational mode. This approach gives rise to a very
complex picture (at least for large and complex chemical species), so that the
results are sometimes not easily interpreted and comparison between the vibra-
tional behavior of similar species is frequently diffi cult.
However, a group approximation can be frequently used. Thus, the vibrational
modes can often be approximately attributed to the movements of small groups
of atoms. This is possible in particular if very different bonds are present in the
molecule, so that the coupling of their movements is negligible. In this way, it is
possible to “ dissect ” the chemical species under study into “ pieces ” and to consider
separately the vibrations of groups of few atoms (like the functional groups in
organic compounds). This makes possible a satisfactory, although approximate,
interpretation of the vibrational spectra of polyatomic molecules and of some
crystals, and allows easy comparison between the vibrational features of related
chemical species.
In the case of crystalline solids, more than one equivalent structural unit may be
present in the primitive cell. This results in splittings of the fundamental vibrational
modes of these units. In the case of many crystalline solid materials “ covalent ”
units (e.g. oxo - anions for oxo - salts) are present, together with other groups bonded
by ionic bonds (e.g. the cations in the oxo - salts). According to the above group
approximation, the internal vibrations of the covalent units can be considered sepa-
rately from their external vibrations ( hindered rotations and translations of the group
that fi nally contribute to the lattice vibrations and to the acoustic modes of the unit
cell) and those of the other units. The presence of a number of covalent structural
units in the primitive cell, causes their internal modes to split.
On the other hand, when the unit cell is centrosymmetric the mutual exclusion
rule is valid, so that Raman active modes are IR - inactive and vice versa. In practice,
for centrosymmetric cells containing N oxo - anions, every internal vibrational
mode of the oxo - anion gives rise to N /2 IR active modes and N /2 Raman active
modes.
Additionally, the TO/LO splitting may be relevant and makes more complex the
identifi cation of the vibrational modes of crystalline solids.
3.3.3
Amorphous Solids
Amorphous solids differ from crystalline solids because no long - range order
occurs. So, variable coupling exists between the vibrational modes of similar or
equivalent structural units. Consequently, amorphous solids can be treated in the
same way as liquids and gases. The vibrational spectra of amorphous materials
can present a smaller number of broader features than those of corresponding
crystalline materials, where crystal coupling effects can produce multiple sharp
features.

3.4
The Skeletal IR Spectra of Metal Oxides
IR spectroscopy is largely used for the characterization of metal oxide catalysts in
relation to their structural features, with additional possible information on their
morphology. Several collections of IR, Raman or both IR and Raman spectra of
inorganic materials and minerals have been published, and are available electroni-
cally. In the following we will briefl y review some of the applications of vibrational
spectroscopies in the characterization of such materials.
3.4.1
Crystalline Simple Anhydrous Oxides
In network oxide structures the metal – oxygen bonds are all almost equivalent, so
that their movements couple extensively. Also stretching and deformation vibra-
tions mix. As a result of this, assignments of the different modes to a particular
vibration can be very uncertain. However, the highest frequency vibrational fea-
tures can be generally associated with stretching modes. The position of these
modes can be assumed to be primarily dependent on the coordination of the metal
ions involved and on the extent of the condensation of the resulting polyhedra, as
shown in Table 3.1 . This approach has been proposed by Tarte [45, 46] and is a
useful approximation for the interpretation of the IR and Raman skeletal spectra
of oxides of unknown structure. On the other hand, and perhaps more correctly,
analysis of the spectra suggests that the higher frequency modes are mainly to be
assigned to motions of the lighter oxygen atoms, so that they can be assigned to
stretchings of oxygen in different coordination states. This alternative approach
proposed fi rst by Beattie and Gilson [47] , is shown in Table 3.2 .
Most simple oxide structures have been the object of extensive studies and
complete vibrational analysis. Some examples are summarized in Table 3.3 .
3.4 The Skeletal IR Spectra of Metal Oxides 111
Table 3.1
Absorption range (cm − 1) of some elements and coordinated compounds.
Atom X Coordination
Tetrahedral XO
4
Octahedral XO
6
Isolated Pure oxide Isolated complex
Ge
IV
800 – 700 700 – 800
∼ 500
Ti
IV
1000 – 650 600 – 300 500 – 400
Al
III
850 – 700 700 – 300 500 – 350
Ga
III
750 – 600 600 – 350
Cr
III
450 – 300
Fe
III
650 – 550 650 – 300 400 – 300
Mg
II
700 – 550 450 – 300
Zn
II
650 – 400
< 300
Fe
II
< 350

112 3 The Use of Infrared Spectroscopic Methods
Table 3.2 Typical oxygen atom vibrational modes for metal oxide structures.
Structure No. of
oxygen
atoms
No. of
vibrational
modes
Stretchings Frequency
region
(cm
− 1
)
Deformations
Terminal
M
=
O
1 3 1 1500 – 800 1 degenerate
MO
2
2 6 asym +
sym
1300 – 700 bending twisting Wagging Rocking
MO
3
3 9 asym(2) +
sym
1300 – 700 2 asym.
bendings
sym.
bending
2 rockings Torsion
Bridging
M
–
O
–
M 1 3
Bent asym +
sym
1200 – 600 rocking
Linear asym bending degenerate
Triply
bridging
M
3
O 1 3
Pyramidal asym(2) +
sym
< 600
Planar asym(2) out - of - plane
3.4.1.1 Oxides of Divalent Elements
According to its very high symmetry, the rock - salt structure taken by several diva-
lent metal oxides, (space group Fm m O3
5
≡
h
, with Z = 4, and with one formula
unit only in the smallest Bravais mcell) only gives rise to one triply degenerate IR
active mode. The TO quoted for MgO at 401 cm
− 1
(see Figure 3.1 a), corresponds
to the lower frequency limit of the refl ectance band while the LO corresponds to
the higher energy limit of the refl ectance, and is quoted at 718 cm
− 1
for MgO (see
Figure 3.1 a). The transmission/absorption IR spectrum of the MgO powder shows
the maximum slightly above ν
TO
(407 cm
− 1
in our spectrum of a polyethylene disk)
and a shoulder near ν
LO
. Other components arise from particles with different
morphologies although the microcrystal powder spectrum of MgO is also affected
by surface relaxation. ν
TO
is found near 330 cm
− 1
for MnO (manganosite) and near
405 cm
− 1
for NiO powder, which also have a rock - salt type structure.
