Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.


The cation - to - anion vibrations (lattice vibrations) are mainly located in the FIR
region and their assignments, based on similar considerations to those reported
for ionic oxides, are frequently diffi cult.
Salts with Planar - Trigonal and Pyramidal
MO
3
n--
Oxo - Anions The movements of the
four atoms present in the
MO
3
n−
oxo - anions give rise to twelve total modes, of
which six are associated with the movements of the anion as a whole (rotations
and translations, which contribute to the lattice vibrations and to the acoustic
modes of the solid). So, six “ internal ” vibrations occur. The highest symmetry
point group for this ions is D
3 h
, occurring when it is planar trigonal and isolated.
This is typical for isolated carbonate, nitrate and orthoborate anions. In this case,
as shown in Table 3.5 , the symmetric stretching gives rise to a non - degenerate
Raman active mode, while the asymmetric stretching is doubly degenerate and
both IR and Raman active. Two deformation modes occur, the out - of - plane defor-
mation ( δ
oop
) which is IR active, and the in - plane deformation ( δ
ip
) which is degen-
erate and both IR and Raman active. By lowering the symmetry, for example to
the C
2v
point group (which occurs when only two of the three oxygen atoms are
Table 3.4 Crystal structures of some solid ternary - oxide
structures with the stoichiometry ABO
3
, and the irreducible
representations of the optical modes.
Structure name Example Space
group
Z
Ilmenite FeTiO
3
R3
C
3
2
i
6 5A
g
(R) + 5E
g
(R) + 4A
u
(IR) + 4E
u
(IR)
LiNbO
3
LiNbO
3
R 3 c
C
3
6
v
6 4A
1
(IR) + 5A
2
(R) + 9E(IR,R)
Cubic
perovskite
SrTiO
3
Pm 3 m O
h1
1 3 F
1u
(IR) + F
2u
(in)
Tetragonal
perovskite
BaTiO
3
P 4 mm
C
4
1
v
1 3A
1
(IR,R) + 4E(IR,R) + B
1
(R)
Orthorombic
perovskite
LaFeO
3
Pnma
D
2
16
h
4 7A
g
(R) + 7B
1g
(R) + 5B
2g
(R) + 5B
3g
(R) +
8A
u
(in) + 7B
1u
(IR) + 9B
2u
(IR) + 9B
3u
(IR)
Rhombohedral
perovskite
LaMnO
3
R3c
D
3
6
d
2 A
1g
(R) + 3A
2g
(in) + 4E
g
(R) + 2A
1u
(in) +
2A
2u
(IR) + 4E
u
(IR)
Calcite CaCO
3
R3c
D
3
6
d
2 A
1g
(R) + 3A
2g
(in) + 4E
g
(R)+2A
1u
(in) +
2A
2u
(IR) + 4E
u
(IR)
Aragonite CaCO
3
Pmcn
D
2
16
h
4 9A
g
(R) + 6B
1g
(R) + 6B
2g
(R) + 9B
3g
(R) +
6A
u
(in) + 8B
1u
(IR) + 8B
2u
(IR) + 5B
3u
(IR)
3.4 The Skeletal IR Spectra of Metal Oxides 123

124 3 The Use of Infrared Spectroscopic Methods
equivalent), the doubly degenerate modes split and all six modes become both IR
and Raman active.
The highest symmetry point group for non - planar pyramidal [MO
3
]
n −
oxo - anions
(such as sulfi te, selenite and tellurite anions) is C
3v
. In this case too all modes are
both IR and Raman active. The symmetric stretching and deformations are non -
degenerate while the asymmetric modes are doubly degenerate. Such a degeneracy
is broken when symmetry is further lowered. Typical positions for the correspond-
ing modes are reported in Table 3.5 . The commonest crystal structures for AMO
3
salts, which have been the object of vibrational studies, are calcite, aragonite,
dolomite and nitrates of divalent metals.
Salts with Tetrahedral
MO
4
n
--
Oxo - Anions Including High - Aluminum - Zeolites Most
of the
MO
4
n−
oxo - anions take a tetrahedral - like coordination. In this case we have
nine internal vibrations plus six external vibrations contributing to lattice vibra-
tions and to acoustic modes. When the ion takes its highest symmetry ( T
d
point
group), the symmetric stretching and the symmetric deformation are Raman
active, the last being doubly degenerate. The asymmetric stretching and deforma-
tion are both IR and Raman active and are triply degenerate. If the symmetry is
lowered, the degeneracies are broken and all modes can become IR and Raman
active. This is shown in Table 3.6 , where the typical positions of such modes
are also reported for different oxo - anions. Examples are magnesium orthovanadate
Mg
3
(VO
4
)
2
[66] and Scheelite type orthomolybdates such as CdMoO
4
[67],
whose IR and Raman skeletal spectra have been studied in detail. The presence
Table 3.5 Normal modes of vibrations and their activity for
isolated
MO
3
n−
oxo - anions, and typical vibrational frequencies.
Normal modes of vibrations and their activity
Planar
ν
sym
δ
oop
ν
asym
δ
ip
D
3 h
′
A
1
(R)
′′
A
1
(IR)
E ′ (IR, R) E ′ (IR, R)
C
2 v
A
1
(IR,R) B
2
(IR,R) A
1
(IR,R)
B
1
(IR,R)
A
1
(IR,R)
B
1
(IR,R)
Pyramidal
ν
sym
δ
sym
ν
asym
δ
asym
C
3 v
A
1
(IR,R) A
1
(IR,R) E (IR, R) E (IR, R)
C
s
A ′ (IR, R) A ′ (IR, R) A ′ (IR, R)
A ″ (IR, R)
A ′ (IR, R)
A ″ (IR, R)
Typical band positions (cm
− 1
)
BO
3
3−
planar
1100 – 900 750 – 650 1500 – 1250 600 – 550
CO
3
2−
planar
1100 – 1050 900 – 850 1480 – 1380 750 – 670
NO
3
−
planar
1080 – 1030 840 – 800 1490 – 1320 750 – 690
SO
3
2−
pyram
.
1000 – 960 670 – 610 1000 – 910 520 – 450
SeO
3
2−
pyram
850 – 780 500 – 400 750 – 700 400 – 350
TeO
3
2−
pyram
800 – 750 430 – 330 730 – 680 350 – 300

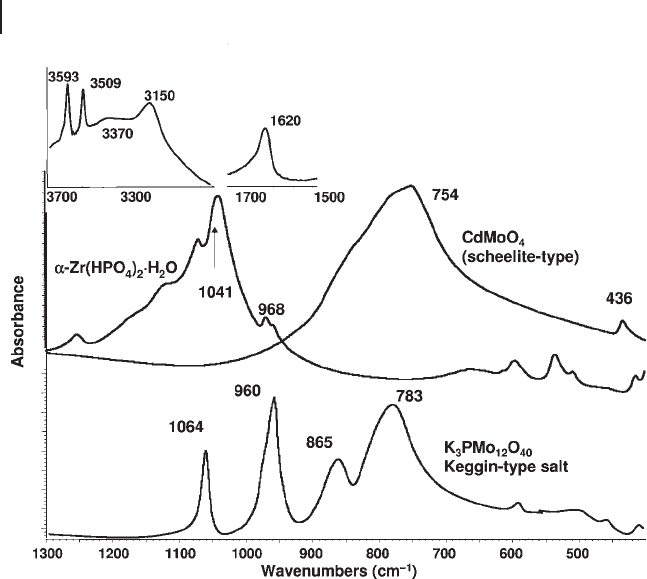
of a strong complex band with a main maximum at 754 cm
− 1
and the absence of
strong bands in the region 1020 – 900 cm
− 1
provides evidence of the absence
of short Mo
=
O bonds, and the substantial equivalence of the four bonds in
the truly tetrahedral molybdate ions in the scheelite structure of CdWO
4
(Figure 3.11 ).
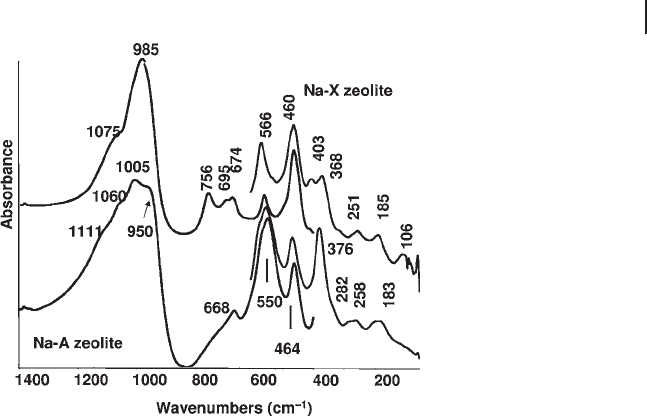
To this family belong high - aluminum zeolites with Si to Al atomic ratio ∼ 1,
which are fundamentally Al, alkali orthosilicates, such as the A zeolites (LTA) and
some faujasites such as NaX zeolite. In Figure 3.12 the IR spectra are shown of
the zeolites NaA (Na - LTA following the IZA international code) and NaX (Na -
FAU), as typical cation - containing zeolites whose composition is, in both cases,
Na
12
Al
12
Si
12
O
48
. The unit cell of dehydrated NaA zeolite (Na
12
(SiO
2
)
12
(AlO
2
)
12
formula) belongs to the Fm c O
h
3 226
6
=≡ space group, with Z = 8. The number
of molecular units in the smallest Bravais cell is 2. Accordingly, 168 atoms are
present in the smallest Bravais cell, 144 of which are framework Si, Al and O
Table 3.6 Normal modes of vibrations and their activity for
tetrahedral oxo - anions, and typical vibrational frequencies.
Normal modes of vibrations and their activity
ν
sym
δ
sym
ν
asym
δ
asym
T
d
A
1
(R) E (R) F
2
(IR,R) F
2
(IR,R)
C
3v
A
1
(IR, R) E (IR, R) A
1
(IR, R) E (IR, R) A
1
(IR, R)
E (IR, R)
C
2v
A
1
(IR, R) A
1
(IR, R)
A
2
(R)
A
1
(IR, R)
B
1
(IR, R)
B
2
(IR, R)
A
1
(IR, R)
B
1
(IR, R)
B
2
(IR, R)
C
1
A (IR, R) 2A (IR, R) 3A (IR, R) 3A (IR, R)
Typical band positions (cm
− 1
)
SiO
4
4−
850 – 800 450 – 300 1000 – 850 650 – 450
GeO
4
4−
800 – 600 350 – 250 850 – 650 550 – 400
PO
4
3−
1000 – 920 420 – 350 1080 – 950 600 – 530
AsO
4
3−
900 – 830 400 – 320 880 – 750 500 – 410
SbO
4
3−
800 – 500 400 – 300 800 – 500 400 – 300
VO
4
3−
915 – 800 500 – 350 900 – 730 500 – 350
NbO
4
3−
800 – 500 400 – 300 800 – 500 400 – 300
SO
4
2−
1070 – 950 520 – 410 1270 – 1030 670 – 570
SeO
4
2−
860 – 810 370 – 300 940 – 840 460 – 390
CrO
4
2−
900 – 830 360 – 330 960 – 860 410 – 330
MoO
4
2−
940 – 860 360 – 310 910 – 740 (360 – 310)
WO
4
2−
960 – 900 355 – 320 870 – 830 (355 – 320)
MnO
4
−
850 – 820 420 – 380 940 – 880 (420 – 380)
ReO
4
−
1000 – 950 350 – 300 950 – 900 350 – 300
3.4 The Skeletal IR Spectra of Metal Oxides 125
126 3 The Use of Infrared Spectroscopic Methods
atoms. Consequently, by applying factor group analysis, 429 optical degrees of
freedom are associated with the zeolite LTA framework (Na ions excluded) giving
rise to the following irreducible representation:
Γ
optg ggg gu
AR Aia ER Fia FR Aia=
()
+
()
+
()
+
()
+
()
+
(
11 11 22 25 25 7
12 121
))
+
()
+
()
+
()
+
()
7142829
212
A ia E ia F IR F ia
uu u u
Thus 28 IR active modes are expected to fall in the regions of the vibrations of
the orthosilicate anions. Of these, we can expect fi ve modes associated with ν
3
(asymmetric stretching) and two modes associated with ν
1
(symmetric stretching),
three modes associated with the symmetric deformation ( ν
2
) and fi ve with the
asymmetric deformation ν
4
, four hindered rotations, four hindered translations,
and, fi nally, fi ve modes associated with Al
–
O tetrahedra. We actually observe at
least 10 components for framework vibrations. Additionally, the low - frequency
modes of Na ions are expected to fall in the FIR region [68] , where several bands
are indeed observed.
The structure of NaX zeolite also belongs to the Fm c O
h
3 226
6
=≡ space group,
with Z = 8. Thus, also in this case we expect 28 IR active fundamentals, while we
observe at least nine components for the framework spectrum.
Figure 3.11 FTIR skeletal spectra of α - Zr(HPO
4
)
2
.
H
2
O,
CdMoO4 (scheelite type) and the Keggin - type heteropolyacid
salt K
3
PMo
1
2
O
40
.
In any case, the spectrum of these high - aluminum zeolites, which can be con-
sidered as zeolitic Na,Al - orthosilicates, differ from those of highly siliceous sili-
cates including most zeolites (which are actually framework silicates), and of silica
polymorphs (Figure 3.9 ) in the absence of the typical Si
–
O
–
Si symmetric stretch-
ing / bending mode occurring near 800 cm
− 1
. They also differ from phyllosilicates
such as kaolinite (Figure 3.9 , upper spectrum) in the absence of the Si
–
(OH)
stretching modes typically found near 900 cm
− 1
.
The tetrahedral oxo - anions can retain one or two protons in the crystal structure,
giving rise to monohydrogen or dihydrogen ortho anions. In this case, at least one
of the oxygen atoms is certainly unequivalent to the others, and the vibrations of
the O
–
H group also appear. As an example, several studies have appeared con-
cerning metal monohydrogen orthophosphates and dihydrogen orthophosphates.
In Figure 3.11 the spectrum of the interesting compound α - Zr(HPO
4
)
2
.
H
2
O is
reported. The band at 968 cm
− 1
is due to the P
–
O stretching of the POH group
while the strong complex band with the maximum at 1040 cm
− 1
is due to PO
3
stretches [69] .
Dimeric Condensed Tetrahedral Anions [X
2
O
7
]
n
-
Salts in which the tetrahedral oxo -
anions are condensed through common oxygen atoms are denoted with the prefi x
pyro - (for dimeric species) or meta - (for polymeric species). The oxo - anions
involved have terminal M
–
O bonds (six in total for dimeric pyro species, two for
each atom for polymeric meta anions), and in addition contain M
–
O
–
M bridges.
Such bridges are generally bent, but they can be sometimes linear, such as in the
cases of some pyrosilicates, pyrogermanates, pyrophosphates and pyroarsenates.
When the bridge is bent it gives rise (like the Si
–
O
–
Si bridges of silica, see above)
to an asymmetric stretching mode, a bending/symmetric stretching mode (both
Figure 3.12 FTIR and FTFIR skeletal spectra of zeolites Na - X
(Na - FAU) and Na - A (Na - LTA), Si/Al ∼ 1 in both cases.
3.4 The Skeletal IR Spectra of Metal Oxides 127

128 3 The Use of Infrared Spectroscopic Methods
are in - plane modes) and an out - of - plane rocking mode. For silicates, the ν
as
Si
–
O
–
Si and ν
sym
Si
–
O
–
SiO fall in the regions 1050 – 900 cm
− 1
and near 650 cm
− 1
,
respectively. According to Lazarev [70] a linear relationship exists between the
bond angle and the parameter ∆ = ( ν
as
− ν
sym
)/( ν
as
+ ν
sym
) × 100. When the bridge
is linear, (as in thortveitite, Sc
2
Si
2
O
7
) the symmetric stretching could become IR
inactive (or very weak if the environment is symmetric).
Several studies have been devoted to compounds containing condensed tetrahe-
dral anions [X
2
O
7
]
n −
such as pyrovanadates [66] , dichromates, pyrosulfates, pyrosel-
enates, pyrophosphates and pyroarsenates, pyroniobates, and so on. Typical of
these compounds are the vibrations of the bridging oxygens. Additionally, the
spectra show the vibrations of the terminal MO
3
. Each terminal MO
3
moiety gives
rise to one symmetric and two asymmetric stretchings, one symmetric and two
asymmetric bendings, as well as to one twisting and two rocking modes.
In the case of vanadyl pyrophosphate, a very relevant oxidation catalyst, the IR
spectra also provide information on the morphology, allowing the distinction of
very active catalysts from less active, highly crystalline, materials [71] .
Salts with Polymeric Tetrahedral Oxo - Ions: Metasilicates, Layer Silicates and Framework
Silicates including Highly Siliceous Zeolites
The vibrational spectra of the
different kinds of chain and ring silicates are dominated by the vibrations of the
Si
–
O
–
Si bridges (as already discussed for amorphous silicas and for pyrosilicates,
see above) and of Si
–
O – terminal bonds. The difference between the “ network
silicate structure ” of silica forms and the layer silicate of kaolinite is mainly shown
by the presence, in the case of kaolinite, of strong bands with maxima at 940 and
913 cm
− 1
, typically due to Si
–
(OH) “ terminal ” stretchings. In this region silicas
and network silicates do not absorb. Layer silicates also present the absorptions of
Si
–
O
–
Si bridges and of terminal silanol groups Si
–
OH. Both terminal silicate
bonds Si
–
O
−
M
+
and silanols give rise to strong IR bands in the region 950 –
1000 cm
− 1
which frequently have signifi cant intensity in the Raman spectra also.
Cyclic structures and particular conformations of the chains can give rise to char-
acteristic features, particularly in the lower frequency regions (Si
–
O
–
Si rockings
and lattice modes). Terminal silanols, such those present in layer silicates, also
give rise to OH stretching modes strong in IR, such as those of kaolinite (Figure
3.9 ).
Highly siliceous zeolites (with Si/Al ratios >> 1) are microporous framework
alumino - silicate materials. Discussion of the framework skeletal vibrations of
highly siliceous zeolites is similar to that reported above for silicas. The addition
of aluminum in the framework causes shifts in the positions of the sole band. In
particular, the asymmetric Si
–
O
–
Si stretching modes of framework silicates,
usually observed as a complex very strong absorption in the region 1200 – 1000 cm
− 1
,
tend to shift down a little with Al for Si substitution.
Salts with Condensed Octahedral - Like Oxo - Anions Chains of oxo - anions can “ con-
dense ” giving rise to octahedral - like chains or multiple chains. This is the case,
for example, for several metavanadates forming double chains [(V
2
O
6
)
2 −
]
n
such as

magnesium metavanadate. In this case vibrational features appear due to triply
bridging O atoms and to pairs of short divanadyl VO
2
bonds [66] . Similarly, “ ortho-
tungstate ions ” , such as those of metal tungstates in the wolframite structure (e.g.
CdWO
4
), condense giving rise to single chains of corner - sharing octahedra. Cor-
respondingly, the spectra can be interpreted in terms of bridging W
–
O
–
W oxygens
and of pairs of ditungstyl WO
2
terminal groups [67] . Also, the so - called Keggin - type
heteropoly - salts such as K
3
[PMo
12
O
40
] are of this type: the anion, in fact, presents
12 distorted MoO
6
octahedra surrounding a central phosphate tetrahedron. Every
molybdenum atom presents one terminal short Mo
=
O “ double bond ” which is
responsible for the strong stretching band at 960 cm
− 1
(Figure 3.11 ), with addi-
tional Mo
–
O
–
Mo bridges (asymmetric stretching at 865 cm
− 1
) and also oxygen
atoms triply bridging between one P and two Mo atoms (mainly responsible for
the strongest band 783 cm
− 1
. The band at 1064 cm
− 1
is essentially due to P
–
O
asymmetric stretching of the orthophosphate group.
3.4.3
Amorphous versus Crystalline Oxide Materials
In many cases, the skeletal spectra of amorphous oxides are similar to those of
the corresponding crystalline materials, with broader and less - resolved features.
This is the case, for example, in amorphous versus crystalline V
2
O
5
[72] . In a few
cases, however, amorphous material may also have relatively unusual features,
different from those of the crystalline counterparts. For example, amorphous
alumina is characterized by only octahedral cation coordination, in contrast to
transitional aluminas which also have tetrahedral Al coordination. The skeletal IR
spectrum of amorphous alumina is characterized by a strong band near 550 cm
− 1
,
and by the absence of the feature in the region near 800 cm
− 1
, associated with AlO
4
tetrahedra, present in crystalline transitional aluminas [73] .
3.5
Skeletal Spectra of Precursors for Metal Oxide Catalysts
Oxide catalysts may be obtained by thermal decomposition of precipitated precur-
sors. These materials, usually easily decomposable solids, may be salts such as
carbonates and nitrates, whose skeletal spectroscopic features have been discussed
above. Alternatively they may be simple or complex hydroxides.
3.5.1
Metal Hydroxides
The hydroxides in their solid crystalline structures generally form by condensed
polyhedra containing the metal element surrounded by oxygen atoms, with hydro-
gen atoms located externally to their polymeric structures and involved in strong
hydrogen bonding. According to this picture, the skeletal spectra of hydroxides
3.5 Skeletal Spectra of Precursors for Metal Oxide Catalysts 129

130 3 The Use of Infrared Spectroscopic Methods
show features typically due to the OH groups, and features to be assigned to the
vibrations of MO
x
polyhedra. For the latter features, the same considerations
already made for oxides are valid.
The vibrations of hydroxyl groups are typically composed of O
–
H stretches
(3800 – 2000 cm
− 1
, depending upon the extent of H - bonding), in - plane bending
(1200 – 800 cm
− 1
) and out - of - plane deformation (generally below 1000 cm
− 1
). The
multiplicity of such modes depends upon the number of hydroxyl groups present
in the smallest Bravais cell and in the coupling of their vibrations, and is also
dependent on the hydrogen - bonding patterns.
The centrosymmetric structures of LiOH and NaOH, both containing two
formula units per unit cell, gives rise to two OH stretching modes, one of which
is IR active and the other Raman active. In the case of KOH, whose structure is
not centrosymmetric and contains two formula units per unit cell, a split of both
IR and Raman active peaks is detectable. The positions of these peaks (in the
3680 – 3600 cm
− 1
region) shows that no hydrogen bonding occurs in these
structures.
The structure of brucite Mg(OH)
2
is assumed by most divalent hydroxides. Every
layer of Mg(OH)
2
contains MgO
6
octahedra with three triply bridging hydroxyl
groups pointing up and three pointing down, alternately. The unit cell of the Brucite
structure cointains only one Mg(OH)
2
unit. So, twelve optical modes should exist.
Two OH stretching modes exist, one Raman active (symmetric stretching, A
1g
,
3655 cm
− 1
) and one IR active (asymmetric OH stretching, A
2u
, 3700 cm
− 1
). The posi-
tions of these modes shows that no hydrogen bonds occur in this structure. Addi-
tional two doubly degenerate deformation modes occur, again one is Raman active
(E
g
) and one is IR active (E
u
). Finally, two IR active modes (A
2u
+ E
u
) and two Raman
active modes (A
1g
+ E
g
) are associated with vibrations of the MgO
6
octahedra. In the
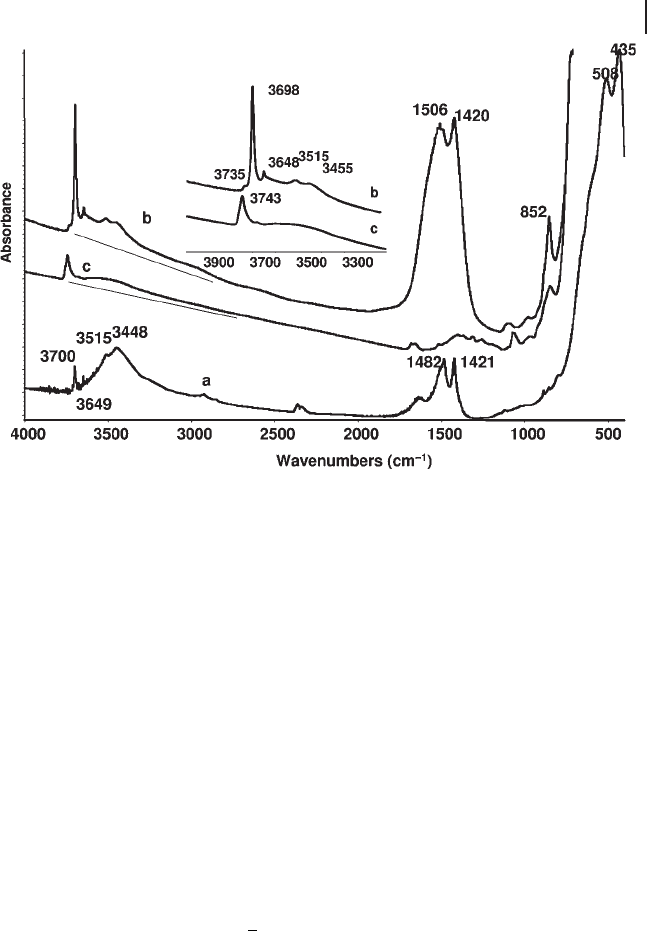
Figure 3.13 the spectra of a KBr disk (a) and of a pure powder pressed disk (b) of
a commercial “ MgO ” sample is shown. The spectra show well the band at 3700 –
3698 cm
− 1
of Mg(OH)
2
impurity in the sample. The pure powder spectrum also
shows a peak at 3648 cm
− 1
, which probably corresponds to the Raman mode, slightly
shifted and activated in IR owing to its location on a surface layer.
The goethite or diaspore structure of α - AlOOH, α - FeOOH, α - CrOOH and α -
GaOOH have orthorhombic unit cells, belonging to Pbnm D
h
==
2
16
62 space group
with Z = 4. The overall unit cell and the smallest Bravais cell coincide and contain
16 atoms; thus 45 vibrational modes are expected. All atoms occupy the 4c Wyckoff
position in the cell on a refl ection plane ( C
s
site symmetry). The irreducible rep-
resentation for the IR optical modes of α - MeOOH is as follows, separating the
modes due to the hydrogen - bonded OH groups from those of the Me
–
O
skeleton:
Γ
Me O u u u
BIR BIR BIR
−
=
()
+
()
+
()
525
123
Γ
OH u u u
BIR RIR BIR
−
=
()
+
()
+
()
212
123
Consequently, the IR spectrum is expected to contain 17 fundamental modes,
only partly resolved. A typical feature of the spectra of these compounds is the
presence of two IR - active OH stretches, giving rise to a strong band with a shoulder
at the higher frequency in the IR spectra (3126, 3050 cm
− 1
for goethite; 3000,
2920 cm
− 1
for diaspore; 2913, 2840 cm
− 1
for α - GaOOH) as well as two strong
bending modes (891, 796 cm
− 1
for goethite, 1080, 970 cm
− 1
for diaspore, 1019,
952 cm
− 1
for α - GaOOH), which have been discussed in terms of the geometry of
the hydrogen - bonding situations. α - (Cr,Fe)OOH [74] and α - (Fe,Ga)OOH [75] ,
forming goethite - type solid solutions, have been also characterized by IR.
X - ray diffraction analysis indicates that boehmite γ - AlOOH and lepidocrocite γ -
FeOOH crystallize in the space group Cmcm n D
h
≡≡.63
2
17
, with Z = 4. However,
such analysis does not reveal the position of the hydrogen atoms. Instead, vibra-
tional spectroscopies can be used to obtain such information. The IR spectra are
dominated by two well - split OH stretches (3305, 3090 cm
− 1
for boehmite) and two
OH deformations (1170 and 1074 cm
− 1
), see Figure 3.6 .
In Figure 3.5 the spectrum of the hydroxy - chloride paratacamite, Cu
2
Cl(OH)
3
, is
shown, together with that of its decomposition product CuO. The crystal structure
of paratacamite belongs to the R
3
space group n.148
3
2
= C
i
. The number of molecu-
lar units in the smallest Bravais cell is 8, so that this cell contains 16 copper atoms,
24 oxygen and hydrogen atoms and 8 chlorine atoms. This gives rise to a very
complex vibrational structure, with 213 vibrational freedom degrees, giving rise to
142 vibrational modes of which 72 are Raman active and 70 are IR active. In particu-
lar, 24 OH stretching modes are expected (12 IR active and 12 Raman active)
together with 24 degenerate OH deformation modes (again 12 IR active and 12
Raman active), 8 Cu
–
Cl stretching modes (4 IR and 4 R), and also 8 Cu - Cl deforma-
tions (4 IR and 4 R), and fi nally 78 Cu
–
O modes (40 Raman active and 38 IR active).
Figure 3.13 FTIR spectra of a “ MgO ” commercial sample:
(a) KBr pressed disk; (b) pure powder pressed disk outgassed
at 473 K; (c) pure powder pressed disk outgassed at 873 K.
3.5 Skeletal Spectra of Precursors for Metal Oxide Catalysts 131

132 3 The Use of Infrared Spectroscopic Methods
It is obvious that most of the modes are superimposed. The IR spectrum clearly
shows at least four of the 12 IR active OH stretching modes resolved (see the insert
in Figure 3.3 ) while 12 maxima can be distinguished in the region 1200 – 700 cm
− 1
,
which could correspond to the resolution of all the IR - active OH bending modes.
Below 60 cm
− 1
several maxima are also observed, due to Cu
–
O and Cu
–
Cl vibra-
tions. The features observed in the region 1700 – 1300 cm
− 1
are either due to harmon-
ics of lower frequency vibrations or to water and carbonate impurities.
3.5.2
Hydrated Compounds
Gas - phase or isolated water molecules belong to the C
2v
point group and give rise
to three optical modes 2A
1
+ B
1
, all both IR and Raman active. The two stretches
are at 3756 cm
− 1
(B
1
, asymmetric stretch) and at 3657 cm
− 1
(A
1
, symmetric stretch)
while the bending mode (A
1
) is at 1594 cm
− 1
. When water molecules are incorpo-
rated into a crystal, their six external modes (arising from frustrated rotations and
translations) contribute to lattice modes called “ librations ” of water molecules
(wagging, rocking and twisting modes) and to the acoustic modes. When water is
coordinated through oxygen lone pairs to cations (aquo - complexes) the metal –
oxygen stretching mode and the corresponding deformation modes appear and
couple with the other metal - to - ligand vibrations. The metal – oxygen stretches are
most usually located below 600 cm
− 1
. At slightly higher frequencies (900 – 500 cm
− 1
)
the wagging and twisting modes of coordinated water molecules become internal
vibrations of the aquo - complex. Coordination per se causes relatively weak perturba-
tions on the stretching and bending modes of water. However, coordination is fre-
quently associated with additional hydrogen bonding of water, with other water
molecules or with anions present in the crystal structure. This can result in strong
shifts down (down to 2000 cm
− 1
) and broadening of the stretching absorptions while
the bending modes are not very sensitive and always fall in the region 1700 –
1550 cm
− 1
. Asymmetric hydrogen bonding (bonding to only one of the two protons
of water, gives rise to particular spectra where sharp high - frequency peaks together
with broad low - frequency OH stretching modes components are present together.
This occurs for α - Zr(HPO
4
)
2
.
H
2
O (Figure 3.11 ) where two very sharp OH stretching
modes at 3593, 3509 cm
− 1
, are due to the free OH groups of the two molecules in
the smallest Bravais cell while broader bands at 3370 and 3150 cm
− 1
are due to the
H - bonded OH groups of water and the hydrogenphosphate ions [76] . Protonated
forms of water such as the oxonium ion H
3
O
+
[77] have also been investigated.
3.5.3
Layered Double Hydroxides
Complex precipitates, such as layered double hydroxides, are frequently prepared
as precursors of oxide catalysts. Hydrotalcite is a hydrated hydroxy - carbonate
mineral with the formula Mg
6
Al
2
(OH)
16
CO
3
· 4 H
2
O. It represents the better known
and most popular member of a family of layered double metal hydroxide com-
