Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.


3.6 The IR Spectroscopy of Adsorbed Probe Molecules for Surface Chemistry Characterization 153
The Olefi n Polymerization Method Olefi ns are very reactive towards the electro-
philic attack of a Br ø nsted acid, and can undergo proton - catalyzed cationic polym-
erization at low temperature. The faster this phenomenon occurs, the stronger the
Br ø nsted acid and the more electron - rich is the olefi nic double bond. The experi-
ment must be performed in a medium – low temperature range (e.g. room tem-
perature) with olefi n pressures of the order of 20 – 200 Torr in order to favor the
oligomerization from the point of view of thermodynamics. The observed polym-
erization rate in our experimental conditions follows the order: 1,3 - butadiene >
isobutene > propene > ethylene [140 – 142] . Actually, ethylene polymerization is (in
the conditions used to evaluate the surface acidity of oxide catalysts) only observed
with very strong Br ø nsted acids, while some weak Br ø nsted acids only allow the
polymerization of butadiene. Additionally, it can be observed that very strong
Br ø nsted acids cause the formation of branched polymeric chains from linear
olefi ns, for example polyisobutene formation from both isobutene and from 1 -
butene and likely also from ethylene [143] . Alumina OH groups, although unable
to cause polymerization of the four butene isomers, cause the polymerization of
1,3 - butadiene.
3.6.3.2 The Br ø nsted Acidity of Simple Metal Oxides
It is evident that the ability to protonate a base depends upon the strength of the
base. The ability to protonate pyridine can be taken as the discriminatory behavior
to defi ne an oxide catalyst as a Br ø nsted acid. Typical ionic oxides, such as alkaline
earth oxides, cupric oxide, zinc oxide, aluminas, gallias, ferric oxides, chromic
oxide (Cr
2
O
3
), titanias, zirconias, ceria and thoria, even when highly hydroxylated,
are not able to protonate pyridine. Also, low - valency typical covalent oxides, such
as silicas (including silicated oxides), germania and boria - containing catalysts, do
not protonate pyridine. Consequently, such oxides are either weakly or non -
Br ø nsted acidic. On the contrary, pyridine is protonated on higher oxidation state
oxides such as vanadia, niobia, molybdena and tungsta, either in bulk form or
when supported. Also, materials containing surface chromic anhydride (CrO
3
),
bulk or supported phosphates and sulfated oxides protonate pyridine. This is
associated with the presence of M
=
O double bonds and the possibility of delocal-
izing the anionic charge after dissociation [101] . Olefi n oligomerization tests agree
with studies performed using protonable bases, showing that surfaces that are able
to protonate pyridine also cause propene oligomerization, while surfaces that
protonate piperidine only, such as alumina, are not able to oligomerize propene
but may cause 1,3 - butadiene oligomerization (Table 3.9 ).
3.6.3.3 The Br ø nsted Acidity of Protonic Zeolites
The strong Br ø nsted acid strength of the bridging OH groups of zeolites is
confi rmed by adsorption of basic probes followed by different techniques. Quite
strong bases such as pyridine are easily protonated, as shown in the spectra on
the left hand side of Figure 3.23 , where the bands of pyridinium ions (1636,
1625, 1547, 1491 cm
− 1
) are strongly predominant after pyridine adsorption on
H - MFI. Weak bases such as nitriles and CO hydrogen bond with these OH

154 3 The Use of Infrared Spectroscopic Methods
groups with a strong shift down of the ν OH mode. In Figure 3.21 , the spectra
of H - MFI before and after low - temperature adsorption of CO are reported. The
band at 3615 cm
− 1
, due to bridging OH groups, shifts down to near 3300 cm
− 1
,
with a ∆ ν ∼ 315 cm
− 1
, evidencing the strong acidity of these groups. In parallel,
the CO triple - bond stretching shifts up from 2138 cm
− 1
for the liquid like species
to > 2175 cm
− 1
.
Most data agree that when the Al content is relatively low, the amount of
Br ø nsted sites in zeolites actually strictly depends on Al concentration, according
to theory. The ratio between catalytically active sites and Al ions ranges apparently
from 80 to 100% for highly siliceous extraframework - species - free zeolites.
Many investigations, also using IR spectroscopic methods, have been performed
with the aim of revealing the degree of acid strength of different zeolites. Experi-
mental as well as theoretical data [77] show that besides the interactions of the
functional groups of the probe molecules with the zeolite ’ s Br ø nsted sites, the van
der Waals interactions of other unreactive groups of atoms with the zeolite cavity
walls may be very relevant, and participate in the stabilization of the adsorbed
species [100] . These interactions may vary signifi cantly as a function of the type
of zeolite and the dimension and shape of the cavities as well as the Al and proton
content and the presence of extra - framework species. Also, they depend on the
size and shape of the probe molecule. These “ confi nement effects ” make the cavi-
ties of the single zeolite structures unique solvation and reactivity environments
and play an important role in catalysis by zeolites. They also account for the dis-
crepancies among the acid strengths measured using different probes and differ-
ent techniques.
In the case of zeolites, studies have also been performed to distinguish sites
located at the external surface from those present in the cavities, and also to dis-
tinguish the position in different cavities for zeolites with a complex pore struc-
ture. This can be performed using molecules having different molecular sizes
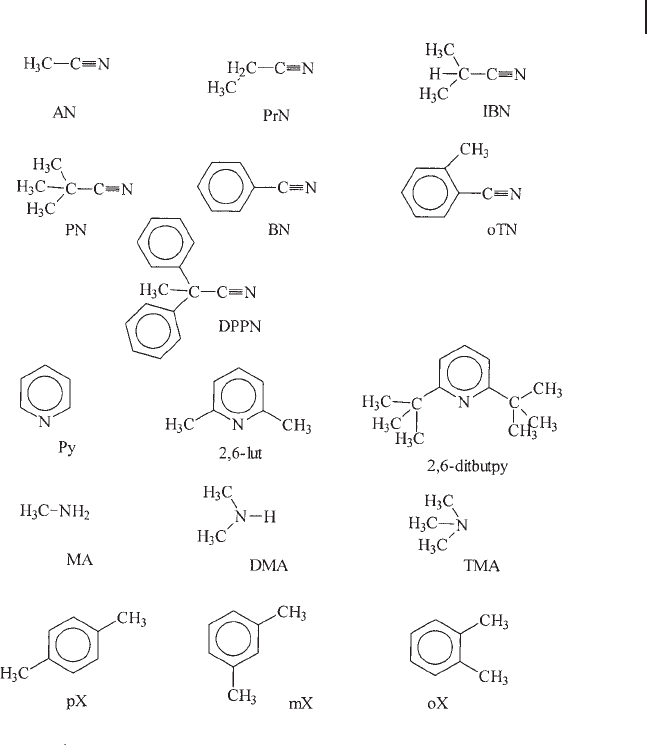
but similar chemical behavior. Some of the useful molecules are shown in
Scheme 3.6 .
Internal versus External and Extraframework Sites in Zeolite Acid Catalysis: the Use
of Hindered Basic Probes
Catalytically active sites also exist on the external surface
and at the pore mouth of zeolite crystals. These sites are considered to be respon-
sible for unwanted non - selective catalysis. On the other hand, H - zeolites also cata-
lyze reactions of molecules that do not enter the cavities because of their larger
size. So, the external surface of zeolites is certainly active in acid catalysis. Addi-
tionally, the bulk and surface Si/Al compositions of a zeolite could be different
and different preparation procedures can be chosen to modify this ratio.
The external surfaces of H - FER [143 – 145] , H - MFI [146, 147] and H - MOR [147,
148] have been studied by IR spectroscopy of adsorbed hindered aliphatic nitriles,
pyridine [145, 146] , lutidine and aromatic hydrocarbons [149] . Terminal silanols
and Lewis acid sites exist at the external surface of H - FER, H - MFI and H - MOR.
Interestingly, the acidity of the external silanol OH groups of zeolites can be
enhanced with respect to those of silica, appearing similar to those of SA. The very
3.6 The IR Spectroscopy of Adsorbed Probe Molecules for Surface Chemistry Characterization 155
strong bridging Br ø nsted acid sites, however, do not apparently exist at the external
surface, being totally confi ned to the internal surface.
Zeolite catalysts are frequently applied after treatments that tend to increase
their stability and also to further enhance surface acidity and shape selectivity
effects. These treatments, such as steam dealumination, can cause a decrease in
the framework Al content and the release of aluminum - containing species from
the framework. This can contribute to the stability of the framework, but extra -
framework species can also contain additional catalytically active acid sites. These
particles can also narrow the size of the zeolite channels or of their mouths, so
improving the shape selectivity effects. Extra - framework material ( EF ) can also
Scheme 3.6 Probe molecules for the study of
localization of active sites in microporous
materials: AN = acetonitrile; PrN =
propionitrile; IBN = isobutyronitrile; PN =
pivalonitrile (2,2 - dimethyl - propionitrile);
BN = benzonitrile; oTN = ortho - toluonitrile;
DPPN = 2,2 - diphenylpropionitrile; py =
pyridine; 2,6 - lut = 2,6 - dimethylpyridine, 2,6 -
lutidine; 2,6,ditbpy = 2,6, di - tert - butyl - pyridine;
MA = methylamine; DMA = dimethyl amine;
TMA = trimethylamine; pX, mX, oX = para - ,
meta - and ortho - xylene.
156 3 The Use of Infrared Spectroscopic Methods
arise from the preparation or the activation procedure or by addition of other
components by impregnation or ion exchange. The presence of EF material gives
rise to the presence of strong additional bands in the IR OH - stretching spectrum.
In general, IR bands above 3750 cm
− 1
, and in the region 3730 – 3650 cm
− 1
in pro-
tonic zeolites, is attributed to OH groups on EF materials.
Some authors believe that EF material is released at the external surface of zeo-
lites. The use of hindered nitriles, however, demonstrated that the EF material
produced by thermal treatment in H - MOR is in the interior of the side pockets
[150] . Similarly in a sample of H - MFI, EF material was found to be located in the
interior of the channels [150] .
IR Determination of the Location of Acid Sites and of the Diffusivity of Large Molecules
in Protonic Zeolites with Complex Pore Structure
The use of hindered nitriles as
probe molecules can be used to to distinguish Br ø nsted sites located in different
cavities in complex pore zeolites. The framework of the FER zeolite gives rise to
two kinds of channels, one of which is a 10 - membered ring, and the other a smaller
8 - membered ring. H - FER sample shows a single band due to bridging OH groups
centered at 3595 cm
− 1
. Acetonitrile ( AN ) perturbs this band strongly, while propio-
nitrile ( PN ) does not perturb it at all, being unable to enter either type of cavity.
Consequently, we attempted to distinguish the sites in the two channels by using
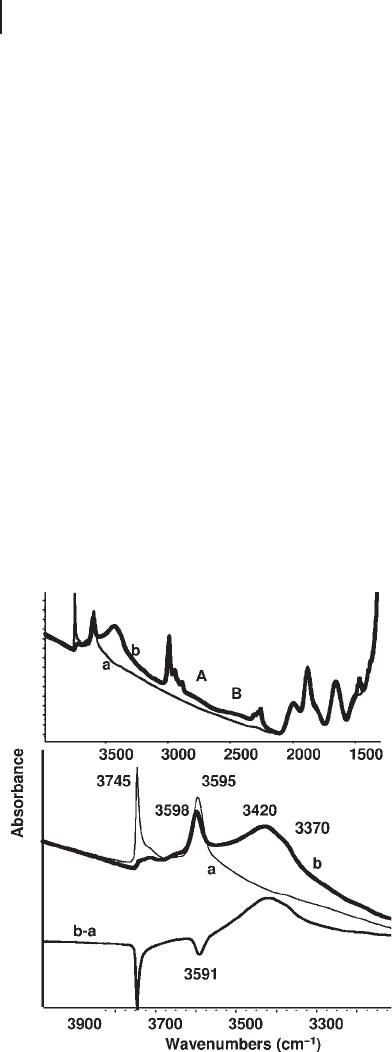
isobutyronitrile ( IBN ) as a probe (Figure 3.24 ). Upon IBN adsorption, the band of
Figure 3.24 FTIR spectra of H - FER zeolite (Zeolyst, Si/Al =
27.5) (a) after activation; (b) after contact with IBN vapour.
b − a: subtraction spectrum.

3.6 The IR Spectroscopy of Adsorbed Probe Molecules for Surface Chemistry Characterization 157
the external terminal silanols is fully perturbed and shifted to 3420 cm
− 1
with a
component near 3370 cm
− 1
. IBN interacts also with part of the bridging OH groups
of H - FER. Upon adsorption of IBN in fact, the band of bridging OH groups
decreases only slightly in intensity. Part of it is unperturbed and does not change
its shape and breadth. Accordingly, a weak A,B,C pattern appears, due to the strong
interaction of part of the H - FER bridging OH groups with IBN. The maximum of
the unperturbed band of the bridging OH groups is at 3598 cm
− 1
, while the
maximum of the band of the OH groups which are perturbed by IBN (read as a
minimum in the subtraction spectrum) is at 3591 cm
− 1
. We consequently assign
the band at 3591 cm
− 1
to the OH groups located in the 10 - membered ring channel
(accessible to IBN) and that at 3598 cm
− 1
(inaccessible to IBN) to the OH groups
located in the 8 - membered ring channels. Analysis of the intensities of the two
bands indicates that they are not far from the ratio 1 : 1.
Similar studies have been performed on H - BEA [151] , allowing the distinction
of the sites in the two channels of this structure, and on H - MOR [149, 150] where
three different types of OH group have been distinguished, located in the main
channels, in the intersection between main channels and side pockets and in the
side pockets. In the case of H - Y faujasite, the sites located in the supercages and
in the sodalite cages are easily distinguished. Using trimethylamine as a probe, a
third component, which is not perturbed at all by the probe, at 3501 cm
− 1
, assigned
to OH groups in the hexagonal prisms, is also observed [152] .
These studies also allowed the determination of the diffusivity of molecules
having different steric hindrance in the cavities of these zeolites, as summarized
in Table 3.10 .
3.6.4
Spectroscopic Detection and Characterization of the Surface Lewis Acid Sites
As already mentioned, coordinatively unsaturated cations exposed at the surface
of ionic oxides give rise to surface Lewis acid sites. Basic molecules can, conse-
quently, interact with these sites by forming a new coordination bond, so complet-
ing or increasing the overall coordination at the surface cation, as shown in
structure I of Scheme 3.5 . Upon this interaction, electrons transfer from the basic
molecules towards the catalyst surface. This electronic perturbation (and some-
times the molecular symmetry lowering) arising from this contact are the causes
of a vibrational perturbation of the adsorbate. In most cases, the vibrational per-
turbation only consists in shifts of some vibrational frequencies, the more pro-
nounced the stronger the interaction, that is, the greater the Lewis strength of the
surface site. Consequently, the shift of the position of some very sensitive bands
of the adsorbate upon adsorption can be taken as a measure of the Lewis acid
strength of the surface sites.
As an example, in Figure 3.14 the spectra of pyridine molecularly adsorbed on
β - Ga
2
O
3
are shown. The spectra are due to molecular Lewis - bonded pyridine,
without any absorption assignable to pyridinium ions. It is evident that the 8a and
19b pyridine bands (1580 and 1438 cm
− 1
in the liquid) are shifted signifi cantly
158 3 The Use of Infrared Spectroscopic Methods
Table 3.10 Summary of the characteristics of protonic zeolites and on the diffusion of molecular probes in their cavities
as measured by IR spectroscopy at room temperature.
Code Channels/cages Channel size
( Å )
IR ν OH
(cm
− 1
)
CN
CN
CN
FER 8 - ring channel [010]
3.5 × 4.8
3598 E E D D D D
10 - ring channel [001]
4.2 × 5.4
3591 E E E D D D
MFI 10 - ring channel [100]
(sinusoidal)
5.1 × 5.5 ∼ 3610
channel
E E E E D D
10 - ring channel [010]
(straight)
5.3 × 5.6 ∼ 3620
intersect
E E E E D D
BEA 12 - ring channels
[001]
5.6 × 5.6
3609 E E E E D D
12 - ring channels
[100]
6.6 × 6.7
3628, 3608,
3590
E E E E E D
MOR 8 - ring compressed
channels [001]
2.6 × 5.7
3588
side - pocket
E D D D D D
8 - ring side pockets
[010]
3.4 × 4.8
3609
intersect
E D D D D D
12 - ring main
channels [001]
6.5 × 7.0
3605 main
chann
E E E E E D
FAU Hexagonal prisms
accessed through
6 - ring channels
ca 2.7 × 2.7
3501 D D D D D D
Sodalite cages
accessed through
6 - ring channels
ca 2.7 × 2.7
3553 D D D D D D
Supercages accessed
through 12 - ring main
channels [111]
7.4 × 7.4
3625 E E E E E E
E = easy; D = diffi cult

3.6 The IR Spectroscopy of Adsorbed Probe Molecules for Surface Chemistry Characterization 159
upwards upon adsorption. The 8a mode is clearly multiple (three components can
be distinguished, at 1622, 1615 – 1605 and ∼ 1590 cm
− 1
, due to multiple adsorption
sites. The behavior observed with β - Ga
2
O
3
is very similar to that observed on
transitional aluminas (see below).
Similar experiments can be performed using nitriles as probe molecules. These
molecules are weaker as bases and this sometimes allows a better resolution in
the detection of the Lewis sites. Analysis is based on the study of the C
≡
N stretch-
ing region. In this region liquid acetonitrile shows a strong doublet at 2294,
2254 cm
− 1
, where the latter band is defi nitely stronger than the former. This
doublet is due to the Fermi resonance between the C
≡
N stretching and a δ CH
3
+
ν C
–
C combination. The virtual position of the CN stretching fundamental shifts
upwards by increasing electron withdrawal from the N lone pair upon adsorption,
and this causes the experimental position of both components of the doublet to
shift up. Simultaneously, however, the relative intensities of the two components
progressively inverts. Deuteroacetonitrile CD
3
CN and other nitriles have single CN
stretchings. They can also be used to determine the location of Lewis sites in pores
and cavities.
Low - temperature adsorption of CO is widely used as an acid site probe. In Figure
3.3 the spectra of CO over different solids are compared. The CO stretching band
may shift strongly up when it bonds as terminal carbonyl species on highly acidic
cationic centers, and can shift down signifi cantly when it bridges over two or three
metal atoms over an extended metal surface. In Table 3.11 the position of some
sensitive bands over oxide surfaces is reported, allowing the measurement of their
Lewis acidity.
3.6.4.1 The Lewis Acid Sites of Aluminas and SA s
The catalytic activity of transitional aluminas ( γ − , η − , δ − , θ − Al
2
O
3
) are undoubtedly
mostly related to the Lewis acidity of a small number of low coordination surface
aluminum ions, as well as to the high ionicity of the surface Al
–
O bond [101] .
Alumina ’ s Lewis sites have been well characterized by adsorption of several probes.
They are the strongest among metal oxides. The number of such very strong Lewis
sites present on transitional alumina surfaces depend on the dehydroxylation
degree (depending on the activation temperature) and on the particular phase and
preparation.
It seems that, although the different alumina spinel - type phases react a little
differently to outgassing, the density of the strongest Lewis acid sites tends to
decrease a little as the calcination temperature of the alumina increases (i.e. upon
the sequence γ → δ → θ , which is also a sequence of decreasing surface area). As
a result of this the number of strongest acid sites per gram signifi cantly decreases
in this sequence, although catalyst stability increases.
Although it is clear that surface Lewis acid sites on alumina are due to coordi-
natively unsaturated Al
3+
ions, it is not fully clear what is the coordination of such
surface ions. Most authors agree that at least three different types of Lewis acid
sites (weak, medium, strong) exist on transitional aluminas, arising in some way
from the two or three coordinations of the ions in the bulk spinel - type structure,
160 3 The Use of Infrared Spectroscopic Methods
namely octahedral and tetrahedral (normal spinel positions) and trigonal. Pyridine
adsorption produces three components for the 8a vibrations at 1624, 1618 and
1597 cm
− 1
, attributed to three diffent Lewis bonded species. Liu and Truitt [126]
emphasized the close proximity of Lewis acid sites to surface OH groups while in
their study Lundie and coworkers [153] identifi ed four different Lewis acid sites
arising from coordinatively unsaturated octahedral (the weakest) and tetrahedral
sites (the three strongest), three of which are considered to be associated with three
different types of hydroxyl groups.
Very strong Lewis acid sites can be detected on the surface of SA, characterized
by ν CO of adsorbed carbon monoxide at 2230 cm
− 1
(Figure 3.21 ), as well as by the
8a mode of adsorbed pyridine at 1625 cm
− 1
(Figure 3.23 ) They are certainly due to
highly uncoordinated Al ions and correspond to the strongest Lewis sites of tran-
sitional alumina or perhaps are even stronger, owing to the induction effect of the
covalent silica matrix. This makes SA also a very strong catalyst for Lewis acid
catalyzed reactions.
3.6.4.2 Lewis Acidity of Other Ionic Oxides
The strongest Lewis acidic oxides in normal circumstances are alumina and gallia,
that is, oxides of elements at the limit of the metallic character. The same elements
also give rise to halides characterized by even stronger Lewis acidity. Medium to
Table 3.11 Position (cm
− 1
) of the sensitive IR bands of adsorbed basic probe molecules on
different catalyst surfaces. The Lewis acid strength roughtly decreases from top to bottom.
Adsorbate CO Pivalonitrile Pyridine Ammonia Adsorbing
site type
IR mode
ν C O ν CN
8a
δ
sym
NH
3
AlF
3
2309, 2305 1627
IV
Al
3+
Zeolites (external surface) 2230 2300 1625 Masked
IV
Al
3+
Silica - alumina 2235 2296 1625 Masked
IV
Al
3+
γ - Al
2
O
3
2235 2296 1625 1295
IV
Al
3+
2210 – 2190 1615 1265
IV
Al
3+
1595 1220
VI
Al
3+
2170
Alumina - pillared
montmorillonite
2290 1625 Masked
IV
Al
3+
Acid - treated montmorillonite 2295 1625 Masked
IV
Al
3+
SiO
2
–
TiO
2
2226 w 2308 w
1610
Masked Ti
4+
2208 2285
WO
3
, unsupported = 2290 1613 1275, 1222
VI
WO
4+
ZrO
2
2195 1606 1210, 1160 Zr
4+
2170
Sulfated zirconia 2160 1606 1210, 1150 Zr
4+
TiO
2
anatase 2208 2285 1610 1225
V
Ti
4+
2182 2260 1185
VI
Ti
4+
Liquids 2143 2236 1583 1054

3.6 The IR Spectroscopy of Adsorbed Probe Molecules for Surface Chemistry Characterization 161
medium - strong Lewis acidity is found for other ionic oxides such as zirconias,
titanias, iron oxides, and so on.
3.6.4.3 Lewis Acidity of Highly Covalent Oxides
Lewis acidity is not usually observed for the covalent oxides of non - metal elements.
Interestingly in the case of silica, Lewis acidity is generally not found but it can
appear after very harsh pretreatment under outgassing [154] . The polarizing power
of the “ cations ” in the covalent oxide, is higher than 8. This shows that the reason
for the absence of Lewis acidity on the surface of these oxides is the diffi culty of
breaking the M
–
(OH) bonds, which are so covalent owing to the strong polarizing
power of the cation that would result by dissociation, with water desorption. Addi-
tionally, when Si(OH) groups actually break, couples of them tend to give rise to
“ strained ” Si
–
O
–
Si bridges, becuase of the strength of Si
–
O bonds, neutralizing
Lewis acidic coordinatively unsaturated Si ions. On the contrary, dehydroxylation
of ionic oxides is easier, and results in the generation of Lewis acid sites that are
suffi ciently stable to stay as such under relatively mild conditions.
On germania, Lewis acidity is generally not found. Oxides of transition metals
in very high oxidation states, such as vanadia, niobia, tungsta and molybdena,
show strong Lewis acidity, attributed to the coordinative unsaturation of vanadyl
[82] , niobyl [83] , tungstyl [84] and molybdenyl ions at the surface. The supported
oxides of pentavalent vanadium and niobium and of hexavalent tungsten and
molybdenum also display strong Lewis acidity [99] .
3.6.4.4 Lewis Acidity of Protonic Zeolites
Studies of adsorbed hindered molecules demonstrate that in non - defective zeolites
quite strong Lewis acidity is usually present at the external surface [149 – 152] .
Additional Lewis acidity occurs due to extra - framework aluminum oxide species
[100, 153] .
3.6.5
Determination of the Oxidation State of Cationic Centers
Carbon monoxide is a very weak base, widely used for the surface characterization
of cationic centers on metal oxide surfaces [155] . The electronic structure of CO
implies a triple bond between C and O, according to the stretching frequency
measured at 2143 cm
− 1
for the free molecule in the gas. In principle, the 14 elec-
trons are distributed symmetrically between C and O atoms, so that the lower
positive charge of the C nucleus with respect to O implies the formation of a dipole
with the negative charge at the C atom, in spite of the lower electronegativity of C
with respect to O. For this reason, the CO molecule tends to interact through the
C end with cationic centers. This interaction is rather weak, usually completely
reversible by outgassing at ambient temperature and should be studied at room
or lower temperature (e.g. at liquid nitrogen temperature, 77 K). According to
theoretical calculations, this interaction is a simple polarization, with no formation
of a true coordinative σ bond with the cationic center. This interaction tends to
162 3 The Use of Infrared Spectroscopic Methods
increase the CO bond order, so that the CO stretching frequency tends to increase.
Accordingly, the experimental measure of the CO stretching frequency for CO
interacting with surface cations can be taken as a measure of the polarizing power
of the cation or, in other terms, of its Lewis acidity (see Table 3.11 ).
However, when the cation or the metal atom contains, besides empty orbitals,
full or partly fi lled d - type orbitals, they can interact with the empty π
*
- type orbitals
of CO, via a π - type electron backdonation from the metal to CO. This implies that
these antibonding orbitals become partly fi lled, so that the bond order and the CO
stretching frequency is decreased by this last interaction. In this case, the interac-
tion can become very strong and very stable metal – carbonyl complexes can be
formed. The experimental CO stretching frequency in this case is a complex func-
tion of the electron accepting power of the cation (Lewis acidity) and of its π - type
electron donating power. Accordingly, the CO stretching frequency of CO adsorbed
on several transition metal cations is very informative concerning the oxidation
state of the adsorbing ion.
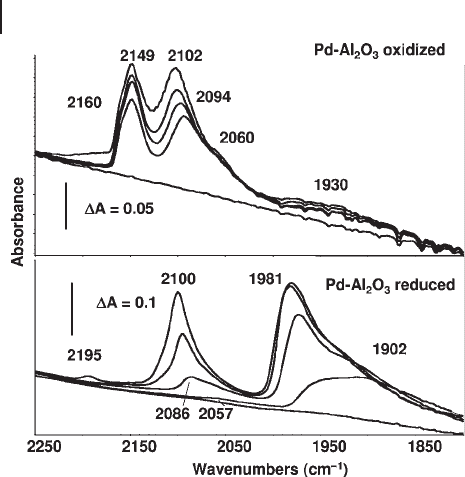
In Figure 3.25 the IR spectra of CO adsorbed at low temperature over a Pd/ γ -
Al
2
O
3
catalyst after oxidizing (upper spectra) and reducing pre - treatments (lower
spectra) are reported. In the oxidized catalysts a strong band composed of a main
maximum at 2149 cm
− 1
and a pronounced shoulder at 2160 cm
− 1
is evident. This
Figure 3.25 FTIR spectra of carbon monoxide adsorbed on
PdO
x
/ γ - Al
2
O
3
catalyst, after previous calcination in air at 673 K
(upper spectra) and after reduction in hydrogen (1 atm) at
673 K (lower spectra). The fi rst spectra in each series has been
obtained in contact with 10 Torr CO at 130 K for 5 min. The
other spectra were recorded upon outgassing (10
− 3
Torr) on
warming to 270 K.
